Alzheimer’s Disease Pathology in Middle Aged and Older People with HIV: Comparisons with Non-HIV Controls on a Healthy Aging and Alzheimer’s Disease Trajectory and Relationships with Cognitive Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. NNTC Cohort
2.2. ADRC Cohort
2.3. Neuropathological Characterization in NNTC and ADRC
2.4. Statistical Analyses
3. Results
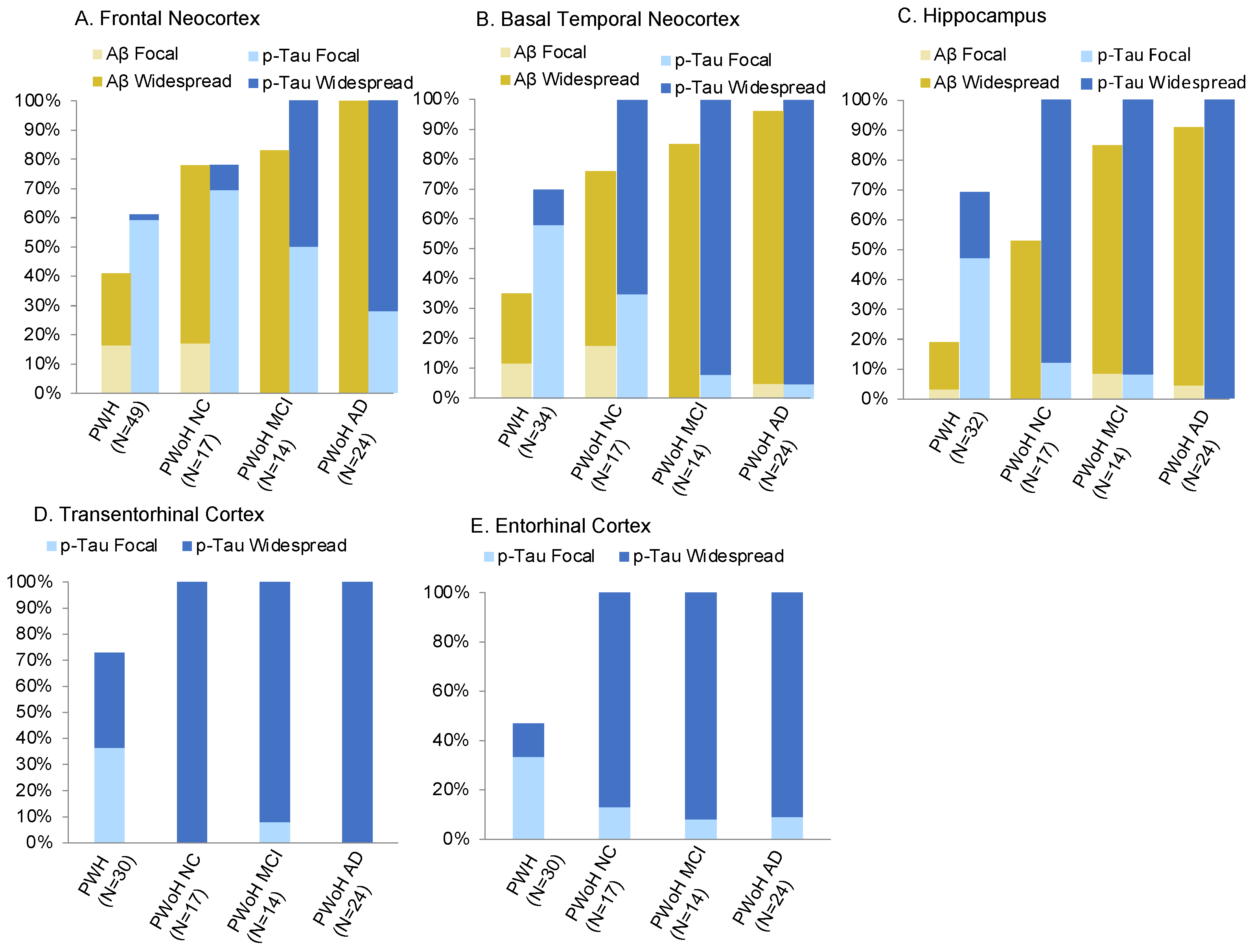
3.1. Region-Specific Aβ Prevalence
3.2. Region-Specific p-Tau Prevalence
3.3. Prevalence of Conjoint Aβ and p-Tau Positivity
3.4. Comparison of AD Pathology between PWH and PWoH
3.5. Relationship between AD Pathology and Antemortem Cognitive Function among PWH
3.6. AD Pathology Prevalence in Women versus Men with HIV
3.7. AD Pathology and Cognitive Function Relationship in Women versus Men with HIV
3.7.1. Women with HIV
3.7.2. Men with HIV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HIV Surveillance Report, 2020; Vol. 33. Published May 2022. Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 1 November 2022).
