Efficiency of Interferon-γ in Activating Dendritic Cells and Its Potential Synergy with Toll-like Receptor Agonists
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culturing JAWSII Murine Dendritic Cells
2.3. Verifying the Expression of TLRs
2.4. Determining the Time to Treat Dendritic Cells
2.5. Evaluating the Efficiency of IFN-γ and TLR Agonists in Activating Dendritic Cells
2.6. Exploring the Potential of IFN-γ and TLR Agonists as Complementary Systems
2.7. Visualization of Dendritic Cell Activation
2.8. Exploring the Potential of IFN-γ, Poly I:C, and R848 Applied Simultaneously
2.9. Statistical Analysis
3. Results
3.1. TLR Expression by JAWSII Murine Dendritic Cells
3.2. Response Time of JAWSII Dendritic Cells to Stimulation
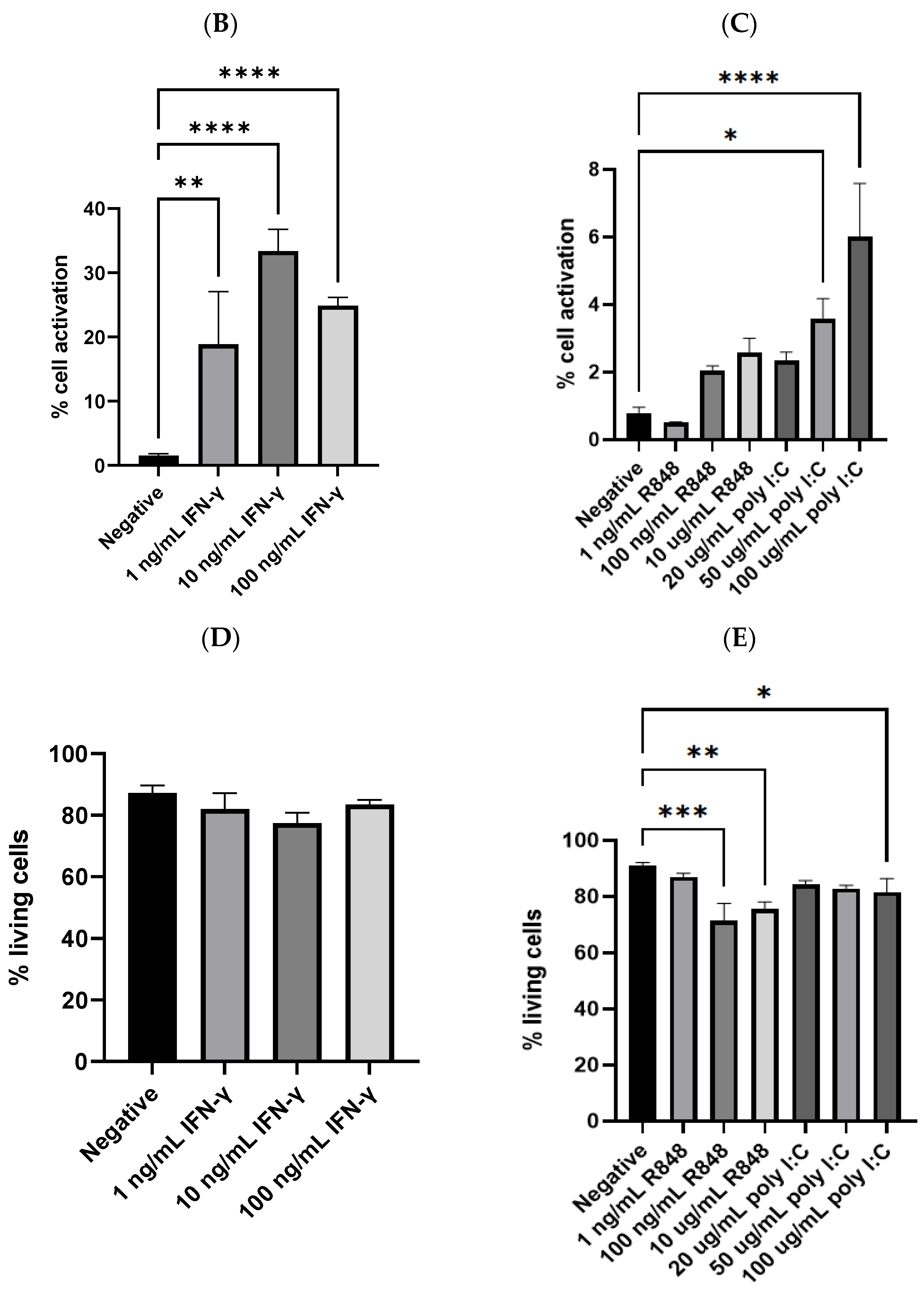
3.3. Efficiency of IFN-γ or Individual TLR Agonist in Activating Dendritic Cells
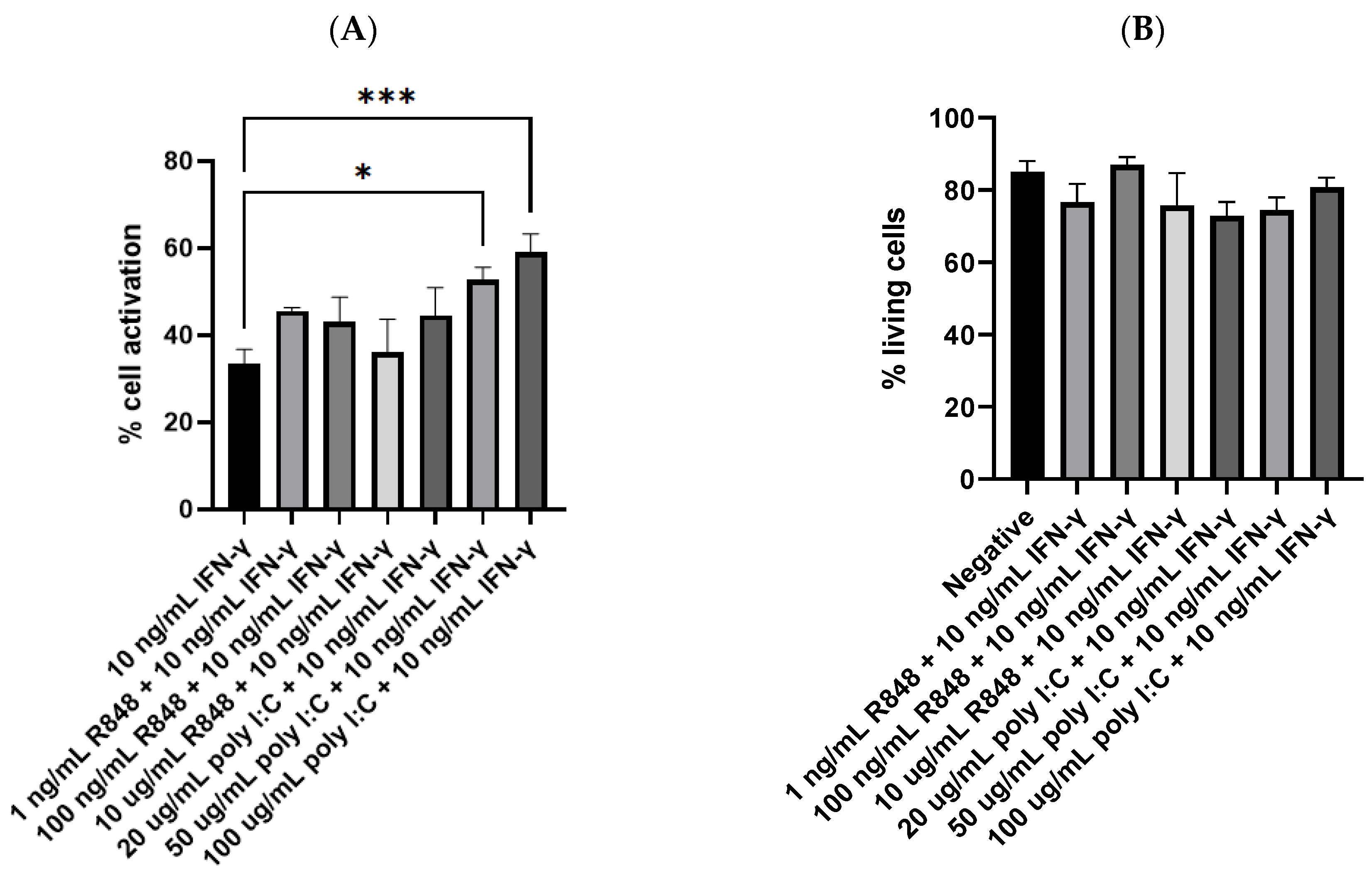
3.4. Potency of IFN-γ and TLR Agonists as Dual, Complementary Systems
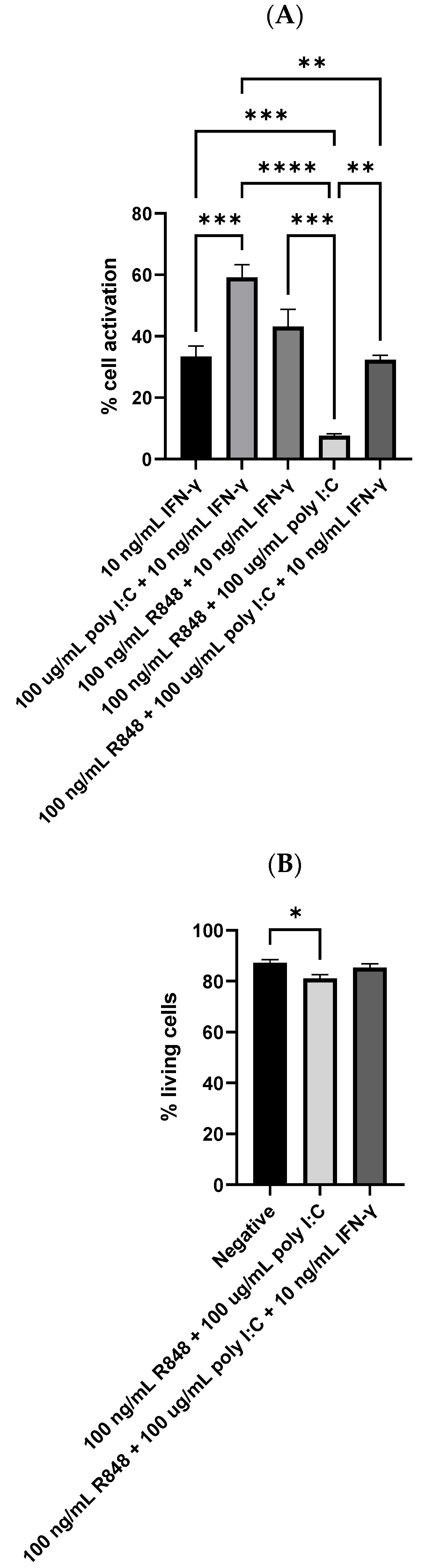
3.5. Dendritic Cell Reaction to IFN-γ, Poly I:C, and R848 Tri-Stimulator System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Hu, H.G.; Li, Y.M. Emerging Adjuvants for Cancer Immunotherapy. Front. Chem. 2020, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; MacLeod, M.K.; Kappler, J.W.; Marrack, P. Immune mechanisms of protection: Can adjuvants rise to the challenge? BMC Biol. 2010, 8, 37. [Google Scholar]
- Wang, Z.B.; Xu, J. Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant-Antigen Codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef]
- Chen, X.; Pravetoni, M.; Bhayana, B.; Pentel, P.R.; Wu, M.X. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine 2012, 31, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pravetoni, M.; Vervacke, J.S.; Distefano, M.D.; Tucker, A.M.; Laudenbach, M.; Pentel, P.R. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS ONE 2014, 9, e96547. [Google Scholar] [CrossRef]
