California Serogroup Viruses in a Changing Canadian Arctic: A Review
Abstract
:1. Introduction
2. The Viruses
2.1. History of SSHV and JCV
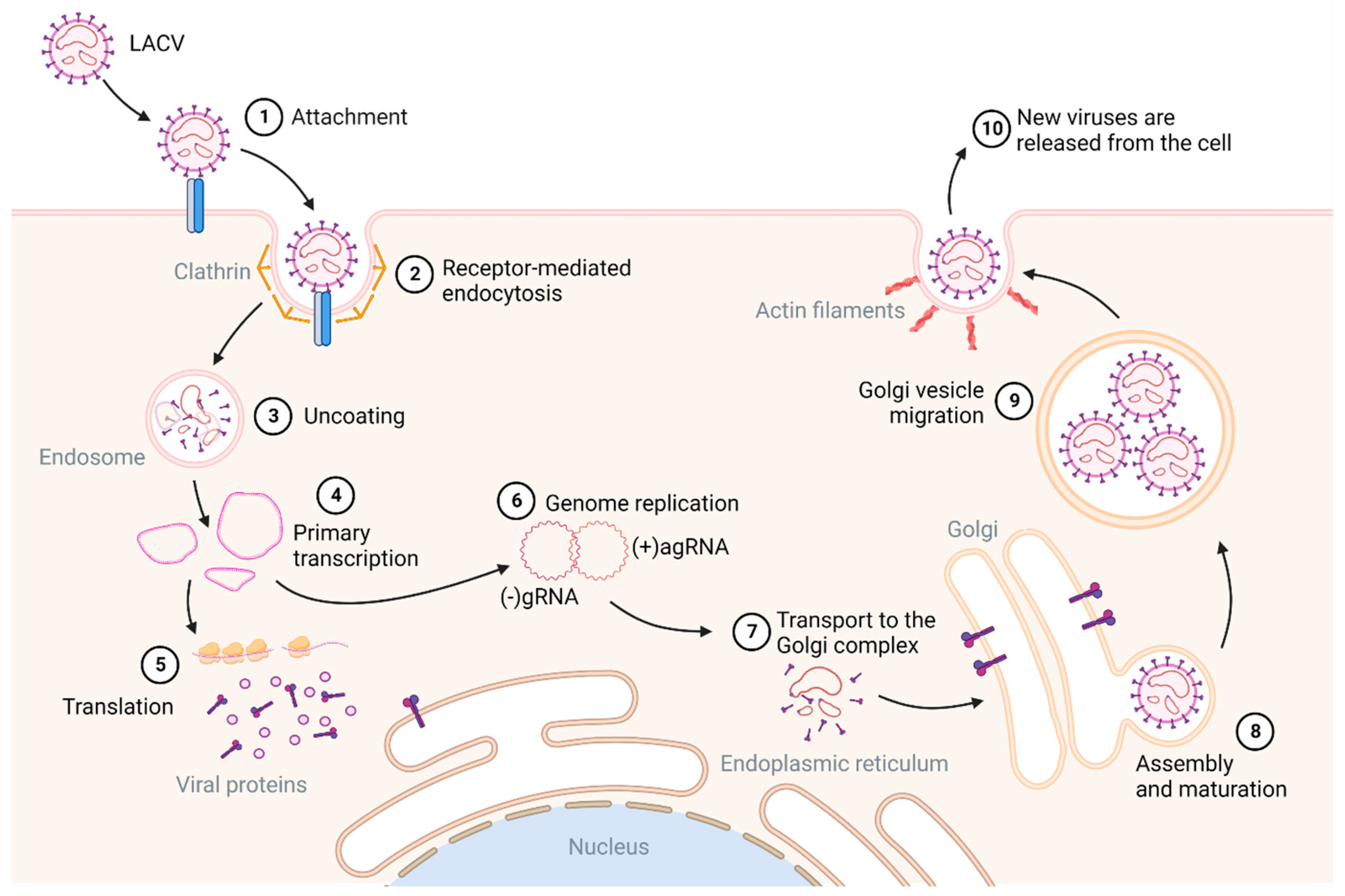
2.2. Genome Structure and Replication
2.3. Pathogenesis, Human Infection, and Host Responses
2.4. Animal Models and Therapeutics Developments
3. The Vectors
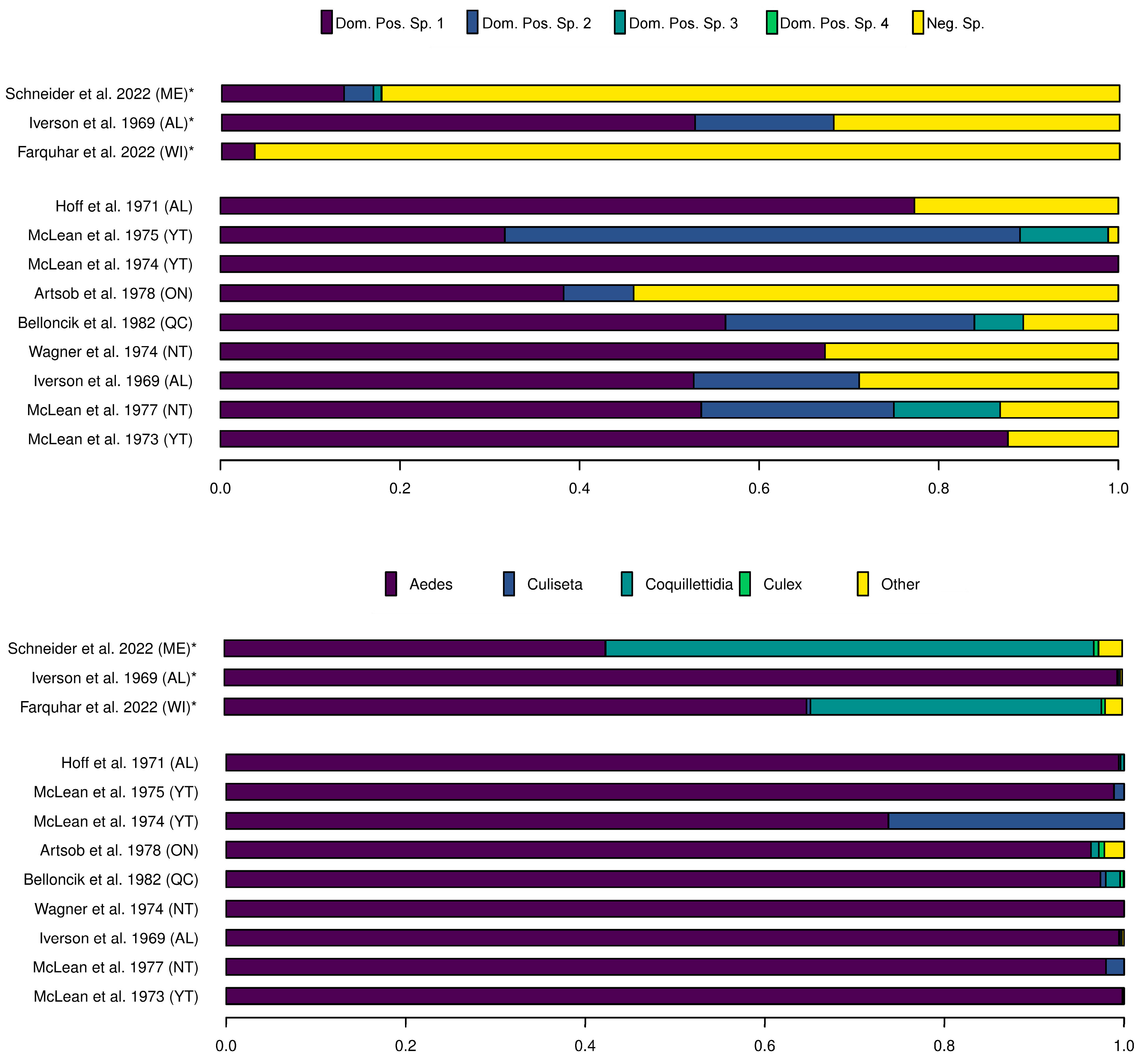
3.1. Distribution and Abundance of Potential Vector Species
3.2. Transovarial Transmission and Temperature Thresholds
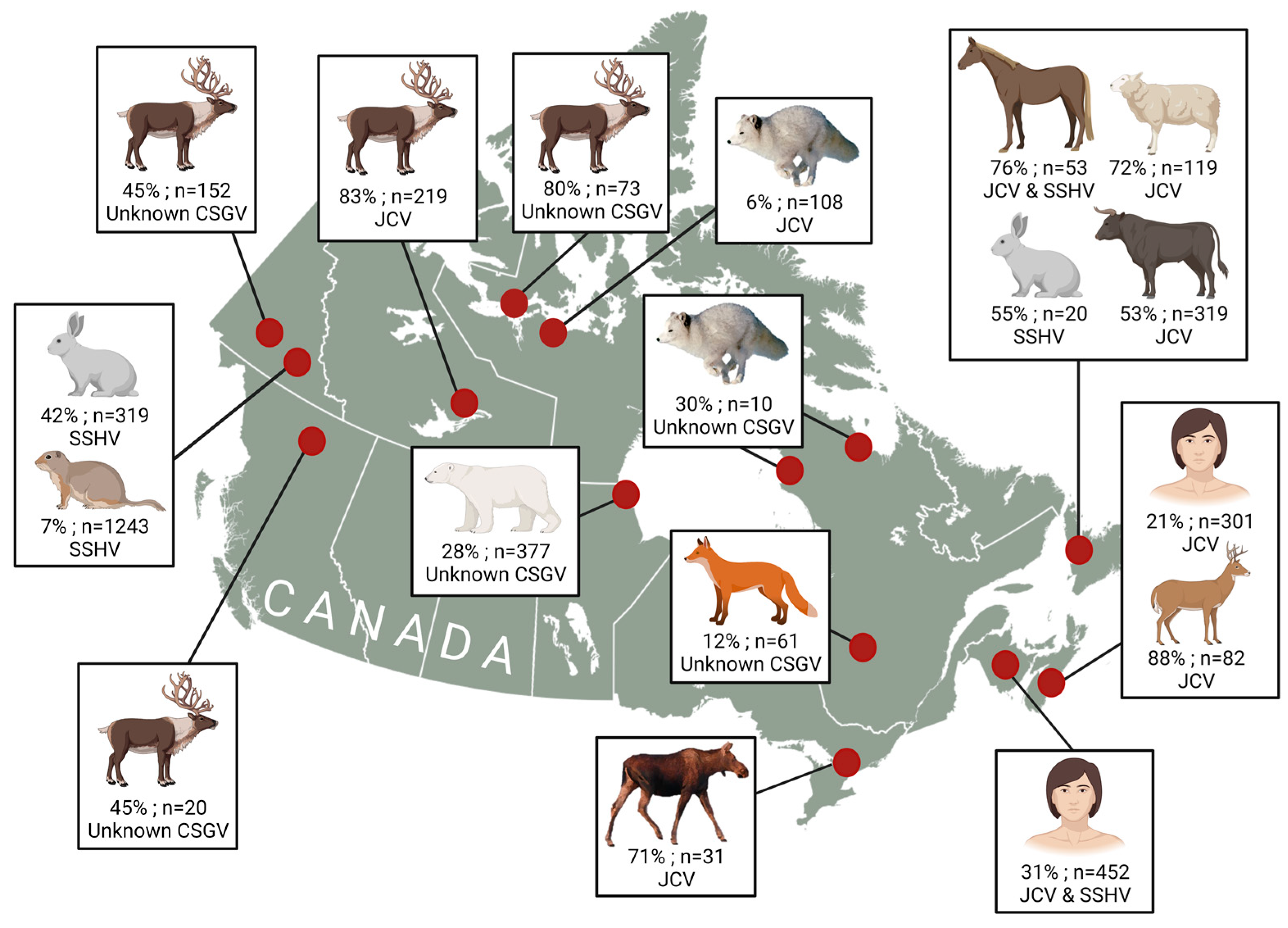
4. Mammalian Hosts
5. Climate Change
6. Public Health Messaging
Author Contributions
Funding
Conflicts of Interest
References
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Lemieux, A.; Colby, G.A.; Poulain, A.J.; Aris-Brosou, S. Viral spillover risk increases with climate change in High Arctic lake sediments. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221073. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.R.; Adkins, S.; Alkhovskiy, S.; Beer, M.; Blair, C.; Calisher, C.H.; Drebot, M.; Lambert, A.J.; de Souza, W.M.; Marklewitz, M.; et al. ICTV Virus Taxonomy Profile: Peribunyaviridae. J. Gen. Virol. 2020, 101, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Meier-Stephenson, V.; Langley, J.M.; Drebot, M.; Artsob, H. Encephalitis in the summer: A case of snowshoe hare (California serogroup) virus infection in Nova Scotia. Can. Commun. Dis. Rep. 2007, 33, 23–26. [Google Scholar] [PubMed]
- Evans, A.B.; Winkler, C.W.; Peterson, K.E. Differences in Neuropathogenesis of Encephalitic California Serogroup Viruses. Emerg. Infect. Dis. 2019, 25, 728–738. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Newhouse, V.F.; Thomas, L.A. Isolation of California encephalitis virus from the blood of a snowshoe hare (Lepus americanus) in western Montana. Am. J. Epidemiol. 1961, 73, 344–349. [Google Scholar] [CrossRef]
- Fauvel, M.; Artsob, H.; Calisher, C.H.; Davignon, L.; Chagnon, A.; Skvorc-Ranko, R.; Belloncik, S. California group virus encephalitis in three children from Quebec: Clinical and serologic findings. Can. Med. Assoc. J. 1980, 122, 60–62, 64. [Google Scholar]
- Drebot, M.A. Vector-borne diseases in Canada: Emerging mosquito-borne bunyaviruses in Canada. Can. Commun. Dis. Rep. 2015, 41, 117–123. [Google Scholar] [CrossRef]
- Artsob, H. Arbovirus activity in Canada. In Hemorrhagic Fever with Renal Syndrome, Tick- and Mosquito-Borne Viruses; Springer: Vienna, Austria, 1991; pp. 249–258. [Google Scholar]
- National Research Council of Canada. Biting Flies in Canada: Health Effects and Economic Consequences; NRCC Publication No. 19248; NRCC: Ottawa, ON, Canada, 1982; pp. 1–157. [Google Scholar]