- Alisky, J.M. The Coming Problem of HIV-Associated Alzheimer’s Disease. Med. Hypoth. 2007, 69, 1140–1143. [Google Scholar] [CrossRef]
- Cohen, R.A.; Seider, T.R.; Navia, B. HIV Effects on Age-Associated Neurocognitive Dysfunction: Premature Cognitive Aging or Neurodegenerative Disease? Alzheimer’s Res. Ther. 2015, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Cruse, B.; Cysique, L.A.; Markus, R.; Brew, B.J. Cerebrovascular Disease in HIV-Infected Individuals in the Era of Highly Active Antiretroviral Therapy. J. Neurovirol. 2012, 18, 264–276. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Stoff, D.M.; Goodkin, K.; Jeste, D.; Marquine, M. Redefining Aging in HIV Infection Using Phenotypes. Curr. HIV/AIDS Rep. 2017, 14, 184–199. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.; Levine, A.J. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J. Infect. Dis. 2015, 212, 1563–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, M.; Chartrand-Lefebvre, C.; Baril, J.-G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; Routy, J.-P.; et al. The Canadian HIV and Aging Cohort Study—Determinants of Increased Risk of Cardio-Vascular Diseases in HIV-Infected Individuals: Rationale and Study Protocol. BMC Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories from 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.C.; Price, J.L. Pathologic Correlates of Nondemented Aging, Mild Cognitive Impairment, and Early-Stage Alzheimer’s Disease. J. Mol. Neurosci. 2001, 17, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Achim, C.L.; Adame, A.; Dumaop, W.; Everall, I.P.; Masliah, E. Neurobehavioral Research Center Increased Accumulation of Intraneuronal Amyloid Beta in HIV-Infected Patients. J. Neuroimmune Pharmacol. 2009, 4, 190–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esiri, M.M.; Biddolph, S.C.; Morris, C.S. Prevalence of Alzheimer Plaques in AIDS. J. Neurol. Neurosurg. Psychiatry 1998, 65, 29–33. [Google Scholar] [CrossRef]
- Gelman, B.B.; Schuenke, K. Brain Aging in Acquired Immunodeficiency Syndrome: Increased Ubiquitin-Protein Conjugate Is Correlated with Decreased Synaptic Protein but Not Amyloid Plaque Accumulation. J. Neurovirol. 2004, 10, 98–108. [Google Scholar] [CrossRef]
- Green, D.A.; Masliah, E.; Vinters, H.V.; Beizai, P.; Moore, D.J.; Achim, C.L. Brain Deposition of Beta-Amyloid Is a Common Pathologic Feature in HIV Positive Patients. AIDS 2005, 19, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Rempel, H.C.; Pulliam, L. HIV-1 Tat Inhibits Neprilysin and Elevates Amyloid Beta. AIDS 2005, 19, 127–135. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Bell, J.E. Brain Viral Burden, Neuroinflammation and Neurodegeneration in HAART-Treated HIV Positive Injecting Drug Users. J. Neurovirol. 2014, 20, 28–38. [Google Scholar] [CrossRef]
- Morgello, S.; Cortes, E.P.; Gensler, G.; Meloni, G.; Jacobs, M.M.; Murray, J.; Borukov, V.; Crary, J.F. HIV Disease Duration, but Not Active Brain Infection, Predicts Cortical Amyloid Beta Deposition. AIDS 2021, 35, 1403–1412. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Moore, D.J.; Gouaux, B.; Soontornniyomkij, B.; Sinsheimer, J.S.; Levine, A.J. Associations of Regional Amyloid-β Plaque and Phospho-Tau Pathology with Biological Factors and Neuropsychological Functioning among HIV-Infected Adults. J. Neurovirol. 2019, 25, 741–753. [Google Scholar] [CrossRef]
- Umlauf, A.; Soontornniyomkij, B.; Sundermann, E.E.; Gouaux, B.; Ellis, R.J.; Levine, A.J.; Moore, D.J.; Soontornniyomkij, V. Risk of Developing Cerebral β-Amyloid Plaques with Posttranslational Modification among HIV-Infected Adults. AIDS 2019, 33, 2157–2166. [Google Scholar] [CrossRef]
- Solomon, I.H.; De Girolami, U.; Chettimada, S.; Misra, V.; Singer, E.J.; Gabuzda, D. Brain and Liver Pathology, Amyloid Deposition, and Interferon Responses among Older HIV-Positive Patients in the Late HAART Era. BMC Infect. Dis. 2017, 17, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.; Meloni, G.; Cortes, E.P.; KimSilva, A.; Jacobs, M.; Ramkissoon, A.; Crary, J.F.; Morgello, S. Frontal Lobe Microglia, Neurodegenerative Protein Accumulation, and Cognitive Function in People with HIV. Acta Neuropathol. Commun. 2022, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Ances, B.M.; Christensen, J.J.; Teshome, M.; Taylor, J.; Xiong, C.; Aldea, P.; Fagan, A.M.; Holtzman, D.M.; Morris, J.C.; Mintun, M.A.; et al. Cognitively Unimpaired HIV-Positive Subjects Do Not Have Increased 11C-PiB: A Case-Control Study. Neurology 2010, 75, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, J.H.; Eftychiou, N.; Schuerer, M.; Rullmann, M.; Barthel, H.; Sabri, O.; Gisslen, M.; Zetterberg, H.; Blennow, K.; O’Brien, C.; et al. Clinical Utility of β-Amyloid PET Imaging in People Living with HIV with Cognitive Symptoms. J. Acquir. Immune Defic. Syndr. 2021, 87, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Howdle, G.C.; Quidé, Y.; Kassem, M.S.; Johnson, K.; Rae, C.D.; Brew, B.J.; Cysique, L.A. Brain Amyloid in Virally Suppressed HIV-Associated Neurocognitive Disorder. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e739. [Google Scholar] [CrossRef]
