A Proteomic Analysis of Detergent-Resistant Membranes in HIV Virological Synapse: The Involvement of Vimentin in CD4 Polarization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and viral Infection
2.2. Isolation of DRM
2.3. 2D DIGE and LC-MS
2.4. Western Blotting
2.5. Confocal Microscopy
2.6. Flow Cytometry (FCM)
3. Results
3.1. Accumulation of Lipid Raft Markers at HIV-1 VS
3.2. Proteomic Analysis of the DRM Fractions of Jurkat Cells Forming HIV-1 VS
3.3. Identification of Proteins Enriched in the DRM upon Formation of HIV-1 VS
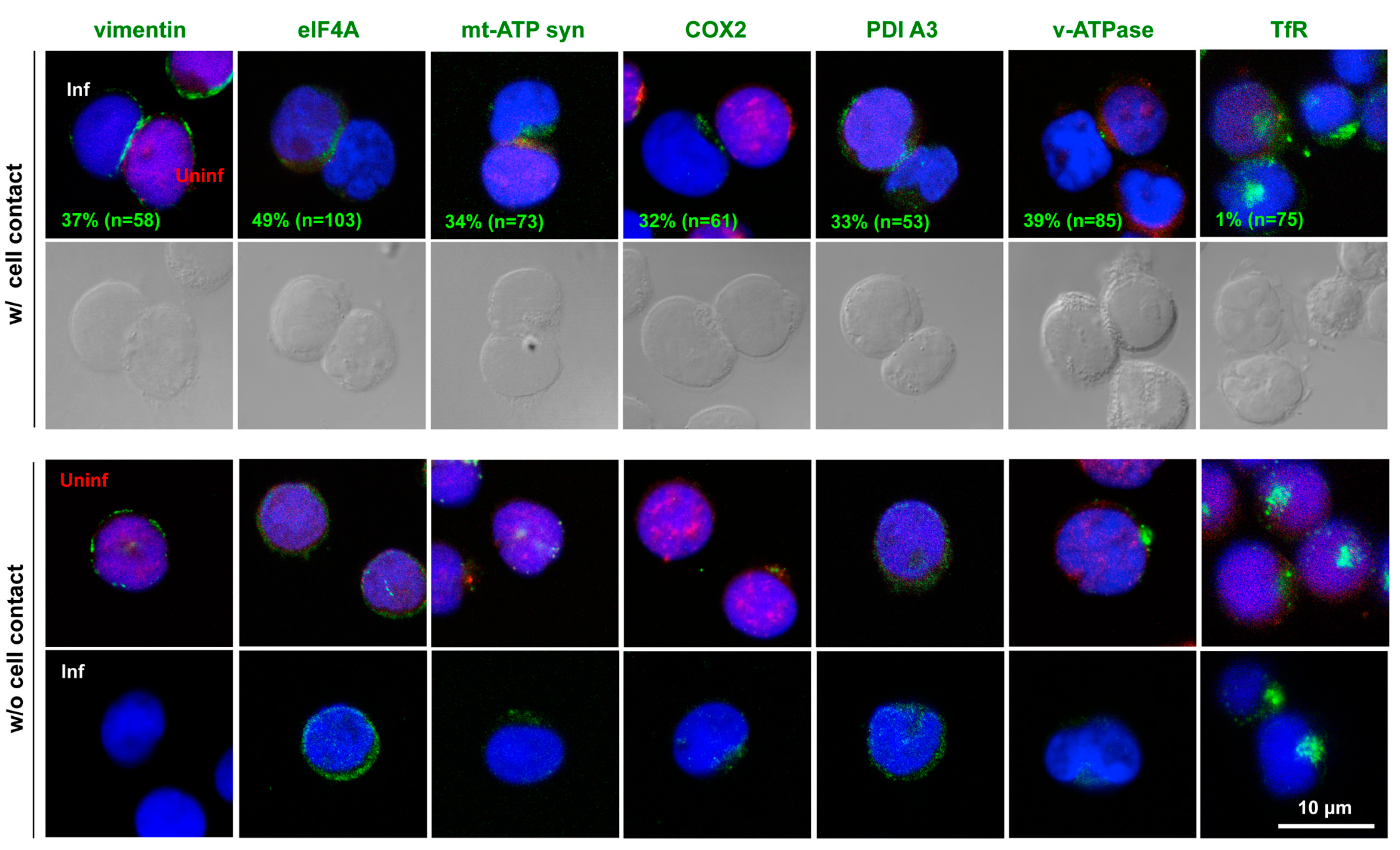
3.4. Polarized Localization of the Identified Molecules to HIV-1 VS
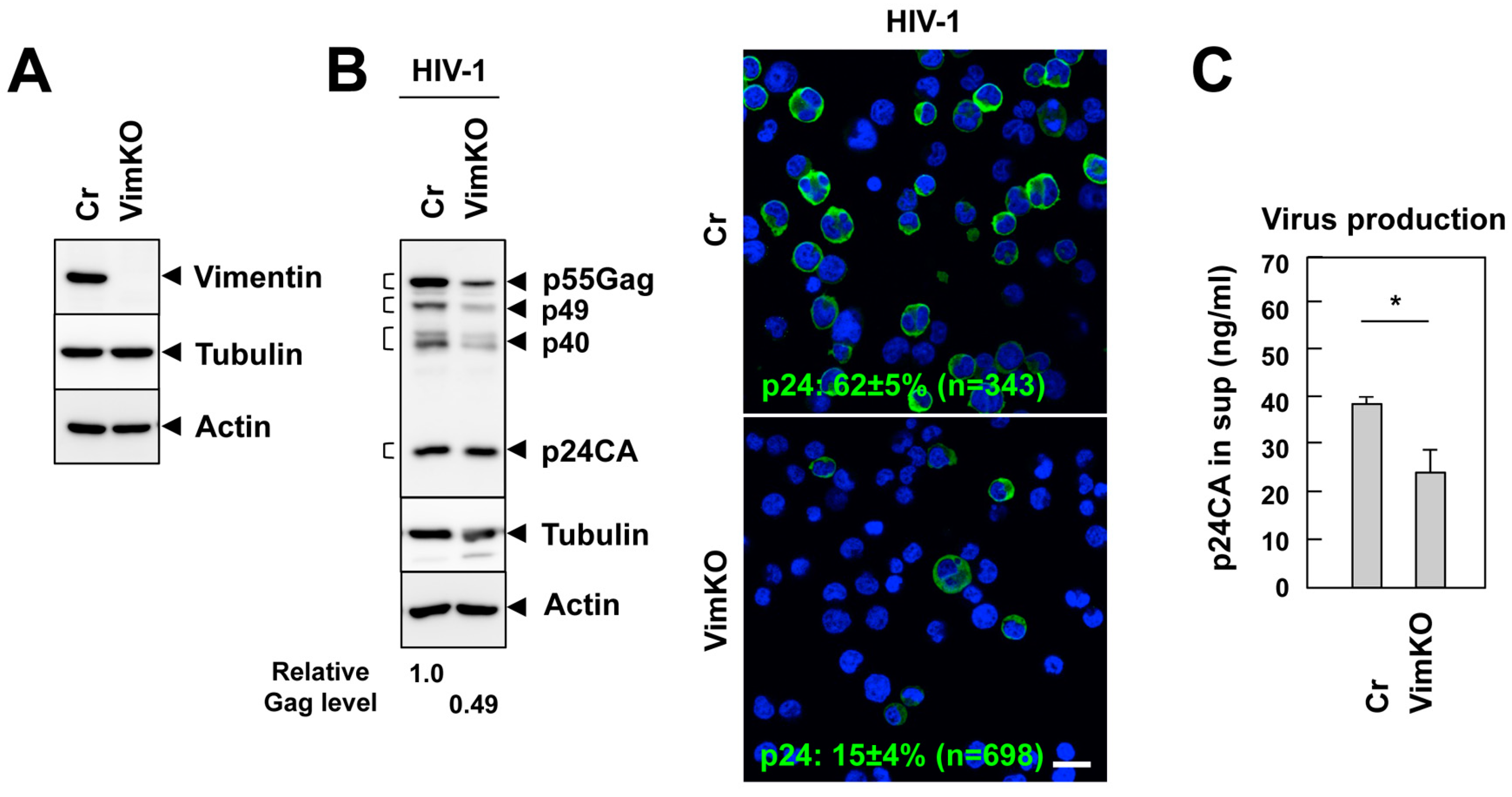
3.5. Vimentin Supports HIV-1 Infection
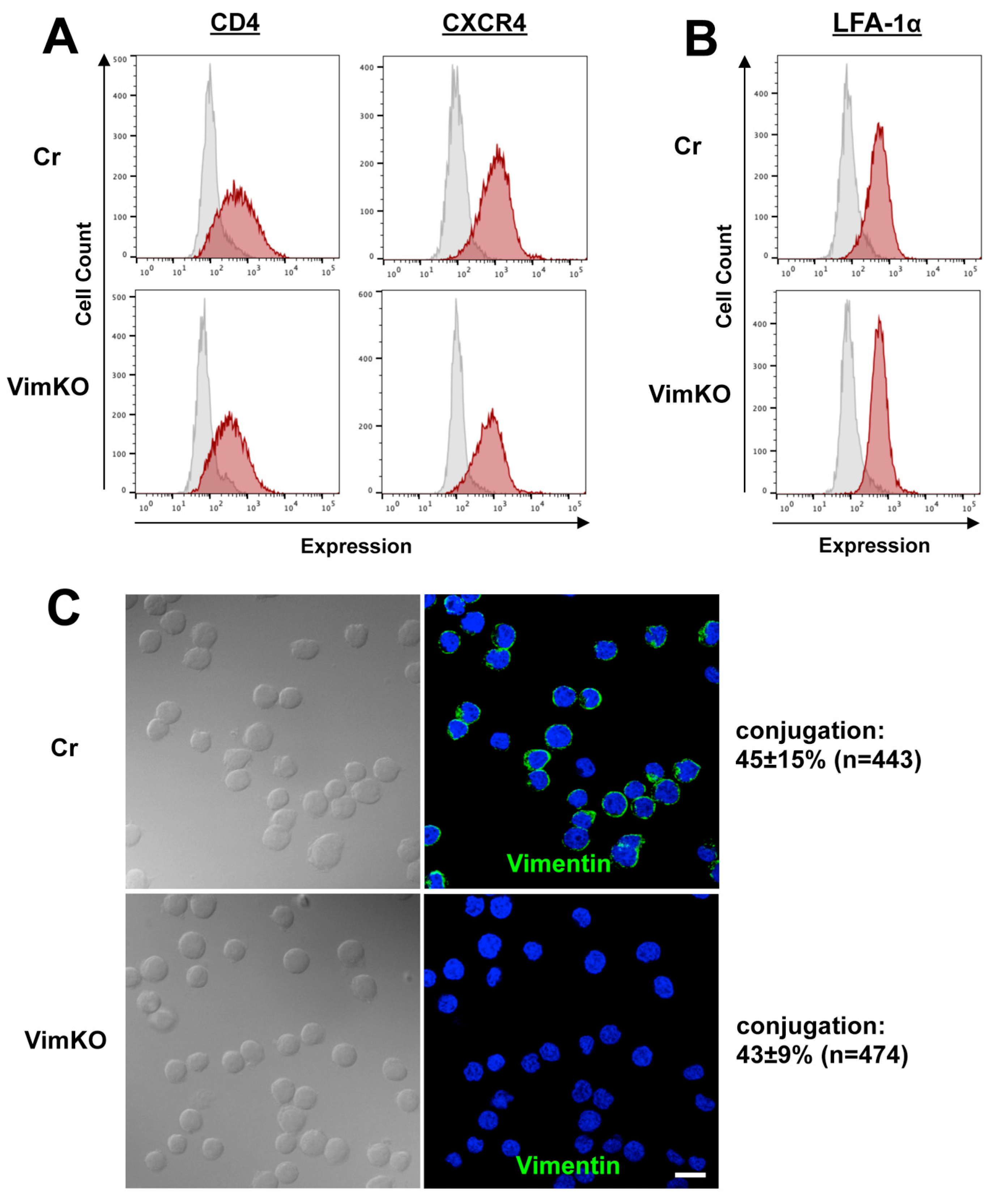
3.6. Little Effect of Vimentin Knockout on Cell Adhesion and Receptor Expression
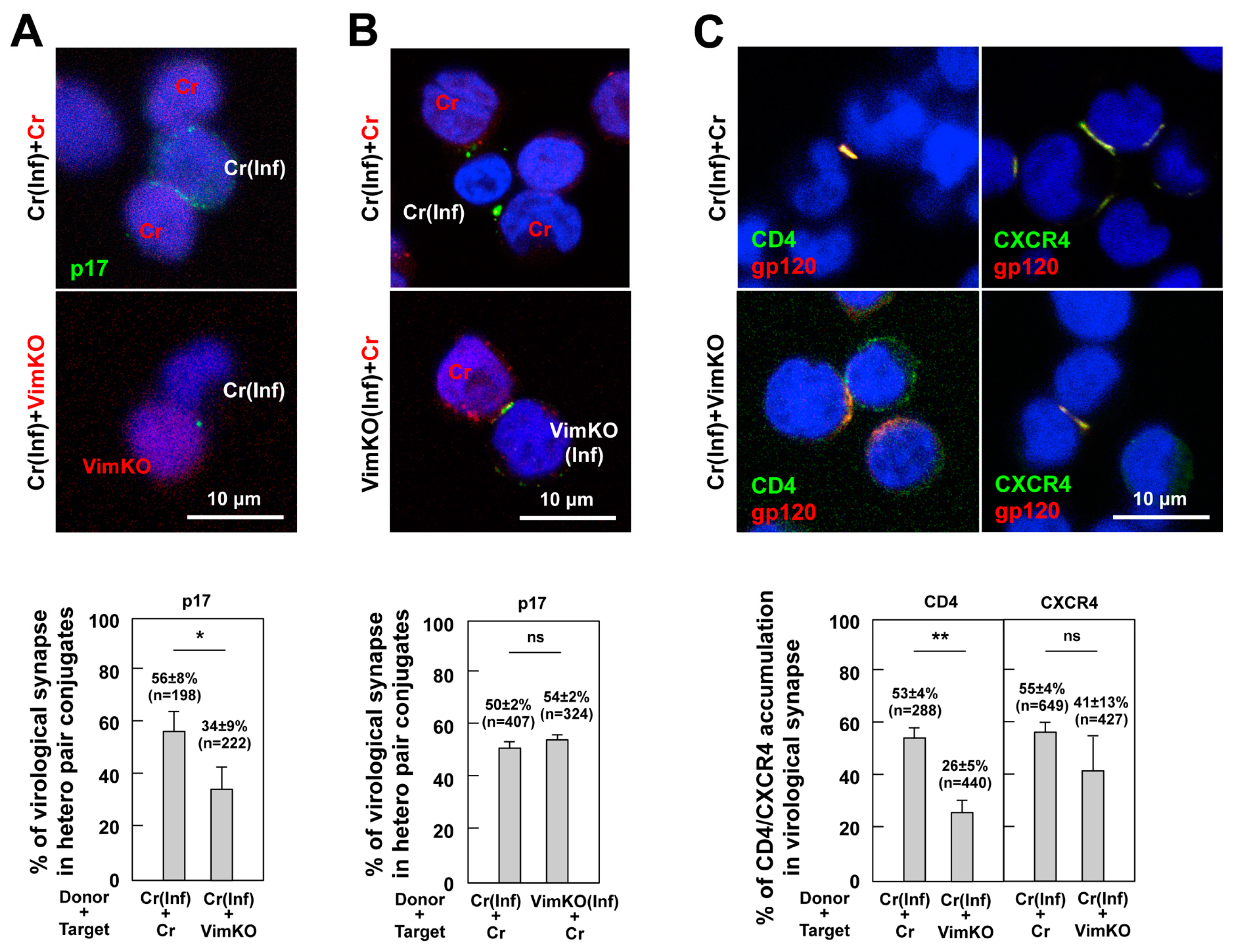
3.7. Reduction in CD4 and CXCR4 Accumulation to HIV-1 VS in Vimentin-Deficient Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, P.; Agosto, L.M.; Ilinskaya, A.; Dorjbal, B.; Truong, R.; Derse, D.; Uchil, P.D.; Heidecker, G.; Mothes, W. Cell-to-Cell Transmission Can Overcome Multiple Donor and Target Cell Barriers Imposed on Cell-Free HIV. PLoS ONE 2013, 8, e53138. [Google Scholar] [CrossRef] [Green Version]
- Jolly, C.; Kashefi, K.; Hollinshead, M.; Sattentau, Q.J. HIV-1 Cell to Cell Transfer across an Env-induced, Actin-dependent Synapse. J. Exp. Med. 2004, 199, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Hubner, W.; Spinelli, M.A.; Chen, B.K. Predominant Mode of Human Immunodeficiency Virus Transfer between T Cells Is Mediated by Sustained Env-Dependent Neutralization-Resistant Virological Synapses. J. Virol. 2007, 81, 12582–12595. [Google Scholar] [CrossRef] [Green Version]