- Martin, M.L.; Lindsey-Regnery, H.; Sasso, D.R.; McCormick, J.B.; Palmer, E. Distinction between Bunyaviridae genera by surface structure and comparison with Hantaan virus using negative stain electron microscopy. Arch. Virol. 1985, 86, 17–28. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Bishop, D.H. Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J. Virol. 1978, 28, 417–419. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Bishop, D.L. M viral RNA segment of bunyaviruses codes for two glycoproteins, G1 and G2. J. Virol. 1979, 30, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Fuller, F.; Bhown, A.S.; Bishop, D.H.L. Bunyavirus Nucleoprotein, N, and a Non-structural Protein, NSS, Are Coded by Overlapping Reading Frames in the S RNA. J. Gen. Virol. 1983, 64, 1705–1714. [Google Scholar] [CrossRef]
- Fuller, F.; Bishop, D.H. Identification of virus-coded nonstructural polypeptides in bunyavirus-infected cells. J. Virol. 1982, 41, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Scarano, F.; Beaty, B.; Sundin, D.; Janssen, R.; Endres, M.J.; Nathanson, N. Genetic determinants of the virulence and infectivity of La Crosse virus. Microb. Pathog. 1988, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ogg, M.M.; Patterson, J.L. RNA binding domain of Jamestown Canyon virus S segment RNAs. J. Virol. 2007, 81, 13754–13760. [Google Scholar] [CrossRef]
- Bowen, M.D.; Jackson, A.O.; Bruns, T.D.; Hacker, D.L.; Hardy, J.L. Determination and comparative analysis of the small RNA genomic sequences of California encephalitis, Jamestown Canyon, Jerry Slough, Melao, Keystone and Trivittatus viruses (Bunyaviridae, genus Bunyavirus, California serogroup). J. Gen. Virol. 1995, 76, 559–572. [Google Scholar] [CrossRef]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef]
- Fazakerley, J.K.; Gonzalez-Scarano, F.; Strickler, J.; Dietzschold, B.; Karush, F.; Nathanson, N. Organization of the middle RNA segment of snowshoe hare bunyavirus. Virology 1988, 167, 422–432. [Google Scholar] [CrossRef]
- Gonzalez-Scarano, F.; Janssen, R.S.; Najjar, J.A.; Pobjecky, N.; Nathanson, N. An avirulent G1 glycoprotein variant of La Crosse bunyavirus with defective fusion function. J. Virol. 1985, 54, 757–763. [Google Scholar] [CrossRef]
