Immune Reconstitution and Safe Metabolic Profile after the Switch to Bictegravir/Emtricitabine/Tenofovir Alafenamide Fumarate among Virologically Controlled PLWH: A 96 Week Update from the BICTEL Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Ethical Aspects
3. Results
3.1. Population Features
3.2. Virologic Efficacy
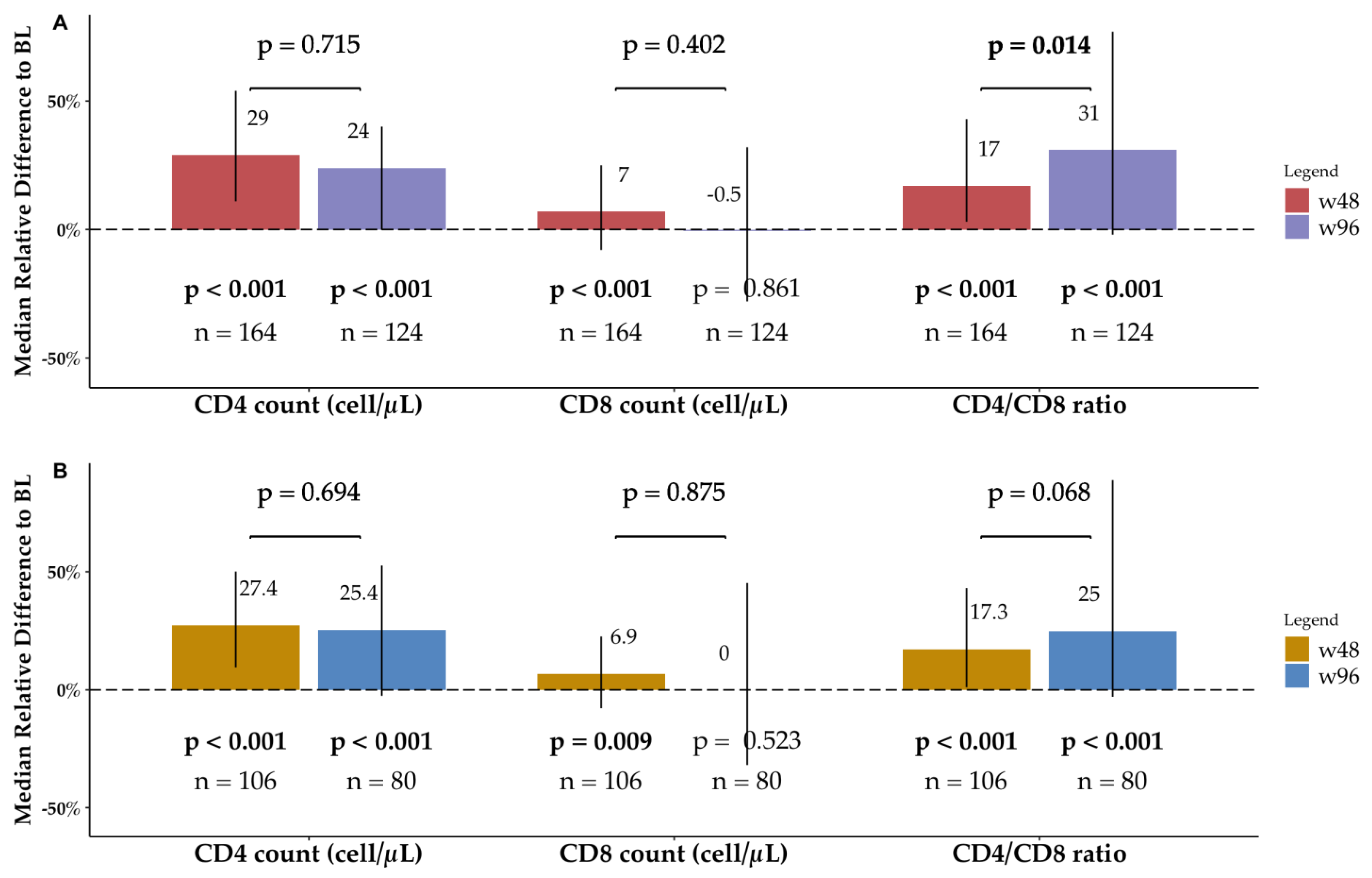
3.3. Immunologic Profile
3.3.1. Overall BICTEL Cohort
3.3.2. PLWH Older than 55
3.3.3. Immune Changes among Time-Points as Function of BL Immune Status
3.4. Metabolic Profile
3.4.1. Lipidic Profile, Body Weight, and BMI
3.4.2. Renal Function
3.4.3. Hepatic Function
3.5. Safety and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilead Sciences. Biktarvy® (Bictegravir, Emtricitabine, and Tenofovir Alafenamide): US Prescribing Information. 2018; p. 369. Available online: https://www.accessdata.fda.gov/ (accessed on 1 January 2023).