- Rudnicka, D.; Feldmann, J.; Porrot, F.; Wietgrefe, S.; Guadagnini, S.; Prevost, M.-C.; Estaquier, J.; Haase, A.T.; Sol-Foulon, N.; Schwartz, O. Simultaneous Cell-to-Cell Transmission of Human Immunodeficiency Virus to Multiple Targets through Polysynapses. J. Virol. 2009, 83, 6234–6246. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Eng, E.T.; Law, K.; Gordon, R.E.; Rice, W.J.; Chen, B.K. Visualization of HIV T Cell Virological Synapses and Virus-Containing Compartments by Three-Dimensional Correlative Light and Electron Microscopy. J. Virol. 2017, 91, e01605-16. [Google Scholar] [CrossRef] [Green Version]
- Geijtenbeek, T.B.H.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.M.H.A.; Nottet, H.S.L.M.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D. Dendritic Cells and HIV-1 Trans-Infection. Viruses 2010, 2, 1704–1717. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.; Wu, L.; Bohks, S.M.; KewalRamani, V.N.; Unutmaz, D.; Hope, T.J. Recruitment of HIV and Its Receptors to Dendritic Cell-T Cell Junctions. Science 2003, 300, 1295–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, C.; Mitar, I.; Sattentau, Q.J. Adhesion Molecule Interactions Facilitate Human Immunodeficiency Virus Type 1-Induced Virological Synapse Formation between T Cells. J. Virol. 2007, 81, 13916–13921. [Google Scholar] [CrossRef] [Green Version]
- Vasiliver-Shamis, G.; Tuen, M.; Wu, T.W.; Starr, T.; Cameron, T.O.; Thomson, R.; Kaur, G.; Liu, J.; Visciano, M.L.; Li, H.; et al. Human Immunodeficiency Virus Type 1 Envelope gp120 Induces a Stop Signal and Virological Synapse Formation in Noninfected CD4+ T Cells. J. Virol. 2008, 82, 9445–9457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiliver-Shamis, G.; Cho, M.W.; Hioe, C.E.; Dustin, M.L. Human Immunodeficiency Virus Type 1 Envelope gp120-Induced Partial T-Cell Receptor Signaling Creates an F-Actin-Depleted Zone in the Virological Synapse. J. Virol. 2009, 83, 11341–11355. [Google Scholar] [CrossRef] [Green Version]
- Vasiliver-Shamis, G.; Dustin, M.; Hioe, C. HIV-1 Virological Synapse is not Simply a Copycat of the Immunological Synapse. Viruses 2010, 2, 1239–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starling, S.; Jolly, C. LFA-1 Engagement Triggers T Cell Polarization at the HIV-1 Virological Synapse. J. Virol. 2016, 90, 9841–9854. [Google Scholar] [CrossRef] [Green Version]