- Shi, X.; Kohl, A.; Léonard, V.H.J.; Li, P.; McLees, A.; Elliott, R.M. Requirement of the N-Terminal Region of Orthobunyavirus Nonstructural Protein NSm for Virus Assembly and Morphogenesis. J. Virol. 2006, 80, 8089–8099. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Rozhon, E.J.; Klimas, R.A.; El Said, L.H.; Shope, R.E.; Bishop, D.H. Evidence from recombinant bunyavirus studies that the M RNA gene products elicit neutralizing antibodies. Virology 1980, 102, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.J.; Jacoby, D.R.; Janssen, R.S.; Gonzalez-Scarano, F.; Nathanson, N. The Large Viral RNA Segment of California Serogroup Bunyaviruses Encodes the Large Viral Protein. J. Gen. Virol. 1989, 70, 223–228. [Google Scholar] [CrossRef]
- Mincer, J.; Materniak, S.; Dimitrova, K.; Wood, H.; Iranpour, M.; Dibernardo, A.; Loomer, C.; Drebot, M.A.; Lindsay, L.R.; Webster, D. Jamestown Canyon and snowshoe hare virus seroprevalence in New Brunswick. Off. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2021, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.; Wudel, B.; Kadkhoda, K.; Keynan, Y. Snowshoe Hare Virus Causing Meningoencephalitis in a Young Adult From Northern Manitoba, Canada. Open. Forum Infect. Dis. 2017, 4, ofx150. [Google Scholar] [CrossRef] [PubMed]
- Windhaber, S.; Xin, Q.; Lozach, P.-Y. Orthobunyaviruses: From Virus Binding to Penetration into Mammalian Host Cells. Viruses 2021, 13, 872. [Google Scholar] [CrossRef]
- Patterson, J.L.; Holloway, B.; Kolakofsky, D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J. Virol. 1984, 52, 215–222. [Google Scholar] [CrossRef]
- Lozach, P.-Y.; Kühbacher, A.; Meier, R.; Mancini, R.; Bitto, D.; Bouloy, M.; Helenius, A. DC-SIGN as a Receptor for Phleboviruses. Cell. Host Microbe 2011, 10, 75–88. [Google Scholar] [CrossRef]
- Léger, P.; Tetard, M.; Youness, B.; Cordes, N.; Rouxel, R.N.; Flamand, M.; Lozach, P.Y. Differential Use of the C-Type Lectins L-SIGN and DC-SIGN for Phlebovirus Endocytosis. Traffic 2016, 17, 639–656. [Google Scholar] [CrossRef]
- Klimstra, W.B.; Nangle, E.M.; Smith, M.S.; Yurochko, A.D.; Ryman, K.D. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 2003, 77, 12022–12032. [Google Scholar] [CrossRef]
- Hofmann, H.; Li, X.; Zhang, X.; Liu, W.; Kühl, A.; Kaup, F.; Soldan, S.S.; González-Scarano, F.; Weber, F.; He, Y.; et al. Severe Fever with Thrombocytopenia Virus Glycoproteins Are Targeted by Neutralizing Antibodies and Can Use DC-SIGN as a Receptor for pH-Dependent Entry into Human and Animal Cell Lines. J. Virol. 2013, 87, 4384–4394. [Google Scholar] [CrossRef]