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nieves, H.A.; Torrez, T.W.; Langridge, W.H. The Role of Dendritic Cell Maturation in the Induction of Insulin-Dependent Diabetes Mellitus. Front. Immunol. 2017, 8, 327. [Google Scholar] [CrossRef]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell. Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Zanetti, B.F.; Ferreira, C.P.; Vasconcelos, J.R.C.; Han, S.W. Adjuvant properties of IFN-γ and GM-CSF in the scFv6.C4 DNA vaccine against CEA-expressing tumors. Gene Ther. 2023, 30, 41–50. [Google Scholar] [CrossRef]
- Kumar, M.; Behera, A.K.; Hu, J.; Lockey, R.F.; Mohapatra, S.S. IFN-gamma and IL-12 plasmid DNAs as vaccine adjuvant in a murine model of grass allergy. J. Allergy Clin. Immunol. 2001, 108, 402–408. [Google Scholar] [CrossRef]
- Tovey, M.G.; Lallemand, C. Adjuvant activity of cytokines. Methods Mol. Biol. 2010, 626, 287–309. [Google Scholar]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar]
- van Slooten, M.L.; Hayon, I.; Babai, I.; Zakay-Rones, Z.; Wagner, E.; Storm, G.; Kedar, E. Immunoadjuvant activity of interferon-gamma-liposomes co-administered with influenza vaccines. Biochim. Biophys. Acta 2001, 1531, 99–110. [Google Scholar] [CrossRef]
- McCormick, A.L.; Thomas, M.S.; Heath, A.W. Immunization with an interferon-gamma-gp120 fusion protein induces enhanced immune responses to human immunodeficiency virus gp120. J. Infect. Dis. 2001, 184, 1423–1430. [Google Scholar] [CrossRef]
- Long, J.E.; Huang, L.N.; Qin, Z.Q.; Wang, W.Y.; Qu, D. IFN-gamma increases efficiency of DNA vaccine in protecting ducks against infection. World J. Gastroenterol. 2005, 11, 4967–4973. [Google Scholar] [CrossRef]
- Gnjatic, S.; Sawhney, N.B.; Bhardwaj, N. Toll-like receptor agonists: Are they good adjuvants? Cancer J. 2010, 16, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Jeong, J.H.; Yook, S. Immunoadjuvants for cancer immunotherapy: A review of recent developments. Acta Biomater. 