- Brew, B.J.; Crowe, S.M.; Landay, A.; Cysique, L.A.; Guillemin, G. Neurodegeneration and Ageing in the HAART Era. J. Neuroimmune Pharmacol. 2009, 4, 163–174. [Google Scholar] [CrossRef]
- Brew, B.J.; Letendre, S.L. Biomarkers of HIV Related Central Nervous System Disease. Int. Rev. Psychiatry 2008, 20, 73–88. [Google Scholar] [CrossRef]
- Everall, I.; Vaida, F.; Khanlou, N.; Lazzaretto, D.; Achim, C.; Letendre, S.; Moore, D.; Ellis, R.; Cherner, M.; Gelman, B.; et al. Cliniconeuropathologic Correlates of Human Immunodeficiency Virus in the Era of Antiretroviral Therapy. J. Neurovirol. 2009, 15, 360–370. [Google Scholar] [CrossRef]
- Patrick, C.; Crews, L.; Desplats, P.; Dumaop, W.; Rockenstein, E.; Achim, C.L.; Everall, I.P.; Masliah, E. Increased CDK5 Expression in HIV Encephalitis Contributes to Neurodegeneration via Tau Phosphorylation and Is Reversed with Roscovitine. Am. J. Pathol. 2011, 178, 1646–1661. [Google Scholar] [CrossRef]
- Anthony, I.C.; Ramage, S.N.; Carnie, F.W.; Simmonds, P.; Bell, J.E. Accelerated Tau Deposition in the Brains of Individuals Infected with Human Immunodeficiency Virus-1 before and after the Advent of Highly Active Anti-Retroviral Therapy. Acta Neuropathol. 2006, 111, 529–538. [Google Scholar] [CrossRef]
- Fields, J.A.; Swinton, M.K.; Soontornniyomkij, B.; Carson, A.; Achim, C.L. Beta Amyloid Levels in CSF of HIV-Infected People Vary by Exposure to Antiretroviral Therapy. AIDS 2020, 34, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Fagan, A.M.; Holtzman, D.M.; Morris, J.C.; Teshome, M.; Shah, A.R.; Kauwe, J.S.K. CSF Biomarkers of Alzheimer Disease in HIV-Associated Neurologic Disease. Neurology 2009, 73, 1982–1987. [Google Scholar] [CrossRef]
- Gisslén, M.; Krut, J.; Andreasson, U.; Blennow, K.; Cinque, P.; Brew, B.J.; Spudich, S.; Hagberg, L.; Rosengren, L.; Price, R.W.; et al. Amyloid and Tau Cerebrospinal Fluid Biomarkers in HIV Infection. BMC Neurol. 2009, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Jessen Krut, J.; Mellberg, T.; Price, R.W.; Hagberg, L.; Fuchs, D.; Rosengren, L.; Nilsson, S.; Zetterberg, H.; Gisslén, M. Biomarker Evidence of Axonal Injury in Neuroasymptomatic HIV-1 Patients. PLoS ONE 2014, 9, e88591. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.; Gisslen, M.; Zetterberg, H.; Fuchs, D.; Shacklett, B.L.; Hagberg, L.; Yiannoutsos, C.T.; Spudich, S.S.; Price, R.W. Cerebrospinal Fluid (CSF) Neuronal Biomarkers across the Spectrum of HIV Infection: Hierarchy of Injury and Detection. PLoS ONE 2014, 9, e116081. [Google Scholar] [CrossRef]
- Brew, B.J.; Pemberton, L.; Blennow, K.; Wallin, A.; Hagberg, L. CSF Amyloid Beta42 and Tau Levels Correlate with AIDS Dementia Complex. Neurology 2005, 65, 1490–1492. [Google Scholar] [CrossRef]
- Cooley, S.A.; Strain, J.F.; Beaumont, H.; Boerwinkle, A.H.; Doyle, J.; Morris, J.C.; Benzinger, T.L.; Ances, B.M. Tau Positron Emission Tomography Binding Is Not Elevated in HIV-Infected Individuals. J. Infect. Dis. 2019, 220, 68–72. [Google Scholar] [CrossRef]
- Dreyer, A.J.; Munsami, A.; Williams, T.; Andersen, L.S.; Nightingale, S.; Gouse, H.; Joska, J.; Thomas, K.G.F. Cognitive Differences between Men and Women with HIV: A Systematic Review and Meta-Analysis. Arch. Clin. Neuropsychol. 2022, 37, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Neigh, G.N.; Sundermann, E.E.; Xu, Y.; Scully, E.P.; Maki, P.M. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr. Psychiatry Rep. 2019, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.F.; Mormino, E.C.; Rabin, J.S.; Hohman, T.J.; Landau, S.; Hanseeuw, B.J.; Jacobs, H.I.L.; Papp, K.V.; Amariglio, R.E.; Properzi, M.J.; et al. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. 2019, 76, 542–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohman, T.J.; Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Gifford, K.A.; Bush, W.S.; Chibnik, L.B.; Mukherjee, S.; et al. Sex-Specific Association of Apolipoprotein e with Cerebrospinal Fluid Levels of Tau. JAMA Neurol. 2018, 75, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D. Sex Modifies the APOE-Related Risk of Developing Alzheimer Disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oveisgharan, S.; Arvanitakis, Z.; Yu, L.; Farfel, J.; Schneider, J.A.; Bennett, D.A. Sex Differences in Alzheimer’s Disease and Common Neuropathologies of Aging. Acta Neuropathol. 2018, 136, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.F.; Scott, M.R.; Jacobs, H.I.L.; Schultz, A.P.; Properzi, M.J.; Amariglio, R.E.; Hohman, T.J.; Mayblyum, D.V.; Rubinstein, Z.B.; Manning, L.; et al. Sex Mediates Relationships Between Regional Tau Pathology and Cognitive Decline. Ann. Neurol. 2020, 88, 921–932. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Panizzon, M.; Chen, X.; Andrews, M.; Galasko, D.; Banks, S.J. Sex Differences in Alzheimer’s-Related Tau Biomarkers and the Mediating Effect of Testosterone. Biol. Sex Differ. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Biegon, A.; Rubin, L.H.; Lipton, R.B.; Landau, S.; Maki, P.M. Alzheimer’s Disease Neuroimaging Initiative Does the Female Advantage in Verbal Memory Contribute to Underestimating Alzheimer’s Disease Pathology in Women versus Men? J. Alzheimers Dis. 2017, 56, 947–957. [Google Scholar] [CrossRef]