- Gilead Sciences. Biktarvy 50 mg/200 mg/25 mg Film-Coated Tab-Lets: EU Summary of Product Characteristics. 2018. Available online: http://www.371ema.europa.eu/ (accessed on 1 January 2023).
- Markham, A. Bictegravir: First Global Approval. Drugs 2018, 78, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Tsiang, M.; Jones, G.S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R.A.; Stepan, G.; Stray, K.M.; et al. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob. Agents Chemother. 2016, 60, 7086–7097. [Google Scholar] [CrossRef] [PubMed]
- Di Perri, G. Tenofovir alafenamide (TAF) clinical pharmacology. Le Infez. Med. 2021, 29, 526–529. [Google Scholar]
- Daar, E.S.; DeJesus, E.; Ruane, P.; Crofoot, G.; Oguchi, G.; Creticos, C.; Rockstroh, J.K.; Molina, J.-M.; Koenig, E.; Liu, Y.-P.; et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e347–e356. [Google Scholar] [PubMed]
- Kityo, C.; Hagins, D.; Koenig, E.; Avihingsanon, A.; Chetchotisakd, P.; Supparatpinyo, K.; Gankina, N.; Pokrovsky, V.; Vo-ronin, E.; Stephens, J.L.; et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide in women [poster no. 500]. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 4–7 March 2018. [Google Scholar]
- Molina, J.-M.; Ward, D.; Brar, I.; Mills, A.; Stellbrink, H.J.; López-Cortés, L.; Ruane, P.; Podzamczer, D.; Brinson, C.; Custodio, J.; et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-380 blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e357–e365. [Google Scholar]
- Pepperrell, T.; Hill, A.; Moorhouse, M.; Clayden, P.; McCann, K.; Sokhela, S.; Serenata, C.; Venter, W.D.F. Phase 3 trials of new antiretrovirals are not representative of the global HIV epidemic. J. Virus Erad. 2020, 30, 70–73. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef]
- Smit, M.; Brinkman, K.; Geerlings, S.; Smit, C.; Thyagarajan, K.; van Sighem, A.; de Wolf, F.; Hallett, T.B. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect. Dis. 2015, 15, 810–818. [Google Scholar] [CrossRef]
- Horberg, M.A.; Hurley, L.B.; Klein, D.B.; Towner, W.J.; Kadlecik, P.; Antoniskis, D.; Mogyoros, M.; Brachman, P.S.; Remmers, C.L.; Gambatese, R.C.; et al. The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDS 2015, 29, 582–590. [Google Scholar] [CrossRef]
- Lazzaro, A.; Cacciola, E.G.; Borrazzo, C.; Innocenti, G.P.; Cavallari, E.N.; Mezzaroma, I.; Falciano, M.; Fimiani, C.; Mastroianni, C.M.; Ceccarelli, G.; et al. Switching to a Bictegravir Single Tablet Regimen in Elderly People Living with HIV-1: Data Analysis from the BICTEL Cohort. Diagnostics 2021, 12, 76. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Human Immunodeficiencyvirus-1 Infection: Developing Antiretroviral Drugs for Treatment. 2015. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-immunodeficiency-virus-1-infection-developing-antiretroviral-drugs-treatment (accessed on 1 January 2023).
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Available online: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf (accessed on 31 March 2023).
- European AIDS Clinical Society. Guidelines Version 11.1. 2021. Available online: http://eacsociety.org (accessed on 1 January 2023).