2020, 114, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Tseng, J.C.; Yu, G.Y.; Luo, Y.; Huang, C.F.; Hong, Y.R.; Chuang, T.H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signaling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signaling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Yang, E.; Li, M.M.H. All About the RNA: Interferon-Stimulated Genes That Interfere With Viral RNA Processes. Front. Immunol. 2020, 11, 605024. [Google Scholar] [CrossRef]
- Meraro, D.; Gleit-Kielmanowicz, M.; Hauser, H.; Levi, B.Z. IFN-stimulated gene 15 is synergistically activated through interactions between the myelocyte/lymphocyte-specific transcription factors, PU.1, IFN regulatory factor-8/IFN consensus sequence binding protein, and IFN regulatory factor-4: Characterization of a new subtype of IFN-stimulated response element. J. Immunol. 2002, 168, 6224–6231. [Google Scholar]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Chen, M.; Huang, J.; Yang, X.; Liu, B.; Zhang, W.; Huang, L.; Deng, F.; Ma, J.; Bai, Y.; Lu, R.; et al. Serum starvation induced cell cycle synchronization facilitates human somatic cells reprogramming. PLoS ONE 2012, 7, e28203. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, C.; Rey-Ladino, J.; Yu, H.; Brunham, R.C. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect. Immun. 2008, 76, 2392–2401. [Google Scholar] [CrossRef]
- Schlitzer, A.; Sivakamasundari, V.; Chen, J.; Sumatoh, H.R.; Schreuder, J.; Lum, J.; Malleret, B.; Zhang, S.; Larbi, A.; Zolezzi, F.; et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat. Immunol. 2015, 16, 718–728. [Google Scholar] [CrossRef]
- Zapala, L.; Drela, N.; Bil, J.; Nowis, D.; Basak, G.W.; Lasek, W. Optimization of activation requirements of immature mouse dendritic JAWSII cells for in vivo application. Oncol. Rep. 2011, 25, 831–840. [Google Scholar]
- Zhang, M.; Yan, Z.; Wang, J.; Yao, X. Toll-like receptors 7 and 8 expression correlates with the expression of immune biomarkers and positively predicts the clinical outcome of patients with melanoma. OncoTargets Ther. 2017, 10, 4339–4346. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Dragnev, S.; Thurber, T.; Seed, B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genom. 2008, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [PubMed]