- Groppelli, E.; Starling, S.; Jolly, C. Contact-Induced Mitochondrial Polarization Supports HIV-1 Virological Synapse Formation. J. Virol. 2015, 89, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.F.; Tseng, S.P.; Huang, S.W.; Yen, C.H.; Hong, Y.W.; Chen, M.; Chen, Y.M.A. Mitochondria polarization in the contact regions of virus-infected effector cells during cell-to-cell transmission of HIV-1. AIDS Res. Hum. Retrovir. 2015, 31, 175–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavlovich, A.; Viard, M.; Zhou, M.; Veenstra, T.D.; Wang, J.M.; Gong, W.; Heldman, E.; Blumenthal, R.; Raviv, Y. Ectopic ATP synthase facilitates transfer of HIV-1 from antigen-presenting cells to CD4+ target cells. Blood 2012, 120, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Chamberlain, L.H. Detergents as tools for the purification and classification of lipid rafts. FEBS Lett. 2004, 559, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Foster, L.J.; De Hoog, C.L.; Mann, M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. USA 2003, 100, 5813–5818. [Google Scholar] [CrossRef] [Green Version]
- Razzaq, T.M.; Ozegbe, P.; Jury, E.C.; Sembi, P.; Blackwell, N.M.; Kabouridis, P.S. Regulation of T-cell receptor signaling by membrane microdomains. Immunology 2004, 113, 413–426. [Google Scholar] [CrossRef]
- Kabouridis, P.S. Lipid rafts in T cell receptor signalling (review). Mol. Membr. Biol. 2006, 23, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Katagiri, T.; Kosako, H.; Iida, N.; Hattoris, S. Global analysis of dynamic changes in lipid raft proteins during T-cell activation. Electrophoresis 2007, 28, 2035–2043. [Google Scholar] [CrossRef]
- Jolly, C.; Sattentau, Q.J. Human Immunodeficiency Virus Type 1 Virological Synapse Formation in T Cells Requires Lipid Raft Integrity. J. Virol. 2005, 79, 12088–12094. [Google Scholar] [CrossRef] [Green Version]