- Gardner, C.L.; Ebel, G.D.; Ryman, K.D.; Klimstra, W.B. Heparan sulfate binding by natural eastern equine encephalitis viruses promotes neurovirulence. Proc. Natl. Acad. Sci. USA 2011, 108, 16026–16031. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Ryman, K.D.; Johnston, R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998, 72, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Pobjecky, N.; Smith, J.; Gonzalez-Scarano, F. Biological studies of the fusion function of California serogroup Bunyaviruses. Microb. Pathog. 1986, 1, 491–501. [Google Scholar] [CrossRef]
- Hollidge, B.S.; Nedelsky, N.B.; Salzano, M.V.; Fraser, J.W.; González-Scarano, F.; Soldan, S.S. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 2012, 86, 7988–8001. [Google Scholar] [CrossRef]
- Chu, J.J.; Ng, M.L. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 2004, 78, 10543–10555. [Google Scholar] [CrossRef] [PubMed]
- Nawa, M.; Takasaki, T.; Yamada, K.I.; Kurane, I.; Akatsuka, T. Interference in Japanese encephalitis virus infection of Vero cells by a cationic amphiphilic drug, chlorpromazine. J. Gen. Virol. 2003, 84, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Plassmeyer, M.L.; Soldan, S.S.; Stachelek, K.M.; Martín-García, J.; González-Scarano, F. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 2005, 338, 121–132. [Google Scholar] [CrossRef]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef]
- Dawes, B.E.; Gao, J.; Atkins, C.; Nelson, J.T.; Johnson, K.; Wu, P.; Freiberg, A.N. Human neural stem cell-derived neuron/astrocyte co-cultures respond to La Crosse virus infection with proinflammatory cytokines and chemokines. J. Neuroinflammation 2018, 15, 315. [Google Scholar] [CrossRef]
- Bennett, R.S.; Cress, C.M.; Ward, J.M.; Firestone, C.-Y.; Murphy, B.R.; Whitehead, S.S. La Crosse virus infectivity, pathogenesis, and immunogenicity in mice and monkeys. Virol. J. 2008, 5, 25. [Google Scholar] [CrossRef]
- Winkler, C.W.; Race, B.; Phillips, K.; Peterson, K.E. Capillaries in the olfactory bulb but not the cortex are highly susceptible to virus-induced vascular leak and promote viral neuroinvasion. Acta Neuropathol. 2015, 130, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Artsob, H.; Spence, L.; Caughey, W.C.; Wherrett, J.R. Aseptic meningitis in Ontario. Can. Med. Assoc. J. 1981, 125, 958–962. [Google Scholar] [PubMed]
- Artsob, H.; Spence, L.; Surgeoner, G.; Helson, B.; Thorsen, J.; Grant, L.; Th’ng, C. Snowshoe hare virus activity in Southern Ontario. Can. J. Public. Health 1982, 73, 345–349. [Google Scholar] [PubMed]
- Public Health Agency of Canada. Mosquito-Borne Diseases Surveillance Report: Annual Edition, 2019 Preliminary. Ottawa, Canada. February 2022. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/mosquito-borne-diseases-surveillance-annual-report-2019.html (accessed on 15 January 2023).