- Maggiolo, F.; Rizzardini, G.; Molina, J.M.; Pulido, F.; De Wit, S.; Vandekerckhove, L.; Berenguer, J.; D’Antoni, M.L.; Blair, C.; Chuck, S.K.; et al. Bictegravir/emtricitabine/tenofovir alafenamide in older individuals with HIV: Results of a 96-week, phase 3b, open-label, switch trial in virologically suppressed people ≥65 years of age. HIV Med. 2023, 24, 27–36. [Google Scholar] [CrossRef]
- Andreatta, K.; Willkom, M.; Martin, R.; Chang, S.; Wei, L.; Liu, H.; Liu, Y.-P.; Graham, H.; Quirk, E.; Martin, H.; et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J. Antimicrob. Chemother. 2019, 74, 3555–3564, Erratum in J. Antimicrob. Chemother. 2019, 74, 3646–3647. [Google Scholar] [CrossRef]
- Sax, P.E.; Andreatta, K.; Molina, J.-M.; Daar, E.S.; Hagins, D.; Acosta, R.; D’antoni, M.L.; Chang, S.; Martin, R.; Liu, H.; et al. High efficacy of switching to bictegravir/emtricitabine/tenofovir alafenamide in people with suppressed HIV and preexisting M184V/I. AIDS 2022, 36, 1511–1520. [Google Scholar] [CrossRef]
- Tang, M.W.; Shafer, R.W. HIV-1 antiretroviral resistance: Scientific principles and clinical applications. Drugs 2012, 72, e1–e25. [Google Scholar] [CrossRef]
- Toutain, P.L.; Bousquet-Mélou, A. Plasma terminal half-life. J. Vet. Pharmacol. Ther. 2004, 27, 427–439. [Google Scholar] [CrossRef]
- Parienti, J.-J.; Fournier, A.L.; Cotte, L.; Schneider, M.-P.; Etienne, M.; Unal, G.; Perré, P.; Dutheil, J.-J.; Morilland-Lecoq, E.; Chaillot, F.; et al. Forgiveness of Dolutegravir-Based Triple Therapy Compared With Older Antiretroviral Regimens: A Prospective Multicenter Cohort of Adherence Patterns and HIV-RNA Replication. Open Forum Infect. Dis. 2021, 8, ofab316. [Google Scholar] [CrossRef]
- Elliot, E.; Amara, A.; Jackson, A.; Moyle, G.; Else, L.; Khoo, S.; Back, D.; Owen, A.; Boffito, M. Dolutegravir and elvitegravir plasma concentrations following cessation of drug intake. J. Antimicrob. Chemother. 2016, 71, 1031–1036. [Google Scholar] [CrossRef]
- Benedetti, S.; Altobelli, D.; De Socio, G.; Lanzi, A.; Gamboni, G.; Francisci, D. Real-life monocentric Biktarvy cohort from Perugia. HIV Glasgow 2022, Poster P129. J. Int. AIDS Soc. 2022, 25, e26009. [Google Scholar]
- d’Arminio Monforte, A.; Tavelli, A.; Cingolani, A.; Taramasso, L.; Mussini, C.; Piconi, S.; Calcagno, A.; Orofino, G.; Cicalini, S.; Castagna, A.; et al. Effectiveness of Bictegravir/Emtricitabine/Tenofovir Alafenamide (BIC/FTC/TAF) as Switch Strategy in Virologically Suppressed: Real-World Data from the ICONA Cohort. HIV Glasgow 2022, Poster P098. Available online: https://hivglasgow.org/wp-content/uploads/2023/01/P098_dArminio_Monforte.pdf (accessed on 1 January 2023).
- Trottier, B.; Antinori, A.; De Wet, J.; Duvivier, C.; Elinav, H.; Esser, S.; Ghosn, J.; den Hollander, J.; Lamber, J.; Milinkovic, A.; et al. Bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) for the treatment of people living with HIV: 24-month (24M) analyses by age, race, sex, adherence and late presentation in a multi-country cohort study. HIV Glasgow 2022, Poster P067. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jia2.26009 (accessed on 1 January 2023).