- Digma, L.A.; Madsen, J.R.; Rissman, R.A.; Jacobs, D.M.; Brewer, J.B.; Banks, S.J. Alzheimer’s Disease Neuroimaging Initiative Women Can Bear a Bigger Burden: Ante- and Post-Mortem Evidence for Reserve in the Face of Tau. Brain Commun. 2020, 2, fcaa025. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2018, 14, 367–429. [CrossRef]
- Gao, S.; Hendrie, H.C.; Hall, K.S.; Hui, S. The Relationships between Age, Sex, and the Incidence of Dementia and Alzheimer Disease: A Meta-Analysis. Arch. Gen. Psychiatry 1998, 55, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.; Launer, L.J.; Dewey, M.E.; Letenneur, L.; Ott, A.; Copeland, J.R.M.; Dartigues, J.F.; Kragh-Sorensen, P.; Baldereschi, M.; Brayne, C.; et al. Gender Differences in the Incidence of AD and Vascular Dementia: The EURODEM Studies. Neurology 1999, 53, 1992. [Google Scholar] [CrossRef]
- Miech, R.A.; Breitner, J.C.S.; Zandi, P.P.; Khachaturian, A.S.; Anthony, J.C.; Mayer, L. Incidence of AD May Decline in the Early 90s for Men, Later for Women: The Cache County Study. Neurology 2002, 58, 209–218. [Google Scholar] [CrossRef]
- Lin, K.A.; Choudhury, K.R.; Rathakrishnan, B.G.; Marks, D.M.; Petrella, J.R.; Doraiswamy, P.M. Marked Gender Differences in Progression of Mild Cognitive Impairment over 8 Years. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2015, 1, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Sundermann, E.E.; Maki, P.M.; Rubin, L.H.; Lipton, R.B.; Landau, S.; Biegon, A. Female Advantage in Verbal Memory. Neurology 2016, 87, 1916–1924. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, J.Z.K.; Berg, J.-L.; Cummings, J.L.; Banks, S.J. Moderating Effects of Sex on the Impact of Diagnosis and Amyloid Positivity on Verbal Memory and Hippocampal Volume. Alzheimer’s Res. Ther. 2017, 9, 72. [Google Scholar] [CrossRef]
- Barnes, L.L.; Wilson, R.S.; Bienias, J.L.; Schneider, J.A.; Evans, D.A.; Bennett, D.A. Sex Differences in the Clinical Manifestations of Alzheimer Disease Pathology. Arch. Gen. Psychiatry 2005, 62, 685–691. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Bondi, M.W.; Campbell, L.M.; Gouaux, B.; Moore, R.C.; Soontornniyomkij, V.; Moore, D.J. Distinguishing Amnestic Mild Cognitive Impairment From HIV-Associated Neurocognitive Disorders. J. Infect. Dis. 2021, 224, 435–442. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff, F.J.; Becker, J.T.; Belle, S.H.; Nebes, R.D.; Holland, A.L.; Boller, F. Cognitive Deficits and Clinical Diagnosis of Alzheimer’s Disease. Neurology 1987, 37, 1119–1124. [Google Scholar] [CrossRef]
- Morgello, S.; Gelman, B.B.; Kozlowski, P.B.; Vinters, H.V.; Masliah, E.; Cornford, M.; Cavert, W.; Marra, C.; Grant, I.; Singer, E.J. The National NeuroAIDS Tissue Consortium: A New Paradigm in Brain Banking with an Emphasis on Infectious Disease. Neuropathol. Appl. Neurobiol. 2001, 27, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cysique, L.A.; Franklin, D.; Abramson, I.; Ellis, R.J.; Letendre, S.; Collier, A.; Marra, C.; Clifford, D.; Gelman, B.; McArthur, J.; et al. Normative Data and Validation of a Regression Based Summary Score for Assessing Meaningful Neuropsychological Change. J. Clin. Exp. Neuropsychol. 2011, 33, 505–522. [Google Scholar] [CrossRef] [Green Version]
- Heaton, R.K.; Miller, S.W.; Taylor, M.J.; Grant, I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Program; Psychological Assessment Resources, Inc.: Odessa, FL, USA, 2004. [Google Scholar]
- Norman, M.A.; Moore, D.J.; Taylor, M.; Franklin, D.; Cysique, L.; Ake, C.; Lazarretto, D.; Vaida, F.; Heaton, R.K. HNRC Group Demographically Corrected Norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J. Clin. Exp. Neuropsychol. 2011, 33, 793–804. [Google Scholar] [CrossRef]
- Heaton, R.K.; Grant, I.; Butters, N.; White, D.A.; Kirson, D.; Atkinson, J.H.; McCutchan, J.A.; Taylor, M.J.; Kelly, M.D.; Ellis, R.J. The HNRC 500--Neuropsychology of HIV Infection at Different Disease Stages. HIV Neurobehavioral Research Center. J. Int. Neuropsychol. Soc. 1995, 1, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.L.; Woods, S.P.; Gonzalez, R.; Conover, E.; Marcotte, T.D.; Grant, I.; Heaton, R.K. HNRC Group Predictive Validity of Global Deficit Scores in Detecting Neuropsychological Impairment in HIV Infection. J. Clin. Exp. Neuropsychol. 2004, 26, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, K.; Moore, D.J.; Franklin, D.R.; Clifford, D.B.; Collier, A.C.; Marra, C.M.; Gelman, B.B.; McArthur, J.C.; Morgello, S.; Simpson, D.M.; et al. Defining Neurocognitive Impairment in HIV: Deficit Scores versus Clinical Ratings. Clin. Neuropsychol. 2012, 26, 894–908. [Google Scholar] [CrossRef]
- Galasko, D.; Hansen, L.A.; Katzman, R.; Wiederholt, W.; Masliah, E.; Terry, R.; Hill, L.R.; Lessin, P.; Thal, L.J. Clinical-Neuropathological Correlations in Alzheimer’s Disease and Related Dementias. Arch. Neurol. 1994, 51, 888–895. [Google Scholar] [CrossRef]
- Salmon, D.; Butters, N. Neuropsychological Assessment of Dementia in the Elderly. In Principles of Geriatric Neurology; Katzman, R., Rowe, J.W., Eds.; FA Davis Company: Philadelphia, PA, USA, 1992. [Google Scholar]