- Ono, A.; Freed, E.O. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 2001, 98, 13925–13930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, K.; Weclewicz, K.; Hewson, R.; Suomalainen, M. Human Immunodeficiency Virus Type 1 Assembly and Lipid Rafts: Pr55gag Associates with Membrane Domains That Are Largely Resistant to Brij98 but Sensitive to Triton X-100. J. Virol. 2003, 77, 4805–4817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogue, I.B.; Grover, J.R.; Soheilian, F.; Nagashima, K.; Ono, A. Gag Induces the Coalescence of Clustered Lipid Rafts and Tetraspanin-Enriched Microdomains at HIV-1 Assembly Sites on the Plasma Membrane. J. Virol. 2011, 85, 9749–9766. [Google Scholar] [CrossRef] [Green Version]
- Del Real, G.; Jiménez-Baranda, S.; Lacalle, R.A.; Mira, E.; Lucas, P.; Gómez-Moutón, C.; Carrera, A.C.; Martínez-A, C.; Mañes, S. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J. Exp. Med. 2002, 196, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popik, W.; Alce, T.M.; Au, W.-C. Human Immunodeficiency Virus Type 1 Uses Lipid Raft-Colocalized CD4 and Chemokine Receptors for Productive Entry into CD4+ T Cells. J. Virol. 2002, 76, 4709–4722. [Google Scholar] [CrossRef] [Green Version]
- Popik, W.; Alce, T.M. CD4 receptor localized to non-raft membrane microdomains supports HIV-1 entry. Identification of a novel raft localization marker in CD4. J. Biol. Chem. 2004, 279, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Percherancier, Y.; Lagane, B.; Planchenault, T.; Staropoli, I.; Altmeyer, R.; Virelizier, J.L.; Arenzana-Seisdedos, F.; Hoessli, D.C.; Bachelerie, F. HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J. Biol. Chem. 2003, 278, 3153–3161. [Google Scholar] [CrossRef] [Green Version]
- Krementsov, D.N.; Rassam, P.; Margeat, E.; Roy, N.H.; Schneider-Schaulies, J.; Milhiet, P.-E.; Thali, M. HIV-1 Assembly Differentially Alters Dynamics and Partitioning of Tetraspanins and Raft Components. Traffic 2010, 11, 1401–1414. [Google Scholar] [CrossRef] [Green Version]