- Kehler, D.S.; Milic, J.; Guaraldi, G.; Fulop, T.; Falutz, J. Frailty in older people living with HIV: Current status and clinical management. BMC Geriatr. 2022, 22, 919. [Google Scholar] [CrossRef]
- Kanters, S.; Renaud, F.; Rangaraj, A.; Zhang, K.; Limbrick-Oldfield, E.; Hughes, M.; Ford, N.; Vitoria, M. Evidence synthesis evaluating body weight gain among people treating HIV with antiretroviral therapy-a systematic literature review and network meta-analysis. EClinicalMedicine 2022, 48, 101412. [Google Scholar] [CrossRef] [PubMed]
- Saumoy, M.; Sanchez-Quesada, J.L.; Ordoñez-Llanos, J.; Podzamczer, D. Do All Integrase Strand Transfer Inhibitors Have the Same Lipid Profile? Review of Randomised Controlled Trials in Naïve and Switch Scenarios in HIV-Infected Patients. J. Clin. Med. 2021, 10, 3456. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Calza, S.; Milic, J.; Calcagno, A.; Focà, E.; Rota, M.; Renzetti, S.; Celotti, A.; Siano, M.; Celesia, B.M.; et al. Dolutegravir is not associated with weight gain in antiretroviral therapy experienced geriatric patients living with HIV. AIDS 2021, 35, 939–945. [Google Scholar] [CrossRef] [PubMed]
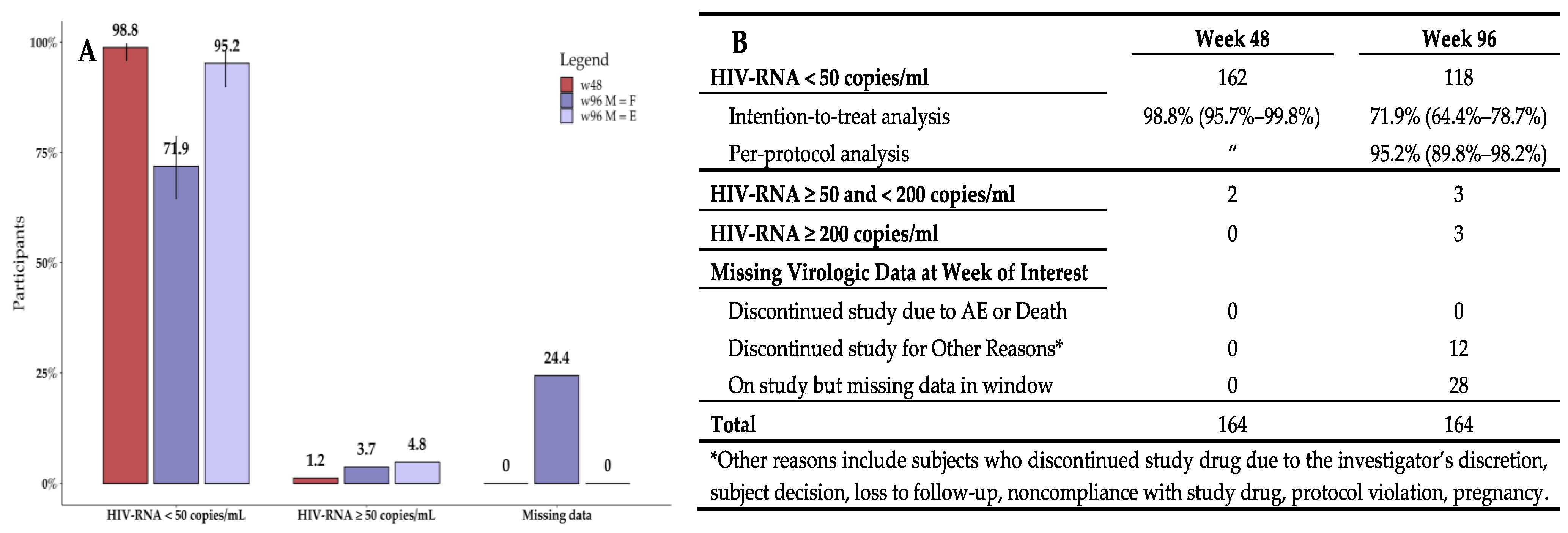



| Overall | Over 55 Years | Under 55 Years | p | Female | Male | p | |
|---|---|---|---|---|---|---|---|
| n = 164 | n = 106 | n = 58 | n = 48 | n = 116 | |||
| m/# (IQR/%) | m/# (IQR/%) | m/# (IQR/%) | m/# (IQR/%) | m/# (IQR/%) | |||
| Sex | |||||||
| Female | 48 (29.3%) | 27 (25.5%) | 21 (36.2%) | 0.156 | 48 (100%) | 0 (0%) | <0.001 |
| Male | 116 (70.7%) | 79 (74.5%) | 37 (63.8%) | 0 (0%) | 116 (100%) | ||
| Age (years) | 57 (47–61) | 59 (57–64) | 41 (37–48) | <0.001 | 56 (46–58) | 57 (47–62) | 0.0893 |
| Smoking (yes) | 108 (65.9%) | 68 (64.2%) | 40 (69.0%) | 0.607 | 28 (58.3%) | 80 (69.0%) | 0.209 |
| Time from HIV-1 diagnosis (years) | 16 (9.5–22) | 19 (12–24) | 12 (6.0–15) | <0.001 | 19 (10–24) | 15 (8.0–21) | 0.113 |
| History of AIDS diagnosis (yes) | 55 (33.5%) | 40 (37.7%) | 15 (25.9%) | 0.166 | 15 (31.3%) | 40 (34.5%) | 0.72 |
| HBV co-infection (yes) | 12 (7.3%) | 8 (7.5%) | 4 (6.9%) | 1 | 2 (4.2%) | 10 (8.6%) | 0.512 |