- Duyckaerts, C.; Delatour, B.; Potier, M.-C. Classification and Basic Pathology of Alzheimer Disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Soontornniyomkij, V.; Moore, D.J.; Gouaux, B.; Soontornniyomkij, B.; Tatro, E.T.; Umlauf, A.; Masliah, E.; Levine, A.J.; Singer, E.J.; Vinters, H.V.; et al. Cerebral β-Amyloid Deposition Predicts HIV-Associated Neurocognitive Disorders in APOE Ε4 Carriers. AIDS 2012, 26, 2327–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alafuzoff, I.; Arzberger, T.; Al-Sarraj, S.; Bodi, I.; Bogdanovic, N.; Braak, H.; Bugiani, O.; Del-Tredici, K.; Ferrer, I.; Gelpi, E.; et al. Staging of Neurofibrillary Pathology in Alzheimer’s Disease: A Study of the BrainNet Europe Consortium. Brain Pathol. 2008, 18, 484–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, K.M.; Brown, G.G.; Grant, I. Analysis of Covariance as a Remedy for Demographic Mismatch of Research Subject Groups: Some Sobering Simulations. J. Clin. Exp. Neuropsychol. 1985, 7, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Grothe, M.J.; Barthel, H.; Sepulcre, J.; Dyrba, M.; Sabri, O.; Teipel, S.J. Alzheimer’s Disease Neuroimaging Initiative In Vivo Staging of Regional Amyloid Deposition. Neurology 2017, 89, 2031–2038. [Google Scholar] [CrossRef] [Green Version]
- Bowman, G.L.; Kaye, J.A.; Moore, M.; Waichunas, D.; Carlson, N.E.; Quinn, J.F. Blood-Brain Barrier Impairment in Alzheimer Disease: Stability and Functional Significance. Neurology 2007, 68, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Nath, A. Human Immunodeficiency Virus (HIV) Proteins in Neuropathogenesis of HIV Dementia. J. Infect. Dis. 2002, 186, S193–S198. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Achim, C.L.; Ge, N.; DeTeresa, R.; Terry, R.D.; Wiley, C.A. Spectrum of Human Immunodeficiency Virus-Associated Neocortical Damage. Ann. Neurol. 1992, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Plessis, S.D.; Vink, M.; Joska, J.A.; Koutsilieri, E.; Stein, D.J.; Emsley, R. HIV Infection and the Fronto-Striatal System: A Systematic Review and Meta-Analysis of FMRI Studies. AIDS 2014, 28, 803–811. [Google Scholar] [CrossRef]
- Ellis, R.; Langford, D.; Masliah, E. HIV and Antiretroviral Therapy in the Brain: Neuronal Injury and Repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ernst, T.; Chang, L.; Jovicich, J.; Ames, N.; Arnold, S. Abnormal Brain Activation on Functional MRI in Cognitively Asymptomatic HIV Patients. Neurology 2002, 59, 1343–1349. [Google Scholar] [CrossRef]
- Israel, S.M.; Hassanzadeh-Behbahani, S.; Turkeltaub, P.E.; Moore, D.J.; Ellis, R.J.; Jiang, X. Different Roles of Frontal versus Striatal Atrophy in HIV-associated Neurocognitive Disorders. Hum. Brain Mapp. 2019, 40, 3010–3026. [Google Scholar] [CrossRef] [Green Version]
- Towgood, K.J.; Pitkanen, M.; Kulasegaram, R.; Fradera, A.; Kumar, A.; Soni, S.; Sibtain, N.A.; Reed, L.; Bradbeer, C.; Barker, G.J.; et al. Mapping the Brain in Younger and Older Asymptomatic HIV-1 Men: Frontal Volume Changes in the Absence of Other Cortical or Diffusion Tensor Abnormalities. Cortex 2012, 48, 230–241. [Google Scholar] [CrossRef]
- Sanford, R.; Fernandez Cruz, A.L.; Scott, S.C.; Mayo, N.E.; Fellows, L.K.; Ances, B.M.; Collins, D.L. Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. J. Acquir. Immune Defic. Syndr. 2017, 74, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Guha, D.; Wagner, M.C.E.; Ayyavoo, V. Human Immunodeficiency Virus Type 1 (HIV-1)-Mediated Neuroinflammation Dysregulates Neurogranin and Induces Synaptodendritic Injury. J. Neuroinflamm. 2018, 15, 126. [Google Scholar] [CrossRef] [Green Version]
- Cysique, L.A.; Moffat, K.; Moore, D.M.; Lane, T.A.; Davies, N.W.S.; Carr, A.; Brew, B.J.; Rae, C. HIV, Vascular and Aging Injuries in the Brain of Clinically Stable HIV-Infected Adults: A (1)H MRS Study. PLoS ONE 2013, 8, e61738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.-C.; Zhang, X.-Y.; Tan, L.; Yu, J.-T. Tauopathies: Mechanisms and Therapeutic Strategies. J. Alzheimers Dis. 2018, 61, 487–508. [Google Scholar] [CrossRef]
- Bell, J.E.; Arango, J.-C.; Anthony, I.C. Neurobiology of Multiple Insults: HIV-1-Associated Brain Disorders in Those Who Use Illicit Drugs. J. Neuroimmune Pharmacol. 2006, 1, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.C.; Sperling, R.A.; Petersen, R.; Sun, C.-K.; Weiner, M.W.; Aisen, P.S. Alzheimer’s Disease Neuroimaging Initiative Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA 2017, 317, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Zhao, L.; Goldstein, F.; Cellar, J.; Lah, J. Biomarkers for Predicting Cognitive Decline in Those with Normal Cognition. J. Alzheimers Dis. 2014, 40, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Tomassen, J.; den Braber, A.; van der Landen, S.M.; Konijnenberg, E.; Teunissen, C.E.; Vermunt, L.; de Geus, E.J.C.; Boomsma, D.I.; Scheltens, P.; Tijms, B.M.; et al. Abnormal Cerebrospinal Fluid Levels of Amyloid and Tau Are Associated with Cognitive Decline over Time in Cognitively Normal Older Adults: A Monozygotic Twin Study. Alzheimers Dement 2022, 8, e12346. [Google Scholar] [CrossRef]