- Nydegger, S.; Khurana, S.; Krementsov, D.N.; Foti, M.; Thali, M. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J. Cell Biol. 2006, 173, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Jolly, C.; Sattentau, Q.J. Human Immunodeficiency Virus Type 1 Assembly, Budding, and Cell-Cell Spread in T Cells Take Place in Tetraspanin-Enriched Plasma Membrane Domains. J. Virol. 2007, 81, 7873–7884. [Google Scholar] [CrossRef] [Green Version]
- Len, A.C.L.; Starling, S.; Shivkumar, M.; Jolly, C. HIV-1 Activates T Cell Signaling Independently of Antigen to Drive Viral Spread. Cell Rep. 2017, 18, 1062–1074. [Google Scholar] [CrossRef] [Green Version]
- Arentz, G.; Weiland, F.; Oehler, M.K.; Hoffmann, P. State of the art of 2D DIGE. Proteom.-Clin. Appl. 2015, 9, 277–288. [Google Scholar] [CrossRef]
- Tsunetsugu-Yokota, Y.; Ishige, M.; Murakami, M. Oral attenuated Salmonella enterica serovar typhimurium vaccine expressing codon-optimized HIV type 1 gag enhanced intestinal immunity in mice. AIDS Res. Hum. Retrovir. 2007, 23, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ferns, R.B.; Partridge, J.C.; Spence, R.P.; Hunt, N.; Tedder, R.S. Epitope location of 13 anti-gag HIV-1 monoclonal antibodies using oligopeptides and their cross reactivity with HIV-2. Aids 1989, 3, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Von Schwedler, U.K.; Stuchell, M.; Müller, B.; Ward, D.M.; Chung, H.Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.P.; Cimbora, D.M.; et al. The protein network of HIV budding. Cell 2003, 114, 701–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbouche, R.; Miquelis, R.; Jones, I.M.; Fenouillet, E. Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 occurs post-CXCR4 binding and is required for fusion. J. Biol. Chem. 2003, 278, 3131–3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandréou, M.J.; Barbouche, R.; Guieu, R.; Rivera, S.; Fantini, J.; Khrestchatisky, M.; Jones, I.M.; Fenouillet, E. Mapping of domains on HIV envelope protein mediating association with calnexin and protein-disulfide isomerase. J. Biol. Chem. 2010, 285, 13788–13796. [Google Scholar] [CrossRef] [Green Version]