- Nanda, N.K.; Birch, L.; Greenberg, N.M.; Prins, G.S. MHC class I and class II molecules are expressed in both human and mouse prostate tumor microenvironment. Prostate 2006, 66, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, P.J. Expression regulation of major histocompatibility complex class I and class II encoding genes. Front. Immunol. 2011, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Wallberg, F.; Tenev, T.; Meier, P. Analysis of Apoptosis and Necroptosis by Fluorescence-Activated Cell Sorting. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087387. [Google Scholar] [CrossRef]
- Moser-Katz, T.; Gavile, C.M.; Barwick, B.G.; Lee, K.P.; Boise, L.H. PDZ Proteins SCRIB and DLG1 Regulate Myeloma Cell Surface CD86 Expression, Growth, and Survival. Mol. Cancer Res. MCR 2022, 20, 1122–1136. [Google Scholar] [CrossRef]
- MacKay, V.L.; Moore, E.E. Immortalized Dendritic Cells. U.S. Patent 5648219, 15 July 1997. [Google Scholar]
- Moore, E.E. Preparation of Immortalized Cells. U.S. Patent 5830682, 3 November 1998. [Google Scholar]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- De Waele, J.; Verhezen, T.; van der Heijden, S.; Berneman, Z.N.; Peeters, M.; Lardon, F.; Wouters, A.; Smits, E. A systematic review on poly(I:C) and poly-ICLC in glioblastoma: Adjuvants coordinating the unlocking of immunotherapy. J. Exp. Clin. Cancer Res. CR 2021, 40, 213. [Google Scholar] [CrossRef]
- Li, Y.G.; Siripanyaphinyo, U.; Tumkosit, U.; Noranate, N.; Nuegoonpipat, A.A.; Pan, Y.; Kameoka, M.; Kurosu, T.; Ikuta, K.; Takeda, N.; et al. Poly (I:C), an agonist of toll-like receptor-3, inhibits replication of the Chikungunya virus in BEAS-2B cells. Virol. J. 2012, 9, 114. [Google Scholar] [CrossRef]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The forgotten relative revindicated. Cell. Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Norgard, M.A.; Zhu, X.; Levasseur, P.R.; Sivagnanam, S.; Liudahl, S.M.; Burfeind, K.G.; Olson, B.; Pelz, K.R.; Ramos, D.; et al. Publisher Correction: The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019, 10, 5257. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, Z.; Li, J.; Long, H.; Wu, Y.; Zhang, Z.; Sun, Y. R848 Is Involved in the Antibacterial Immune Response of Golden Pompano (Trachinotus ovatus) Through TLR7/8-MyD88-NF-κB-Signaling Pathway. Front. Immunol. 2021, 11, 617522. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Suzue, K.; Asai, T.; Takeuchi, T.; Koyasu, S. In vivo role of IFN-gamma produced by antigen-presenting cells in early host defense against intracellular pathogens. Eur. J. Immunol. 2003, 33, 2666–2675. [Google Scholar] [CrossRef]
- Munder, M.; Mallo, M.; Eichmann, K.; Modolell, M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J. Exp. Med. 1998, 187, 2103–2108. [Google Scholar] [CrossRef]
- Rothfuchs, A.G.; Gigliotti, D.; Palmblad, K.; Andersson, U.; Wigzell, H.; Rottenberg, M.E. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 2001, 167, 6453–6461. [Google Scholar]
- Fricke, I.; Mitchell, D.; Mittelstädt, J.; Lehan, N.; Heine, H.; Goldmann, T.; Böhle, A.; Brandau, S. Mycobacteria induce IFN-gamma production in human dendritic cells via triggering of TLR2. J. Immunol. 2006, 176, 5173–5182. [Google Scholar] [CrossRef]
- Lu, R.; Groer, C.; Kleindl, P.A.; Moulder, K.R.; Huang, A.; Hunt, J.R.; Cai, S.; Aires, D.J.; Berkland, C.; Forrest, M.L. Formulation and preclinical evaluation of a toll-like receptor 7/8 agonist as an anti-tumoral immunomodulator. J. Control. Release Off. J. Control. Release Soc. 2019, 306, 165–176. [Google Scholar]
- Hartmann, D.; Schneider, M.A.; Lenz, B.F.; Talmadge, J.E. Toxicity of polyinosinic–polycytidylic acid admixed with poly-L-lysine and solubilized with carboxymethylcellulose in mice. Pathol. Immunopathol. Res. 1987, 6, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Kerr, D.; Stewart, W.E., 2nd; Field, A.K.; Oettgen, H.F. Phase I trials of poly(I,C) complexes in advanced cancer. J. Biol. Response Modif. 1985, 4, 640–649. [Google Scholar]