- Salmon, D.P. Neuropsychological Features of Mild Cognitive Impairment and Preclinical Alzheimer’s Disease. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Farrell, K.; Iida, M.A.; Cherry, J.D.; Casella, A.; Stein, T.D.; Bieniek, K.F.; Walker, J.M.; Richardson, T.E.; White, C.L.; Alvarez, V.E.; et al. Differential Vulnerability of Hippocampal Subfields in Primary Age-Related Tauopathy and Chronic Traumatic Encephalopathy. J. Neuropathol. Exp. Neurol. 2022, 81, 781–789. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Schonhaut, D.R.; Schöll, M.; Lockhart, S.N.; Ayakta, N.; Baker, S.L.; O’Neil, J.P.; Janabi, M.; Lazaris, A.; Cantwell, A.; et al. Tau PET Patterns Mirror Clinical and Neuroanatomical Variability in Alzheimer’s Disease. Brain 2016, 139, 1551–1567. [Google Scholar] [CrossRef] [Green Version]
- Lowe, V.J.; Wiste, H.J.; Senjem, M.L.; Weigand, S.D.; Therneau, T.M.; Boeve, B.F.; Josephs, K.A.; Fang, P.; Pandey, M.K.; Murray, M.E.; et al. Widespread Brain Tau and Its Association with Ageing, Braak Stage and Alzheimer’s Dementia. Brain 2018, 141, 271–287. [Google Scholar] [CrossRef]
- Mattson, M.P.; Haughey, N.J.; Nath, A. Cell Death in HIV Dementia. Cell Death Differ. 2005, 12 (Suppl. S1), 893–904. [Google Scholar] [CrossRef] [Green Version]
- Hategan, A.; Bianchet, M.A.; Steiner, J.; Karnaukhova, E.; Masliah, E.; Fields, A.; Lee, M.-H.; Dickens, A.M.; Haughey, N.; Dimitriadis, E.K.; et al. HIV Tat Protein and Amyloid-β Peptide Form Multifibrillar Structures That Cause Neurotoxicity. Nat. Struct. Mol. Biol. 2017, 24, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- András, I.E.; Rha, G.; Huang, W.; Eum, S.; Couraud, P.-O.; Romero, I.A.; Hennig, B.; Toborek, M. Simvastatin Protects against Amyloid β and HIV-1 Tat-Induced Promoter Activities of Inflammatory Genes in Brain Endothelial Cells. Mol. Pharmacol. 2008, 73, 1424–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Fernandes, N.; Pulliam, L. Profile of Neuronal Exosomes in HIV Cognitive Impairment Exposes Sex Differences. AIDS 2019, 33, 1683–1692. [Google Scholar] [CrossRef]
- Basso, M.R.; Bornstein, R.A. Estimated Premorbid Intelligence Mediates Neurobehavioral Change in Individuals Infected with HIV across 12 Months. J. Clin. Exp. Neuropsychol. 2000, 22, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Farinpour, R.; Miller, E.N.; Satz, P.; Selnes, O.A.; Cohen, B.A.; Becker, J.T.; Skolasky, R.L.; Visscher, B.R. Psychosocial Risk Factors of HIV Morbidity and Mortality: Findings from the Multicenter AIDS Cohort Study (MACS). J. Clin. Exp. Neuropsychol. 2003, 25, 654–670. [Google Scholar] [CrossRef]
- Tsai, A.C.; Burns, B.F.O. Syndemics of Psychosocial Problems and HIV Risk: A Systematic Review of Empirical Tests of the Disease Interaction Concept. Soc. Sci. Med. 2015, 139, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Singer, M. AIDS and the Health Crisis of the U.S. Urban Poor; the Perspective of Critical Medical Anthropology. Soc. Sci. Med. 1994, 39, 931–948. [Google Scholar] [CrossRef] [Green Version]
- Sundermann, E.E.; Heaton, R.K.; Pasipanodya, E.; Moore, R.C.; Paolillo, E.W.; Rubin, L.H.; Ellis, R.; Moore, D.J. Sex Differences in HIV-Associated Cognitive Impairment. AIDS 2018, 32, 2719. [Google Scholar] [CrossRef]
- Rubin, L.H.; Cook, J.A.; Weber, K.M.; Cohen, M.H.; Martin, E.; Valcour, V.; Milam, J.; Anastos, K.; Young, M.A.; Alden, C.; et al. The Association of Perceived Stress and Verbal Memory Is Greater in HIV-Infected versus HIV-Uninfected Women. J. Neurovirol. 2015, 21, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.H.; Cook, J.A.; Springer, G.; Weber, K.M.; Cohen, M.H.; Martin, E.M.; Valcour, V.G.; Benning, L.; Alden, C.; Milam, J.; et al. Perceived and Post-Traumatic Stress Are Associated with Decreased Learning, Memory, and Fluency in HIV-Infected Women. AIDS 2017, 31, 2393. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, A.; Spuch, C.; Penedo, M.A.; Olivares, J.M.; Agís-Balboa, R.C. Important Role of Microglia in HIV-1 Associated Neurocognitive Disorders and the Molecular Pathways Implicated in Its Pathogenesis. Ann. Med. 2021, 53, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.E.; Padgett, C. A Systematic Review of the Association Between Psychological Stress and Dementia Risk in Humans. J. Alzheimers Dis. 2020, 78, 335–352. [Google Scholar] [CrossRef] [PubMed]
| PWH (NNTC Cases, n = 49) | PWoH (ADRC Cases) | Test Statistic, p-Value | |||
|---|---|---|---|---|---|
| NC (n = 20) | MCI (n = 15) | AD (n = 25) | |||
| Demographics | |||||
| Age, mean (SD) | 57.37 (5.04) | 89.10 (6.95) | 89.33 (6.85) | 88.04 (7.44) | F (3,105) = 225.17, p < 0.001 |
| Education years, mean (SD) | 12.69 (2.91) | 14.90 (2.81) | 14.93 (2.71) | 15.08 (3.46) | F (3,105) = 5.00, p < 0.001 |
| Sex, n (% male) | 39 (79.59%) | 7 (35.00%) | 8 (53.33%) | 13 (52.00%) | X2 = 14.08, p = 0.003 |
| Race/ethnicity | |||||
| White, n (%) | 28 (57.14%) | 18 (90.00%) | 15 (100.00%) | 25 (100.00%) | X2 = 26.11, p < 0.001 |
| Black, n (%) | 15 (30.61%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | X2 = 21.30, p < 0.001 |
| Hispanic, n (%) | 6 (12.24%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | X2 = 5.58, p = 0.13 |
| Other, n (%) | 0 (0.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | X2 = 4.49, p = 0.21 |