| Former HCV infection (yes) | 18 (11.0%) | 15 (14.2%) | 3 (5.2%) | 0.116 | 8 (16.7%) | 10 (8.6%) | 0.17 |
| HIV-1-related non-AIDS comorbidities (≥1) | 98 (59.8%) | 73 (68.9%) | 25 (43.1%) | 0.002 | 30 (62.5%) | 68 (58.6%) | 0.727 |
| How many | 1 (0–1) | 1 (0–2.0) | 0 (0–1) | <0.001 | 1 (0–1) | 1 (0–1) | 0.591 |
| Osteoporosis | 27 (16.5%) | 23 (21.7%) | 4 (6.9%) | 0.015 | 8 (16.7%) | 19 (16.4%) | 1 |
| Type 2 Diabetes | 12 (7.3%) | 10 (9.4%) | 2 (3.4%) | 0.217 | 4 (8.3%) | 8 (6.9%) | 0.748 |
| Hypertension | 32 (19.5%) | 26 (24.5%) | 6 (10.3%) | 0.038 | 8 (16.7%) | 24 (20.7%) | 0.667 |
| Major Cardiovascular events | 12 (7.3%) | 12 (11.3%) | 0 (0%) | 0.008 | 1 (2.1%) | 11 (9.5%) | 0.183 |
| Other than ART co-medications | |||||||
| ≥1 | 69 (42.1%) | 49 (46.2%) | 20 (34.5%) | 0.186 | 26 (54.2%) | 43 (37.1%) | 0.056 |
| ≥2 | 41 (25.0%) | 33 (31.1%) | 8 (13.8%) | 0.015 | 12 (25.0%) | 29 (25.0%) | 1 |
| Pre-switch ART regimen | |||||||
| INSTI | 38 (23.2%) | 23 (21.7%) | 15 (25.9%) | 9 (18.8%) | 29 (25.0%) | ||
| NNRTI | 20 (12.2%) | 11 (10.4%) | 9 (15.5%) | 0.545 | 3 (6.3%) | 17 (14.7%) | 0.134 |
| PI | 99 (60.4%) | 68 (64.2%) | 31 (53.4%) | 32 (66.7%) | 67 (57.8%) | ||
| PI+INSTI | 7 (4.3%) | 4 (3.8%) | 3 (5.2%) | 4 (8.3%) | 3 (2.6%) | ||
| TDF-based backbone | 95 (57.9%) | 67 (63.2%) | 28 (48.3%) | 0.071 | 22 (45.8%) | 73 (62.9%) | 0.056 |
| TAF-based backbone | 49 (29.9%) | 26 (24.5%) | 23 (39.7%) | 0.051 | 15 (31.3%) | 34 (29.3%) | 0.852 |
| ABC-based backbone | 5 (3.0%) | 3 (2.8%) | 2 (3.4%) | 1 | 4 (8.3%) | 1 (.9%) | 0.026 |
| Boosted-regimen | 124 (75.6%) | 79 (74.5%) | 45 (77.6%) | 0.708 | 40 (83.3%) | 84 (72.4%) | 0.165 |
| Dual therapy | 10 (6.1%) | 8 (7.5%) | 2 (3.4%) | 0.497 | 4 (8.3%) | 6 (5.2%) | 0.481 |
| Reason to switch | |||||||
| Adherence | 4 (2.4%) | 3 (2.8%) | 1 (1.7%) | 0.536 | 3 (6.3%) | 1 (0.9%) | 0.279 |
| Adverse events | 1 (0.6%) | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (0.9%) | ||
| Proactive | 15 (9.1%) | 7 (6.6%) | 8 (13.8%) | 5 (10.4%) | 10 (8.6%) | ||
| Simplification | 110 (67.1%) | 71 (67.0%) | 39 (67.2%) | 32 (66.7%) | 78 (67.2%) | ||
| Toxicity | 34 (20.7%) | 24 (22.6%) | 10 (17.2%) | 8 (16.7%) | 26 (22.4%) |