- Matkovic, E.; Hoang Johnson, D.K.; Staples, J.E.; Mora-Pinzon, M.C.; Elbadawi, L.I.; Osborn, R.A.; Warshauer, D.M.; Wegner, M.V.; Davis, J.P. Enhanced Arboviral Surveillance to Increase Detection of Jamestown Canyon Virus Infections, Wisconsin, 2011–2016. Am. J. Trop. Med. Hyg. 2019, 100, 445–451. [Google Scholar] [CrossRef]
- Pastula, D.M.; Hoang Johnson, D.K.; White, J.L.; Dupuis, A.P., 2nd; Fischer, M.; Staples, J.E. Jamestown Canyon Virus Disease in the United States-2000–2013. Am. J. Trop. Med. Hyg. 2015, 93, 384–389. [Google Scholar] [CrossRef]
- Vosoughi, R.; Walkty, A.; Drebot, M.A.; Kadkhoda, K. Jamestown Canyon virus meningoencephalitis mimicking migraine with aura in a resident of Manitoba. Can. Med. Assoc. J. 2018, 190, E262. [Google Scholar] [CrossRef]
- Haddow, A.D.; Odoi, A. The Incidence Risk, Clustering, and Clinical Presentation of La Crosse Virus Infections in the Eastern United States, 2003–2007. PLoS ONE 2009, 4, e6145. [Google Scholar] [CrossRef]
- Patriquin, G.; Drebot, M.; Cole, T.; Lindsay, R.; Schleihauf, E.; Johnston, B.L.; Dimitrova, K.; Traykova-Andonova, M.; Mask, A.; Haldane, D.; et al. High Seroprevalence of Jamestown Canyon Virus among Deer and Humans, Nova Scotia, Canada. Emerg. Infect. Dis. J. 2018, 24, 118. [Google Scholar] [CrossRef]
- Sampasa-Kanyinga, H.; Lévesque, B.; Anassour-Laouan-Sidi, E.; Côté, S.; Serhir, B.; Ward, B.J.; Libman, M.D.; Drebot, M.A.; Makowski, K.; Dimitrova, K.; et al. Zoonotic Infections in Communities of the James Bay Cree Territory: An Overview of Seroprevalence. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, 370321. [Google Scholar] [CrossRef]
- Taylor, K.G.; Woods, T.A.; Winkler, C.W.; Carmody, A.B.; Peterson, K.E. Age-dependent myeloid dendritic cell responses mediate resistance to la crosse virus-induced neurological disease. J. Virol. 2014, 88, 11070–11079. [Google Scholar] [CrossRef]
- Bennett, R.S.; Nelson, J.T.; Gresko, A.K.; Murphy, B.R.; Whitehead, S.S. The full genome sequence of three strains of Jamestown Canyon virus and their pathogenesis in mice or monkeys. Virol. J. 2011, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.S.; Gresko, A.K.; Nelson, J.T.; Murphy, B.R.; Whitehead, S.S. A recombinant chimeric La Crosse virus expressing the surface glycoproteins of Jamestown Canyon virus is immunogenic and protective against challenge with either parental virus in mice or monkeys. J. Virol. 2012, 86, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takayama-Ito, M.; Satoh, M.; Kawahara, M.; Kitaura, S.; Yoshikawa, T.; Fukushi, S.; Nakajima, N.; Komeno, T.; Furuta, Y.; et al. Favipiravir treatment prolongs the survival in a lethal mouse model intracerebrally inoculated with Jamestown Canyon virus. PLOS Negl. Trop. Dis. 2021, 15, e0009553. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.D.; Taro, B.S.; Siddharthan, V.; Wang, H.; Smee, D.F.; Christensen, A.J.; Furuta, Y. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antivir. Res. 2008, 80, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Smee, D.F.; Morrey, J.D.; Furuta, Y. Effect of T-705 treatment on western equine encephalitis in a mouse model. Antivir. Res. 2009, 82, 169–171. [Google Scholar] [CrossRef]
- Smee, D.F.; Jung, K.-H.; Westover, J.; Gowen, B.B. 2′-Fluoro-2′-deoxycytidine is a broad-spectrum inhibitor of bunyaviruses in vitro and in phleboviral disease mouse models. Antivir. Res. 2018, 160, 48–54. [Google Scholar] [CrossRef]
- Livonesi, M.C.; De Sousa, R.L.; Badra, S.J.; Figueiredo, L.T. In vitro and in vivo studies of ribavirin action on Brazilian Orthobunyavirus. Am. J. Trop. Med. Hyg. 2006, 75, 1011–1016. [Google Scholar] [CrossRef]
- Sandler, Z.J.; Firpo, M.R.; Omoba, O.S.; Vu, M.N.; Menachery, V.D.; Mounce, B.C. Novel Ionophores Active against La Crosse Virus Identified through Rapid Antiviral Screening. Antimicrob. Agents Chemother. 2020, 64, e00086-20. [Google Scholar] [CrossRef]
- Wood, D.M.; Dang, P.T.; Ellis, R.A. The mosquitoes of Canada. Diptera: Culicidae. In The Insects and Arachnids of Canada; Canadian Government Publishing Centre: Hull, QC, Canada, 1979; p. 106. [Google Scholar]
- Andreadis, T.G.; Anderson, J.F.; Armstrong, P.M.; Main, A.J. Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: A ten-year analysis, 1997–2006. Vector Borne Zoonotic Dis. 2008, 8, 175–188. [Google Scholar] [CrossRef]
- Villeneuve, C.-A.; Buhler, K.J.; Iranpour, M.; Avard, E.; Dibernardo, A.; Fenton, H.; Hansen, C.M.; Gouin, G.-G.; Loseto, L.L.; Jenkins, E.; et al. New Records of California Serogroup Virus in Aedes Mosquitoes and First Detection in Simulioidae Flies from Northern Canada and Alaska. bioRxiv 2021. [Google Scholar] [CrossRef]
- Berry, R.L.; Parsons, M.A.; Restifo, R.A.; Peterson, E.D.; Gordon, S.W.; Reed, M.R.; Calisher, C.H.; Bear, G.T.; Halpin, T.J. California serogroup virus infections in Ohio: An 18-year retrospective summary. Prog. Clin. Biol. Res. 1983, 123, 215–223. [Google Scholar] [PubMed]