- Sanin, D.E.; Prendergast, C.T.; Mountford, A.P. IL-10 Production in Macrophages Is Regulated by a TLR-Driven CREB-Mediated Mechanism That Is Linked to Genes Involved in Cell Metabolism. J. Immunol. 2015, 195, 1218–1232. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 2008, 226, 41–56. [Google Scholar] [CrossRef] [PubMed]




| Genes [Mus musculus] | NCBI Reference | Primer Sequences (5’-3’) | Size (bp) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Tlr3 | NM_126166.5 | GTG AGA TAC AAC GTA GCT GAC TG | TCC TGC ATC CAA GAT AGC AAG T | 162 |
| NM_001357316.1 | ||||
| NM_001357317.1 | ||||
| Tlr4 | NM_021297.3 | ATG GCA TGG CTT ACA CCA CC | GAG GCC AAT TTT GTC TCC ACA | 129 |
| Tlr7 | NM_001290755.1 | ATG TGG ACA CGG AAG AGA CAA | GGT AAG GGT AAG ATT GGT GGT G | 207 |
| NM_001290756.1 | ||||
| NM_133211.4 | ||||
| NM_001290757.1 | ||||
| NM_001290758.1 | ||||
| Tlr8 | NM_133212.3 | GAA AAC ATG CCC CCT CAG TCA | CGT CAC AAG GAT AGC TTC TGG AA | 109 |
| NM_001313760.1 | ||||
| NM_001313760.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Walter, D.L.; Zhang, C. Efficiency of Interferon-γ in Activating Dendritic Cells and Its Potential Synergy with Toll-like Receptor Agonists. Viruses 2023, 15, 1198. https://doi.org/10.3390/v15051198
Bian Y, Walter DL, Zhang C. Efficiency of Interferon-γ in Activating Dendritic Cells and Its Potential Synergy with Toll-like Receptor Agonists. Viruses. 2023; 15(5):1198. https://doi.org/10.3390/v15051198
Chicago/Turabian StyleBian, Yuanzhi, Debra L. Walter, and Chenming Zhang. 2023. "Efficiency of Interferon-γ in Activating Dendritic Cells and Its Potential Synergy with Toll-like Receptor Agonists" Viruses 15, no. 5: 1198. https://doi.org/10.3390/v15051198
APA StyleBian, Y., Walter, D. L., & Zhang, C. (2023). Efficiency of Interferon-γ in Activating Dendritic Cells and Its Potential Synergy with Toll-like Receptor Agonists. Viruses, 15(5), 1198. https://doi.org/10.3390/v15051198