| BL | w48 | w96 | w96 vs. BL | w48 vs. BL | w96 vs. w48 | ||||||
| Absolute Difference | MRD % | p | Absolute Difference | MRD % | p | Absolute Difference | MRD % | p | ||||
| CD4+ T cell count (cell/μL) | 580 (450–750) | 760 (590–1000) | 790 (570–980) | 136 (−1–320) | 24.2 (−0.2–5.4) | <0.001 | 163 (67–281) | 29 (11.1–53.9) | <0.001 | –8 (−126–132) | −1.6 (−19–18) | 0.715 |
| CD8+ T cell count (cell/μL) | 750 (580–1000) | 850 (640–1000) | 790 (550–1100) | −6 (−230–254) | −0.5 (−28.1–31.6) | 0.861 | 56 (−58–183) | 7.1 (−8.3–25.2) | <0.001 | −35 (−290–209) | −5.2 (−32.8–29) | 0.402 |
| CD4+/CD8+ ratio | 0.70 (0.60–0.90) | 0.90 (0.80–1.0) | 1.0 (0.72–1.4) | 0.19 (−0.02–0.6) | 31.4 (−2–77.4) | <0.001 | 0.14 (0.01–0.3) | 17.3 (3.5–43.1) | <0.001 | 0 (−0.2–0.4) | 2.2 (−17.3–4.9) | 0.014 |
| Total Cholesterol (mg/dL) | 190 (170–210) | 180 (160–200) | 180 (160–210) | −4 (−25–16) | −2.5 (−12.9–9.6) | 0.219 | −12 (−24–6) | −6.1 (−13–3.6) | <0.001 | 3.5 (−15–24) | 2.2 (−7.2–14.1) | 0.104 |
| LDL (mg/dL) | 110 (87–130) | 100 (83–130) | 110 (88–140) | −3 (−17–19) | −3.5 (−16–16.4) | 0.545 | −6 (−17–12) | −6 (−18.2–1.7) | 0.034 | 2.9 (−16.2 – 21) | 3.5 (−14–24.4) | 0.204 |
| HDL (mg/dL) | 50 (41–58) | 52 (44–62) | 53 (45–63) | 0 (−7–7) | 0 (−12.1–17) | 0.699 | 3 (−5–8) | 6.5 (−7.8–18.5) | 0.008 | 0 (−9–7) | 0 (−15.8–15.1) | 0.571 |
| TC/HDL ratio | 3.7 (3.0–4.7) | 3.4 (2.8–4.1) | 3.6 (3.0–4.5) | −0.12 (−0.78–0.29) | −2.7 (−23.2–8.6) | 0.069 | −0.22 (−0.88–0.18) | −6.4 (−21.9–6.1) | <0.001 | 0.1 (−0.5–0.7) | 2 (−14.5–22.2) | 0.251 |
| Body Weight (Kg) | 77 (70–84) | 78 (71–85) | 78 (65–84) | 1.3 (−2.5–4) | 1.7 (−2.7–5.6) | 0.246 | 1 (0–3) | 1.6 (0–3.8) | 0.006 | 0 (−5–4) | 0 (−5.8–4.6) | 0.593 |
| BMI (Kg/m2) | 25 (23–28) | 26 (23–29) | 25 (23–27) | 0.55 (−0.43–1.67) | 1.9 (−1.9–6.8) | 0.174 | 0.28 (0–0.88) | 0.9 (0–3.4) | 0.024 | 0.3 (−1.6–1.4) | 1.1 (−5.1–5.4) | 0.990 |
| Creatinine (mg/dL) | 0.94 (0.85–1.1) | 1.0 (0.84–1.1) | 1.0 (0.87–1.2) | 0.05 (−0.03–0.14) | 4.2 (−3.7–15.2) | <0.001 | 0 (−0.2–0.08) | 0 (−2.2–6.8) | 0.034 | 0 (−0.1–0.1) | 1.3 (−5.5–12.9) | 0.076 |
| eGFR | 85 (73–97) | 80 (70–93) | 78 (65–92) | −4.15 (−11.42–3.27) | −5.5 (−14.5–3.6) | 0.002 | −1.6 (−6.35–1) | −1.8 (−7.8–1.3) | <0.001 | –1.8 (−8.1–5.7) | −2.1 (−9.5–7.6) | 0.099 |