- Jouvenet, N. Dynamics of ESCRT proteins. Cell. Mol. Life Sci. 2012, 69, 4121–4133. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortega, C.; Ramírez, A.; Casillas, D.; Paneque, T.; Ubieta, R.; Dubed, M.; Navea, L.; Castellanos-Serra, L.; Duarte, C.; Falcon, V.; et al. Identification of vimentin as a potential therapeutic target against HIV infection. Viruses 2016, 8, 98. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Gonzales, M.; Weksler, B.; Tsuruta, D.; Goldman, R.D.; Yoon, K.J.; Hopkinson, S.B.; Flitney, F.W.; Jones, J.C.R. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 2001, 12, 85–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yang, C.; Kim, E.J.; Jang, J.; Kim, S.J.; Kang, S.M.; Kim, M.G.; Jung, H.; Park, D.; Kim, C. Vimentin filaments regulate integrin-ligand interactions by binding to the cytoplasmic tail of integrin β3. J. Cell Sci. 2016, 129, 2030–2042. [Google Scholar] [CrossRef] [Green Version]
- Ono, A.; Orenstein, J.M.; Freed, E.O. Role of the Gag Matrix Domain in Targeting Human Immunodeficiency Virus Type 1 Assembly. J. Virol. 2000, 74, 2855–2866. [Google Scholar] [CrossRef] [Green Version]
- Ono, A.; Freed, E.O. Cell-Type-Dependent Targeting of Human Immunodeficiency Virus Type 1 Assembly to the Plasma Membrane and the Multivesicular Body. J. Virol. 2004, 78, 1552–1563. [Google Scholar] [CrossRef] [Green Version]
- Hubner, W.; McNerney, G.P.; Chen, P.; Dale, B.M.; Gordon, R.E.; Chuang, F.Y.S.; Li, X.-D.; Asmuth, D.M.; Huser, T.; Chen, B.K. Quantitative 3D Video Microscopy of HIV Transfer Across T Cell Virological Synapses. Science 2009, 323, 1743–1747. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.Z.; Berg, K.B.; Foster, L.J. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 2009, 50, 988–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.J.; Williamson, R.; Schofield, E.; Stephenson, J.; Hanger, D.; Anderton, B. Quantitation of glycogen synthase kinase-3 sensitive proteins in neuronal membrane rafts. Proteomics 2009, 9, 3022–3035. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Mitar, I.; Sattentau, Q.J. Requirement for an Intact T-Cell Actin and Tubulin Cytoskeleton for Efficient Assembly and Spread of Human Immunodeficiency Virus Type 1. J. Virol. 2007, 81, 5547–5560. [Google Scholar] [CrossRef] [Green Version]