- Dieme, C.; Kramer, L.D.; Ciota, A.T. Vector competence of Anopheles quadrimaculatus and Aedes albopictus for genetically distinct Jamestown Canyon virus strains circulating in the Northeast United States. Parasites Vectors 2022, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.F.; Robich, R.M.; Elias, S.P.; Lubelczyk, C.B.; Cosenza, D.S.; Smith, R.P. Jamestown Canyon Virus in Collected Mosquitoes, Maine, United States, 2017–2019. Emerg. Infect. Dis. 2022, 28, 2330–2333. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.; Hanson, R.P.; Papadopoulos, O.; Morris, C.V.; DeFoliart, G.R. Isolation of viruses of the California encephalitis virus group from boreal Aedes mosquitoes. Am. J. Trop. Med. Hyg. 1969, 18, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mokry, J.; Artsob, H.; Ralph, B. Studies on California serogroup virus activity in Newfoundland, Canada, 1980–1983. Mosq. News 1984, 44, 310–331. [Google Scholar]
- Peach, D.A.H.; Poirier, L.M. New distribution records and range extensions of mosquitoes (Diptera: Culicidae) in British Columbia and the Yukon Territory. J. Entomol. Soc. Br. Columbia 2020, 117, 69–74. [Google Scholar]
- Farquhar, M.R.; Thrun, N.B.; Tucker, B.J.; Bartholomay, L.C. Outbreak Investigation: Jamestown Canyon Virus Surveillance in Field-Collected Mosquitoes (Diptera: Culicidae) From Wisconsin, USA, 2018–2019. Front. Public. Health 2022, 10, 818204. [Google Scholar] [CrossRef]
- Belloncik, S.; Poulin, L.; Maire, A.; Aubin, A.; Fauvel, M.; Jousset, F.X. Activity of California encephalitis group viruses in Entrelacs (province of Quebec, Canada). Can. J. Microbiol. 1982, 28, 572–579. [Google Scholar] [CrossRef]
- Hoff, G.L.; Anslow, R.O.; Spalatin, J.; Hanson, R.P. Isolation of Montana snowshoe hare serotype of California encephalitis virus group from a snowshoe hare and Aedes mosquitoes. J. Wildl. Dis. 1971, 7, 28–34. [Google Scholar] [CrossRef]
- Wagner, R.J.; DeJong, C.; Leung, M.K.; McLintock, J.; Iversen, J.O. Isolations of California encephalitis virus from tundra mosquitoes. Can. J. Microbiol. 1975, 21, 574–576. [Google Scholar] [CrossRef]
- McLean, D.M.; Bergman, S.K.A.; Graham, E.A.; Greenfield, G.P.; Olden, J.A.; Patterson, R.D. California Encephalitis Virus prevalence in Yukon mosquitoes during 1973. Can. J. Public. Health 1974, 65, 23–28. [Google Scholar] [PubMed]
- McLean, D.M.; Clarke, A.M.; Goddard, E.J.; Manes, A.S.; Montalbetti, C.A.; Pearson, R.E. California encephalitis virus endemicity in the Yukon Territory, 1972. J. Hyg. 1973, 71, 391–402. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.M.; Grass, P.N.; Judd, B.D.; Ligate, L.V.; Peter, K.K. Bunyavirus isolations from mosquitoes in the western Canadian Arctic. J. Hyg. 1977, 79, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Reidenbach, K.R.; Cook, S.; Bertone, M.A.; Harbach, R.E.; Wiegmann, B.M.; Besansky, N.J. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol. Biol. 2009, 9, 298. [Google Scholar] [CrossRef]
- Boromisa, R.D.; Grimstad, P.R. Virus-vector-host relationships of Aedes stimulans and Jamestown Canyon virus in a northern Indiana enzootic focus. Am. J. Trop. Med. Hyg. 1986, 35, 1285–1295. [Google Scholar] [CrossRef]
- Artsob, H.; Wright, R.; Shipp, L.; Spence, L.; Th’ng, C. California encephalitis virus activity in mosquitoes and horses in southern Ontario, 1975. Can. J. Microbiol. 1978, 24, 1544–1547. [Google Scholar] [CrossRef]
- Bergren, N.A.; Kading, R.C. The Ecological Significance and Implications of Transovarial Transmission among the Vector-Borne Bunyaviruses: A Review. Insects 2018, 9, 173. [Google Scholar] [CrossRef]
- Berry, R.L.; Weigert, B.J.L.; Calisher, C.H.; Parsons, M.A.; Bear, G.T. Evidence for transmission of Jamestown canyon virus, in Ohio. Mosq. News 1977, 37, 494–496. [Google Scholar]
- Kramer, L.D.; Bowen, M.D.; Hardy, J.L.; Reeves, W.C.; Presser, S.B.; Eldridge, B.F. Vector Competence of Alpine, Central Valley, and Coastal Mosquitoes (Diptera: Culicidae) from California for Jamestown Canyon Virus. J. Med. Entomol. 1993, 30, 398–406. [Google Scholar] [CrossRef]
- McLean, D.M.; Bergman, S.K.; Gould, A.P.; Grass, P.N.; Miller, M.A.; Spratt, E.E. California encephalitis virus prevalence throughout the Yukon Territory, 1971–1974. Am. J. Trop. Med. Hyg. 1975, 24, 676–684. [Google Scholar] [CrossRef]
- McLintock, J.; Curry, P.S.; Wagner, R.J.; Leung, M.K.; Iversen, J.O. Isolation of Snowshoe Hare Virus from Aedes implicatus larvae in Saskatchewan. Mosq. News 1976, 36, 233–237. [Google Scholar]
- McLean, D.M.; Clarke, A.M.; Coleman, J.C.; Montalbetti, C.A.; Skidmore, A.G.; Walters, T.E.; Wise, R. Vector capability of Aedes aegypti mosquitoes for California encephalitis and dengue viruses at various temperatures. Can. J. Microbiol. 1974, 20, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.V.; Gasparotto, A.; Liang, P.; Nelder, M.P.; Russell, C.; Hunter, F.F. Discovery of an Aedes (Stegomyia) albopictus population and first records of Aedes (Stegomyia) aegypti in Canada. Med. Vet. Entomol. 2020, 34, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.C.; Olival, K.J.; Perkins, S.L. Molecular identification of host feeding patterns of snow-melt mosquitoes (Diptera: Culicidae): Potential implications for the transmission ecology of Jamestown Canyon virus. J. Med. Entomol. 2010, 47, 226–229. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Frederick, C.; Racine, T.; Kobinger, G.P.; Wong, G. Modeling host-feeding preference and molecular systematics of mosquitoes in different ecological niches in Canada. Acta Trop. 2021, 213, 105734. [Google Scholar] [CrossRef]