| AST (mg/dL) | 21 (17–24) | 20 (17–23) | 21 (18–26) | 1 (−2.5–5) | 5.6 (−12.9–26.1) | 0.365 | −1 (−4–2) | −4.3 (−18.2–14.3) | 0.189 | 1 (−2–5) | 4 (−10–27.8) | 0.423 |
| ALT (mg/dL) | 21 (16–26) | 20 (16–25) | 20 (15–32) | 0 (−6–9) | 0 (−24.8–52.6) | 0.093 | −1 (−4–3) | −4.4 (−18.8–15.1) | 0.073 | 0 (−5–7) | 0 (19.6–36.9) | 0.017 |
| BL | w48 | w96 | w96 vs. BL | w48 vs. BL | w96 vs. w48 | ||||||
| Absolute Difference | MRD % | p | Absolute Difference | MRD % | p | Absolute Difference | MRD % | p | ||||
| CD4+ T cell count (cell/μL) | 580 (460–730) | 760 (550–960) | 770 (550–920) | 155 (11–312) | 25.4 (2.5–52.6) | <0.001 | 160 (53–264) | 27.4 (9.5–5.1) | <0.001 | 15 (−92–119) | 2.7 (−13.2–18.3) | 0.694 |
| CD8+ T cell count (cell/μL) | 750 (580–1000) | 850 (660–1000) | 800 (590–1100) | 0 (−248–370) | 0 (−31.9–45.2) | 0.523 | 50 (−54–141) | 6.9 (−7.8–22.6) | 0.009 | –19 (−273–291) | −3.3 (−34.9–41.4) | 0.875 |
| CD4+/CD8+ ratio | 0.70 (0.60–0.82) | 0.90 (0.80–1.0) | 0.92 (0.62–1.4) | 0.13 (−0.02–0.6) | 25 (−2.9–88.9) | <0.001 | 0.13 (0.01–0.3) | 17.3 (1.1–43.1) | <0.001 | 0 (−0.2–0.4) | 5.5 (−19.9–40) | 0.068 |
| Total Cholesterol (mg/dL) | 190 (170–210) | 180 (160–210) | 190 (160–220) | −6 (−29–21) | −2.5 (−15.6–12.6) | 0.370 | −12 (−23–2) | −6.5 (−13–1.2) | <0.001 | 2 (−16–24) | 0.7 (−7.3–14.3) | 0.328 |
| LDL (mg/dL) | 110 (88–140) | 100 (84–140) | 110 (81–140) | −11 (−20–22) | −7.8 (−16.9–2.7) | 0.517 | −7 (−17–6) | −7 (−18.2–5.1) | 0.021 | 1 (−20–22) | 0.7 (−2.3–27.9) | 0.718 |
| HDL (mg/dL) | 47 (41–57) | 50 (43–60) | 52 (44–63) | 1 (−7–7) | 2 (−13.2–19.7) | 0.722 | 3 (−4–8) | 6.1 (−7.5–19.4) | 0.028 | –1 (−10–8) | −1.6 (−19.3–17) | 0.385 |
| TC/HDL ratio | 3.9 (3.4–4.7) | 3.6 (2.9–4.3) | 3.6 (3.1–4.6) | −0.13 (−0.82–0.43) | −3 (−26.7–11.7) | 0.173 | −0.31 (−0.97–0.16) | −8.1 (−22.7–4.7) | <0.001 | 0.2 (−0.5–0.7) | 5.3 (−15.5–22.8) | 0.235 |
| Body Weight (Kg) | 78 (71–88) | 80 (71–89) | 79 (65–85) | −0.5 (−3–4) | −0.6 (−4.1–5.1) | 1.000 | 1.5 (0–3) | 2 (0–3.8) | 0.026 | −2 (−5–4) | −2.2 (−7.8–5.1) | 0.236 |