- Billadeau, D.D.; Nolz, J.C.; Gomez, T.S. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 2007, 7, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cófreces, N.B.; Baixauli, F.; Sánchez-Madrid, F. Immune synapse: Conductor of orchestrated organelle movement. Trends Cell Biol. 2014, 24, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritzsche, M.; Fernandes, R.A.; Chang, V.T.; Colin-York, H.; Clausen, M.P.; Felce, J.H.; Galiani, S.; Erlenkämper, C.; Santos, A.M.; Heddleston, J.M.; et al. Cytoskeletal actin dynamics shape a ramifying actin network underpinning immunological synapse formation. Sci. Adv. 2017, 3, e1603032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esue, O.; Carson, A.A.; Tseng, Y.; Wirtz, D. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. J. Biol. Chem. 2006, 281, 30393–30399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, J.E.; He, T.; Trejo-Skalli, A.V.; Härmälä-Braskén, A.S.; Hellman, J.; Chou, Y.H.; Goldman, R.D. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 2004, 117, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Omary, M.B.; Ku, N.O.; Tao, G.Z.; Toivola, D.M.; Liao, J. “Heads and tails” of intermediate filament phosphorylation: Multiple sites and functional insights. Trends Biochem. Sci. 2006, 31, 383–394. [Google Scholar] [CrossRef]
- Pallari, H.M.; Eriksson, J.E. Intermediate filaments as signaling platforms. Sci. STKE 2006, 2006, 6–10. [Google Scholar] [CrossRef]
- Robidoux, J.; Kumar, N.; Daniel, K.W.; Moukdar, F.; Cyr, M.; Medvedev, A.V.; Collins, S. Maximal β3-adrenergic regulation of lipolysis involves Src and epidermal growth factor receptor-dependent ERK1/2 activation. J. Biol. Chem. 2006, 281, 37794–37802. [Google Scholar] [CrossRef] [Green Version]

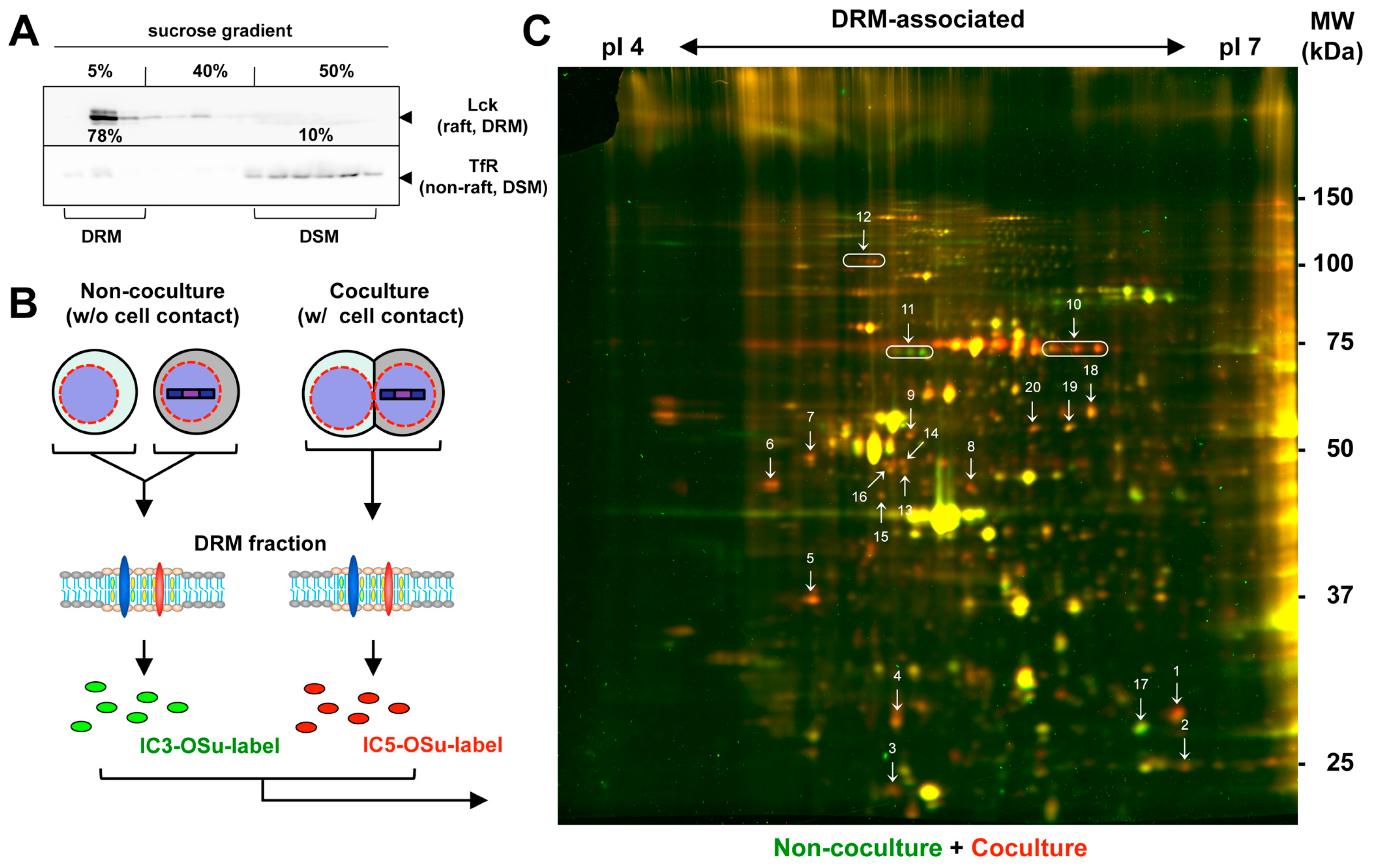

| Spot No. | Name | Accession No. | MW (KDa) | Spot Volume Ratio * |