- Dudley, J.P.; Hoberg, E.P.; Jenkins, E.J.; Parkinson, A.J. Climate Change in the North American Arctic: A One Health Perspective. Ecohealth 2015, 12, 713–725. [Google Scholar] [CrossRef]
- Buhler, K.J.; Dibernardo, A.; Pilfold, N.W.; Harms, N.J.; Fenton, H.; Carriere, S.; Kelly, A.; Schwantje, H.; Aguilar, X.F.; Leclerc, L.M.; et al. Widespread exposure to mosquitoborne California serogroup viruses in caribou, Arctic fox, red fox, and polar bears, Canada. Emerg. Infect. Dis. 2023, 29, 220154. [Google Scholar] [CrossRef]
- Miernyk, K.M.; Bruden, D.; Parkinson, A.J.; Hurlburt, D.; Klejka, J.; Berner, J.; Stoddard, R.A.; Handali, S.; Wilkins, P.P.; Kersh, G.J.; et al. Human Seroprevalence to 11 Zoonotic Pathogens in the U.S. Arctic, Alaska. Vector Borne Zoonotic Dis. 2019, 19, 563–575. [Google Scholar] [CrossRef]
- Goff, G.; Whitney, H.; Drebot, M.A. Roles of Host Species, Geographic Separation, and Isolation in the Seroprevalence of Jamestown Canyon and Snowshoe Hare Viruses in Newfoundland. Appl. Environ. Microbiol. 2012, 78, 6734–6740. [Google Scholar] [CrossRef]
- Grimstad, P.R.; Schmitt, S.M.; Williams, D.G. Prevalence of neutralizing antibody to Jamestown Canyon virus (California group) in populations of elk and moose in northern Michigan and Ontario, Canada. J. Wildl. Dis. 1986, 22, 453–458. [Google Scholar] [CrossRef]
- Neitzel, D.F.; Grimstad, P.R. Serological evidence of California group and Cache Valley virus infection in Minnesota white-tailed deer. J. Wildl. Dis. 1991, 27, 230–237. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Lvov, S.D.; Savage, H.M.; Calisher, C.H.; Smith, G.C.; Lvov, D.K.; Gubler, D.J. Vector and host relationships of California serogroup viruses in western Siberia. Am. J. Trop. Med. Hyg. 1993, 49, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, P.R. California group virus disease. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 2, pp. 99–136. [Google Scholar]
- Bowser, N.H.; Anderson, N.E. Dogs (Canis familiaris) as Sentinels for Human Infectious Disease and Application to Canadian Populations: A Systematic Review. Vet. Sci. 2018, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Artsob, H. Distribution of California serogroup viruses and virus infections in Canada. Prog. Clin. Biol. Res. 1983, 123, 277–290. [Google Scholar]
- Watts, D.M.; Tammariello, R.F.; Dalrymple, J.M.; Eldridge, B.F.; Russell, P.K.; Top, F.H., Jr. Experimental infection of vertebrates of the Pocomoke Cypress Swamp, Maryland with Keystone and Jamestown Canyon viruses. Am. J. Trop. Med. Hyg. 1979, 28, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.K.; Holloway, K.; Dimitrova, K.; Rogers, L.; Chaulk, A.C.; Lang, A.S.; Whitney, H.G.; Drebot, M.A.; Chapman, T.W. The Seasonal Timing of Snowshoe Hare Virus Transmission on the Island of Newfoundland, Canada. J. Med. Entomol. 2017, 54, 712–718. [Google Scholar] [CrossRef]
- Zhang, X.; Flato, G.; Kirchmeier-Young, M.; Vincent, L.; Wan, H.; Wang, X.; Rong, R.; Fyfe, J.; Li, G.; Kharin, V.V. Changes in Temperature and Precipitation Across Canada. In Canada’s Changing Climate Report; Bush, E.L., Lemmen, D.S., Eds.; Government of Canada: Ottawa, ON, Canada, 2019; pp. 112–193. [Google Scholar]
- Bergerud, A.T. Evolving perspectives on caribou population dynamics, have we got it right yet? Rangifer 1996, 16, 95–116. [Google Scholar] [CrossRef]
- Culler, L.E.; Ayres, M.P.; Virginia, R.A. In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proc. R. Soc. B: Biol. Sci. 2015, 282, 20151549. [Google Scholar] [CrossRef]
- Buhler, K.J.B.J.; Fenton, H.; Solomon, I.; Jenkins, E. Revealing Reservoir Potential: Jamestown Canyon Virus Infection in Reindeer (Rangifer tarandus); University of Saskatchewan: Saskatoon, SK, Canada, 2023. [Google Scholar]
- Boutin, S.; Krebs, C.J.; Boonstra, R.; Dale, M.R.T.; Hannon, S.J.; Martin, K.; Sinclair, A.R.E.; Smith, J.N.M.; Turkington, R.; Blower, M.; et al. Population Changes of the Vertebrate Community during a Snowshoe Hare Cycle in Canada’s Boreal Forest. Oikos 1995, 74, 69–80. [Google Scholar] [CrossRef]
- Keith, L.B. Role of Food in Hare Population Cycles. Oikos 1983, 40, 385–395. [Google Scholar] [CrossRef]
- Krebs, C.J. Of lemmings and snowshoe hares: The ecology of northern Canada. Proc. R. Soc. B: Biol. Sci. 2011, 278, 481–489. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, T.P. Home Range and Ecology of Snowshoe Hares in Interior Alaska. J. Mammal. 1965, 46, 406–418. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Brooks, D.R. Evolution in action: Climate change, biodiversity dynamics and emerging infectious disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20130553. [Google Scholar] [CrossRef] [PubMed]
- Annual Climate Trends and Variations Bulletin 2021. Environment and Climate Change Canada. Available online: https://www.canada.ca/en/environment-climate-change/services/climate-change/science-research-data/climate-trends-variability/trends-variations.html (accessed on 15 January 2021).