| APOE ε4 carrier, n (%) | 11 (22.92%) | 4 (21.05%) | 5 (33.33%) | 13 (52.00%) | X2 = 7.56, p = 0.06 |
| WRAT-3 reading subtest, mean (SD) | 47.7 (9.6) | NA | NA | NA | |
| Antemortem Clinical Comorbidities | |||||
| History of alcohol use disorder, n (%) | 28 (60.87%) | 0 (0.00%) | 1 (11.11%) | 1 (8.33%) | X2 = 24.82 p < 0.001 |
| History of substance use disorder, n (%) | 33 (71.74%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | X2 = 40.66 p < 0.001 |
| Major depressive disorder, n (%) | 32 (69.57%) | 1 (14.29%) | 3 (30.00%) | 3 (21.43%) | X2 = 17.62 p < 0.001 |
| Hypertension, n (%) | 12 (33.33%) | 4 (36.36%) | 7 (87.50%) | 6 (60.00%) | X2 = 8.69, p = 0.03 |
| Diabetes, n (%) | 5 (13.89%) | 2 (10.53%) | 1 (8.33%) | 3 (17.65%) | X2 = 1.91, p = 0.59 |
| Hypercholesterolemia, n (%) | 9 (25.00%) | 5 (41.67%) | 8 (88.89%) | 9 (75.00%) | X2 = 16.02, p = 0.001 |
| HIV Disease Characteristics | |||||
| Nadir CD4+ T-cell count (cells/μL), mean (SD) | 97.87 (138.56) | NA | NA | NA | NA |
| Antemortem CD4+ T-cell count (cells/μL), mean (SD) | 198.73 (233.76) | NA | NA | NA | NA |
| Antemortem detectable plasma HIV-1 RNA load (≥50 copies/mL), n (%) | 32 (65.31%) | NA | NA | NA | NA |
| Duration of HIV disease, years, mean (SD) | 14.75 (6.71) | NA | NA | NA | NA |
| Antemortem ART, n (% prescribed) | 34 (85%) | NA | NA | NA | NA |
| PWH (NNTC Cohort) | PWoH NC | PWoH MCI | PWoH AD | |||||
|---|---|---|---|---|---|---|---|---|
| Path+ | Path− | Path+ | Path− | Path+ | Path− | Path+ | Path− | |
| Aβ | ||||||||
| Frontal neocortex | 20 | 29 | 14 | 3 | 12 | 2 | 24 | 0 |
| Basal temporal neocortex | 12 | 22 | 13 | 4 | 12 | 2 | 24 | 0 |
| Hippocampus | 6 | 26 | 9 | 7 | 12 | 2 | 23 | 1 |
| Any brain region | 23 | 16 | 14 | 3 | 12 | 2 | 24 | 0 |
| Widespread Aβ (any brain region) | 12 | 24 | 11 | 6 | 12 | 2 | 24 | 0 |
| p-Tau | ||||||||
| Frontal neocortex | 30 | 19 | 14 | 3 | 14 | 0 | 24 | 0 |
| Basal temporal neocortex | 24 | 10 | 17 | 0 | 14 | 0 | 24 | 0 |
| Trans-entorhinal cortex | 22 | 8 | 17 | 0 | 14 | 0 | 24 | 0 |
| Entorhinal cortex | 14 | 16 | 17 | 0 | 14 | 0 | 24 | 0 |
| Hippocampus | 22 | 10 | 17 | 0 | 14 | 0 | 24 | 0 |
| Any brain region | 41 | 1 | 17 | 0 | 14 | 0 | 24 | 0 |
| Widespread p-Tau (any brain region) | 15 | 16 | 17 | 0 | 13 | 1 | 24 | 0 |
| Learning | Recall | |||||
|---|---|---|---|---|---|---|
| Path+ Mean (SD) | Path− Mean (SD) | ANOVA Results | Path+ Mean (SD) | Path− Mean (SD) | ANOVA Results | |
| Aβ | ||||||
| Frontal neocortex | 38.3 (7.4) | 43.2 (9.8) | F(1,43) = 3.2. p = 0.08 | 37.6 (9.3) | 43.0 (8.9) | F(1,43) = 3.8, p = 0.06 |
| Basal temporal neocortex | 39.3 (8.4) | 44.6 (9.8) | F(1,31) = 2.3, p = 0.14 | 38.9 (8.6) | 43.9 (9.9) | F(1,31) = 2.0, p = 0.16 |
| Hippocampus | 42.3 (7.4) | 42.2 (10.5) | F(1,29) = 0.001, p = 0.98 | 42.3 (7.4) | 42.2 (10.5) | F(1,29) = 0.001, p = 0.98 |
| Any brain region | 39.1 (7.1) | 46.5 (10.5) | F(1,35) = 6.6, p = 0.01 | 38.7 (9.0) | 45.2 (9.7) | F(1,35) = 4.5, p = 0.04 |
| Widespread Aβ any brain region) | 37.5 (8.6) | 44.9 (9.1) | F(1,32) = 4.8, p = 0.04 | 35.8 (8.4) | 44.2 (9.2) | F(1,32) = 6.1, p = 0.02 |
| p-Tau | ||||||
| Frontal neocortex | 42.4 (9.6) | 39.2 (8.2) | F(1,43) = 0.3, p = 0.27 | 42.2 (8.8) | 38.2 (10.0) | F(1,43) = 1.9, p = 0.17 |
| Basal temporal neocortex | 42.5 (10.9) | 9, 43.6 (4.7) | F(1,31) = 0.08, p = 0.77 | 41.5 (10.2) | 44.3 (8.0) | F(1,31) = 0.6, p = 0.46 |
| Transentorhinal cortex | 44.4 (9.9) | 40.1 (10.5) | F(1,27) = 0.99, p = 0.33 | 43.4 (10.4) | 40.7 (9.3) | F(1,27) = 0.4, p = 0.54 |
| Entorhinal cortex | 44.0 (7.7) | 42.8 (12.1) | F(1,27) = 0.1, p = 0.75 | 44.0 (9.5) | 41.7 (10.7) | F(1,27) = 0.4, p = 0.55 |
| Hippocampus | 44.6 (10.1) | 39.5 (8.8) | F(1,29) = 1.9, p = 0.18 | 43.2 (10.2) | 40.1 (9.6) | F(1,29) = 0.6, p = 0.43 |
| Any brain region ¥ | NA | NA | NA | NA | NA | NA |
| Widespread p-Tau (any brain region) | 40.8 (7.9) | 45.3 (11.6) | F(1,28), p = 0.22 | 40.5 (9.4) | 44.3 (10.7) | F(1,28) = 1.1, p = 0.31 |
| Executive Function | Speed of Information Processing | Attention/Working Memory | Verbal Fluency | Motor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Path+ Mean (SD) | Path− Mean (SD) | ANOVA Results | Path+ Mean (SD) | Path− Mean (SD) | ANOVA Results | Path+ Mean (SD) | Path−Mean (SD) | ANOVA Results | Path+ Mean (SD) | Path− Mean (SD) | ANOVA Results | Path+ Mean (SD) | Path− Mean (SD) | ANOVA Results | |
| Aβ | |||||||||||||||
| Frontal neocortex | 53.7 (13.2) | 51.1 (11.1) | F(1,41) = 0.5, p = 0.49 | 43.6 (10.7) | 46.1 (11.0) | F(1,40) = 0.5, p = 0.48 | 45.1 (8.4) | 49.0 (9.8) | F(1,42) = 1.9, p = 0.18 | 45.6 (8.4) | 50.6 (12.7) | F(1,42) = 2.1, p = 0.15 | 38.2 (9.5) | 39.8 (9.8) | F(1,42) = 0.2, p = 0.62 |
| Basal temporal neocortex | 51.6 (15.1) | 54.6 (11.7) | F(1,31) = 0.4, p = 0.54 | 46.7 (6.4) | 49.0 (11.3) | F(1,29) = 0.4, p = 0.55 | 45.7 (11.0) | 48.1 (7.1) | F(1,31) = 0.6, p = 0.46 | 46.8 (10.0) | 53.0 (12.1) | F(1,31) = 2.1, p = 0.16 | 41.3 (9.0) | 41.5 (8.8) | F(1,31) = 0.01, p = 0.93 |
| Hippo-campus | 49.3 (3.7) | 54.7 (14.1) | F(1,29) = 0.7, p = 0.41 | 49.2 (7.2) | 48.7 (10.7) | F(1,27) = 0.01, p = 0.92 | 41.9 (5.8) | 48.3 (8.9) | F(1,29) = 2.4, p = 0.13 | 46.8 (10.5) | 52.2 (12.2) | F(1,29) = 0.8, p = 0.36 | 41.3 (12.6) | 41.3 (8.3) | F(1,29) = 0.0, p = 1.0 |