|---|---|---|---|---|
| 1 | HIV-1 p24CA | NP579880.1 | 25.6 | 2.45 |
| 2 | Unidentified | 1.49 | ||
| 3 | Mitochondrial ATP synthase subunit d | O75947 | 18.5 | 1.66 |
| 4 | Rho GDP-dissociation inhibitor 2 | P52566 | 23 | 1.76 |
| 5 | Charged multivesicular body protein 4B | Q9H444 | 25 | 2.46 |
| 6 | Vimentin | P08670 | 53.7 | 2.66 |
| 7 | Vimentin | P08670 | 53.7 | 1.74 |
| 8 | Eukaryotic initiation factor 4A | P06842 | 46.2 | 2.71 |
| 9 | Vimentin | P08670 | 53.7 | 2.04 |
| 10 | Serum albumin | P02768 | 69.4 | 3.19, 3.49, 3.53 |
| 11 | Lamin B1 | P20700 | 66.4 | −1.11, −2.27, −3.07 |
| 12 | GRIP1-associated protein 1 | Q4V328 | 96 | 1.93, 2.50, 2.39, 3.24 |
| 13 | Mitochondrial Elongation factor Tu | P494111 | 49.5 | 1.37 |
| 14 | 26S protease regulatory subunit 6A | P17980 | 49.2 | 1.5 |
| 15 | unindentified | 1.61 | ||
| 16 | 26S protease regulatory subunit 6B | P43686 | 47.4 | 1.61 |
| 17 | Metaxin 2 | O75431 | 29.8 | −1.84 |
| 18 | Protein disulfide-isomerase A3 | P30101 | 56.8 | 1.58 |
| 19 | V-type proton ATPase subunit B | P21281 | 56.5 | 1.16 |
| 20 | V-type proton ATPase subunit B | P21281 | 56.5 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iida, N.; Kawahara, M.; Hirota, R.; Shibagaki, Y.; Hattori, S.; Morikawa, Y. A Proteomic Analysis of Detergent-Resistant Membranes in HIV Virological Synapse: The Involvement of Vimentin in CD4 Polarization. Viruses 2023, 15, 1266. https://doi.org/10.3390/v15061266
Iida N, Kawahara M, Hirota R, Shibagaki Y, Hattori S, Morikawa Y. A Proteomic Analysis of Detergent-Resistant Membranes in HIV Virological Synapse: The Involvement of Vimentin in CD4 Polarization. Viruses. 2023; 15(6):1266. https://doi.org/10.3390/v15061266
Chicago/Turabian StyleIida, Naoyuki, Madoka Kawahara, Riku Hirota, Yoshio Shibagaki, Seisuke Hattori, and Yuko Morikawa. 2023. "A Proteomic Analysis of Detergent-Resistant Membranes in HIV Virological Synapse: The Involvement of Vimentin in CD4 Polarization" Viruses 15, no. 6: 1266. https://doi.org/10.3390/v15061266