- Bintanja, R. The impact of Arctic warming on increased rainfall. Sci. Rep. 2018, 8, 16001. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.; Berninger, F.; Nagy, L. Impacts of climate change on the tree line. Ann. Bot. 2002, 90, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Jenkins, E.; Epp, T.; Waldner, C.; Curry, P.S.; Soos, C. Climate change and West Nile virus in a highly endemic region of North America. Int. J. Environ. Res. Public. Health 2013, 10, 3052–3071. [Google Scholar] [CrossRef]
- Koltz, A.M.; Culler, L.E. Biting insects in a rapidly changing Arctic. Curr. Opin. Insect Sci. 2021, 47, 75–81. [Google Scholar] [CrossRef]
- Høye, T.T. Arthropods and climate change—Arctic challenges and opportunities. Curr. Opin. Insect Sci. 2020, 41, 40–45. [Google Scholar] [CrossRef]
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 38, 646–656. [Google Scholar] [CrossRef]
- Corbet, P.S.; Danks, H.V. Seasonal emergence and activity of mosquitoes (Diptera: Culicidae) in a high-arctic locality. Can. Entomol. 1973, 105, 837–872. [Google Scholar] [CrossRef]
- Laaksonen, S.; Oksanen, A. Status and review of the vector-borne nematode setaria tundra in finnish cervids. Alces: A J. Devoted Biol. Manag. Moose 2009, 45, 81–84. [Google Scholar]
- DeSiervo, M.H.; Ayres, M.P.; Virginia, R.A.; Culler, L.E. Consumer–resource dynamics in Arctic ponds. Ecology 2020, 101, e03135. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.C., Jr. Egg retention in Anopheles quadrimaculatus say in relation to the physiological age of the mosquito. J. Med. Entomol. 1968, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar]
- Frohne, W.C. The biology of northern mosquitoes. Public. Health Rep. 1956, 71, 616–621. [Google Scholar] [CrossRef]
- Hagan, R.W.; Didion, E.M.; Rosselot, A.E.; Holmes, C.J.; Siler, S.C.; Rosendale, A.J.; Hendershot, J.M.; Elliot, K.S.B.; Jennings, E.C.; Nine, G.A.; et al. Dehydration prompts increased activity and blood feeding by mosquitoes. Sci. Rep. 2018, 8, 6804. [Google Scholar] [CrossRef]
- Bradley, M.; Kutz, S.J.; Jenkins, E.; O’Hara, T.M. The potential impact of climate change on infectious diseases of Arctic fauna. Int. J. Circumpolar Health 2005, 64, 468–477. [Google Scholar] [CrossRef]
- Laaksonen, S.; Pusenius, J.; Kumpula, J.; Venäläinen, A.; Kortet, R.; Oksanen, A.; Hoberg, E. Climate change promotes the emergence of serious disease outbreaks of filarioid nematodes. EcoHealth 2010, 7, 7–13. [Google Scholar] [CrossRef]
- Andreassen, H.P.; Sundell, J.; Ecke, F.; Halle, S.; Haapakoski, M.; Henttonen, H.; Huitu, O.; Jacob, J.; Johnsen, K.; Koskela, E.; et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia 2021, 195, 601–622. [Google Scholar] [CrossRef]
- Laurent, M.; Dickie, M.; Becker, M.; Serrouya, R.; Boutin, S. Evaluating the Mechanisms of Landscape Change on White-Tailed Deer Populations. J. Wildl. Manag. 2021, 85, 340–353. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyman, J.; Snyman, L.P.; Buhler, K.J.; Villeneuve, C.-A.; Leighton, P.A.; Jenkins, E.J.; Kumar, A. California Serogroup Viruses in a Changing Canadian Arctic: A Review. Viruses 2023, 15, 1242. https://doi.org/10.3390/v15061242
Snyman J, Snyman LP, Buhler KJ, Villeneuve C-A, Leighton PA, Jenkins EJ, Kumar A. California Serogroup Viruses in a Changing Canadian Arctic: A Review. Viruses. 2023; 15(6):1242. https://doi.org/10.3390/v15061242
Chicago/Turabian StyleSnyman, Jumari, Louwrens P. Snyman, Kayla J. Buhler, Carol-Anne Villeneuve, Patrick A. Leighton, Emily J. Jenkins, and Anil Kumar. 2023. "California Serogroup Viruses in a Changing Canadian Arctic: A Review" Viruses 15, no. 6: 1242. https://doi.org/10.3390/v15061242
APA StyleSnyman, J., Snyman, L. P., Buhler, K. J., Villeneuve, C.-A., Leighton, P. A., Jenkins, E. J., & Kumar, A. (2023). California Serogroup Viruses in a Changing Canadian Arctic: A Review. Viruses, 15(6), 1242. https://doi.org/10.3390/v15061242