| Any brain region | 21, 53.8 (12.5) | 16, 53.4 (12.1) | F(1,35) = 0.01, p = 0.92 | 20, 44.7 (10.3) | 15, 49.1 (11.8) | F(1,33) = 1.4, p = 0.25 | 21, 46.5 (9.4) | 16, 48.2 (7.0) | F(1,35) = 0.4, p = 0.55 | 21, 47.4 (9.0) | 16, 54.2 (12.8) | F(1,35) = 3.5, p = 0.07 | 21, 39.0 (9.1) | 16, 41.8 (9.7) | F(1,35) = 0.7, p = 0.40 |
| Widespread Aβ any brain region) | 10, 52.4 (14.8) | 24, 53.9 (11.9) | F(1,32) = 0.1, p = 0.76 | 9, 45.1 (7.5) | 23, 49.1 (10.8) | F(1,30) = 1.0, p = 0.32 | 10, 42.9 (8.0) | 24, 49.1 (8.0) | F(1,32) = 4.2, p = 0.05 | 10, 44.5 (9.0) | 24, 53.3 (11.7) | F(1,32) = 4.5, p = 0.04 | 10, 40.0 (10.1) | 24, 41.1 (8.4) | F(1,32) = 0.2, p = 0.67 |
| p-Tau | |||||||||||||||
| Frontal neocortex | 51.0 (11.4) | 54.1 (13.1) | F(1,41) = 0.7, p = 0.42 | 46.9 (10.0) | 41.4 (11.8) | F(1,40) = 2.5, p = 0.12 | 48.6 (8.2) | 45.1 (11.0) | F(1,42) = 1.4, p = 0.24 | 48.8 (10.0) | 48.1 (13.5) | F(1,42) = 0.03, p = 0.85 | 40.0 (9.0) | 37.6 (10.7) | F(1,42) = 0.5, p = 0.46 |
| Basal temporal neocortex | 54.7 (13.7) | 50.7 (10.0) | F(1,31) = 0.6, p = 0.43 | 48.1 (10.0) | 48.7 (10.2) | F(1,29) = 0.02, p = 0.88 | 47.1 (8.9) | 47.7 (7.8) | F(1,31) = 0.03, p = 0.87 | 49.6 (10.7) | 54.4 (14.0) | F(1,31) = 1.1, p = 0.30 | 41.0 (8.8) | 42.4 (8.8) | F(1,31) = 0.1, p = 0.71 |
| Trans-entorhinal cortex | 55.7 (14.2) | 46.9 (6.8) | F(1,27) = 2.5, p = 0.13 | 50.8 (9.4) | 45.1 (11.6) | F(1,25) = 1.7, p = 0.21 | 48.0 (9.0) | 46.3 (8.1) | F(1,27) = 0.2, p = 0.66 | 52.9 (9.6) | 49.1 (17.5) | F(1,27) = 0.5, p = 0.48 | 42.0 (8.3) | 41.9 (9.5) | F(1,27) = 0.001, p = 0.97 |
| Entorhinal cortex | 57.2 (15.8) | 50.2 (9.6) | F(1,27) = 2.1, p = 0.16 | 51.7 (9.5) | 47.5 (10.6) | F(1,25) = 1.1, p = 0.30 | 46.0 (8.6) | 49.1 (8.8) | F(1,27) = 0.9, p = 0.35 | 49.2 (8.8) | 54.5 (13.8) | F(1,27) = 1.5, p = 0.23 | 43.4 (8.0) | 41.0 (8.9) | F(1,27) = 0.5, p = 0.47 |
| Hippo-campus | 55.8 (14.1) | 49.7 (10.1) | F(1,29) = 1.5, p = 0.23 | 49.7 (9.4) | 47.0, (11.6) | F(1,27) = 0.4, p = 0.52 | 46.3 (7.6) | 49.3 (10.9) | F(1,29) = 0.8, p = 0.37 | 53.0 (10.9) | 47.8 (13.9) | F(1,29) = 1.3, p = 0.26 | 43.1 (8.3) | 40.0 (9.4) | F(1,29) = 2.3, p = 0.14 |
| Any brain Region ¥ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Widespread p-Tau (any brain region) | 51.6 (11.1) | 54.9 (15.1) | F(1,28) = 0.4, p = 0.51 | 48.6 (8.3) | 49.6 (11.9) | F(1,26) = 0.1, p = 0.79 | 48.7 (8.1) | 46.9 (9.3) | F(1,28) = 0.3, p = 0.58 | 51.3 (7.8) | 51.7 (15.1) | F(1,28) = 0.01, p = 0.93 | |||
| Brain Region-Specific Pathology Positivity Status | Pathology Positivity Status in Any Brain Region | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive Domain | Frontal Neocortex | Basal Temporal Neocortex | Hippocampus | Trans-Entorhinal Cortex p-Tau | Entorhinal Cortex p-Tau | Any Aβ Pathology | Wide-Spread Aβ Pathology | p-Tau Pathology (Any Brain Region) | Wide-Spread p-Tau Pathology | |||
| Aβ | p-Tau | Aβ | p-Tau | Aβ | p-Tau | |||||||
| Learning | T | - | - | - | - | - | - | - | + | + | - | - |
| Recall | T | - | - | - | - | - | - | - | + | + | - | - |
| Executive Function | - | - | - | - | - | - | - | - | - | - | ||
| Speed of Information Processing | - | - | - | - | - | - | - | - | - | - | - | - |
| Attention/Working Memory | - | - | - | - | - | - | - | - | - | T | - | - |
| Verbal Fluency | - | - | - | - | - | - | - | - | T | + | - | - |
| Motor | - | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundermann, E.E.; Campbell, L.M.; Villers, O.; Bondi, M.W.; Gouaux, B.; Salmon, D.P.; Galasko, D.; Soontornniyomkij, V.; Ellis, R.J.; Moore, D.J. Alzheimer’s Disease Pathology in Middle Aged and Older People with HIV: Comparisons with Non-HIV Controls on a Healthy Aging and Alzheimer’s Disease Trajectory and Relationships with Cognitive Function. Viruses 2023, 15, 1319. https://doi.org/10.3390/v15061319
Sundermann EE, Campbell LM, Villers O, Bondi MW, Gouaux B, Salmon DP, Galasko D, Soontornniyomkij V, Ellis RJ, Moore DJ. Alzheimer’s Disease Pathology in Middle Aged and Older People with HIV: Comparisons with Non-HIV Controls on a Healthy Aging and Alzheimer’s Disease Trajectory and Relationships with Cognitive Function. Viruses. 2023; 15(6):1319. https://doi.org/10.3390/v15061319
Chicago/Turabian StyleSundermann, Erin E., Laura M. Campbell, Olivia Villers, Mark W. Bondi, Ben Gouaux, David P. Salmon, Douglas Galasko, Virawudh Soontornniyomkij, Ronald J. Ellis, and David J. Moore. 2023. "Alzheimer’s Disease Pathology in Middle Aged and Older People with HIV: Comparisons with Non-HIV Controls on a Healthy Aging and Alzheimer’s Disease Trajectory and Relationships with Cognitive Function" Viruses 15, no. 6: 1319. https://doi.org/10.3390/v15061319
APA StyleSundermann, E. E., Campbell, L. M., Villers, O., Bondi, M. W., Gouaux, B., Salmon, D. P., Galasko, D., Soontornniyomkij, V., Ellis, R. J., & Moore, D. J. (2023). Alzheimer’s Disease Pathology in Middle Aged and Older People with HIV: Comparisons with Non-HIV Controls on a Healthy Aging and Alzheimer’s Disease Trajectory and Relationships with Cognitive Function. Viruses, 15(6), 1319. https://doi.org/10.3390/v15061319