| BMI (Kg/m2) | 26 (24–29) | 27 (25–29) | 25 (24–28) | 0 (−1.01–1.55) | 0 (−3.8–6.1) | 0.936 | 0.4 (0–0.9) | 1.6 (0–3.7) | 0.125 | −0.3 (−1.8–1.4) | −1.1 (−6.4–5.3) | 0.554 |
| Creatinine (mg/dL) | 0.90 (0.82–1.0) | 0.98 (0.81–1.1) | 1.0 (0.88–1.2) | 0.05 (−0.03–0.15) | 4.1 (−2.9–16.7) | 0.001 | 0 (−0.03–0.07) | 0 (−3–6.3) | 0.317 | 0 (0–1) | 1.3 (−3.7–16) | 0.061 |
| eGFR | 83 (71–94) | 78 (69–92) | 72 (63–88) | −4.85 (−16.73–3.83) | −6.2 (−17.4–4.1) | 0.016 | −0.7 (−6.1–1.3) | −0.7 (−7.1–1.9) | 0.036 | −2.2 (−12–4.7) | −3.1 (−15.4–6.7) | 0.114 |
| AST (mg/dL) | 21 (18–24) | 20 (17–23) | 21 (19–26) | 1 (−3–5) | 7.7 (−13.6–26) | 0.239 | −1 (−4–2) | −4.7 (−18.4–15.2) | 0.160 | 0 (−2–4) | 0 (−11.9–2.2) | 0.345 |
| ALT (mg/dL) | 21 (17–26) | 21 (16–25) | 19 (15–31) | −1 (−5–7) | −4.8 (−24.4–3.2) | 0.716 | 1 (−4–2) | −5.9 (−18.8–12.5) | 0.251 | –1 (−5–6) | −5.3 (−24.4–30) | 0.970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzaro, A.; Bianchini, D.; Gentilini Cacciola, E.; Mezzaroma, I.; Falciano, M.; Andreoni, C.; Fimiani, C.; Santinelli, L.; Maddaloni, L.; Bugani, G.; et al. Immune Reconstitution and Safe Metabolic Profile after the Switch to Bictegravir/Emtricitabine/Tenofovir Alafenamide Fumarate among Virologically Controlled PLWH: A 96 Week Update from the BICTEL Cohort. Viruses 2023, 15, 1222. https://doi.org/10.3390/v15061222
Lazzaro A, Bianchini D, Gentilini Cacciola E, Mezzaroma I, Falciano M, Andreoni C, Fimiani C, Santinelli L, Maddaloni L, Bugani G, et al. Immune Reconstitution and Safe Metabolic Profile after the Switch to Bictegravir/Emtricitabine/Tenofovir Alafenamide Fumarate among Virologically Controlled PLWH: A 96 Week Update from the BICTEL Cohort. Viruses. 2023; 15(6):1222. https://doi.org/10.3390/v15061222
Chicago/Turabian StyleLazzaro, Alessandro, Diana Bianchini, Elio Gentilini Cacciola, Ivano Mezzaroma, Mario Falciano, Carolina Andreoni, Caterina Fimiani, Letizia Santinelli, Luca Maddaloni, Ginevra Bugani, and et al. 2023. "Immune Reconstitution and Safe Metabolic Profile after the Switch to Bictegravir/Emtricitabine/Tenofovir Alafenamide Fumarate among Virologically Controlled PLWH: A 96 Week Update from the BICTEL Cohort" Viruses 15, no. 6: 1222. https://doi.org/10.3390/v15061222










