Same but Different? Comparing the Epidemiology, Treatments and Outcomes of COVID-19 and Non-COVID-19 ARDS Cases in Germany Using a Sample of Claims Data from 2021 and 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Data
2.2. Codes Regarding Patient Characteristics, Diagnoses, Procedures, and Outcomes
2.3. Parameters, Definitions, and Study Outcomes
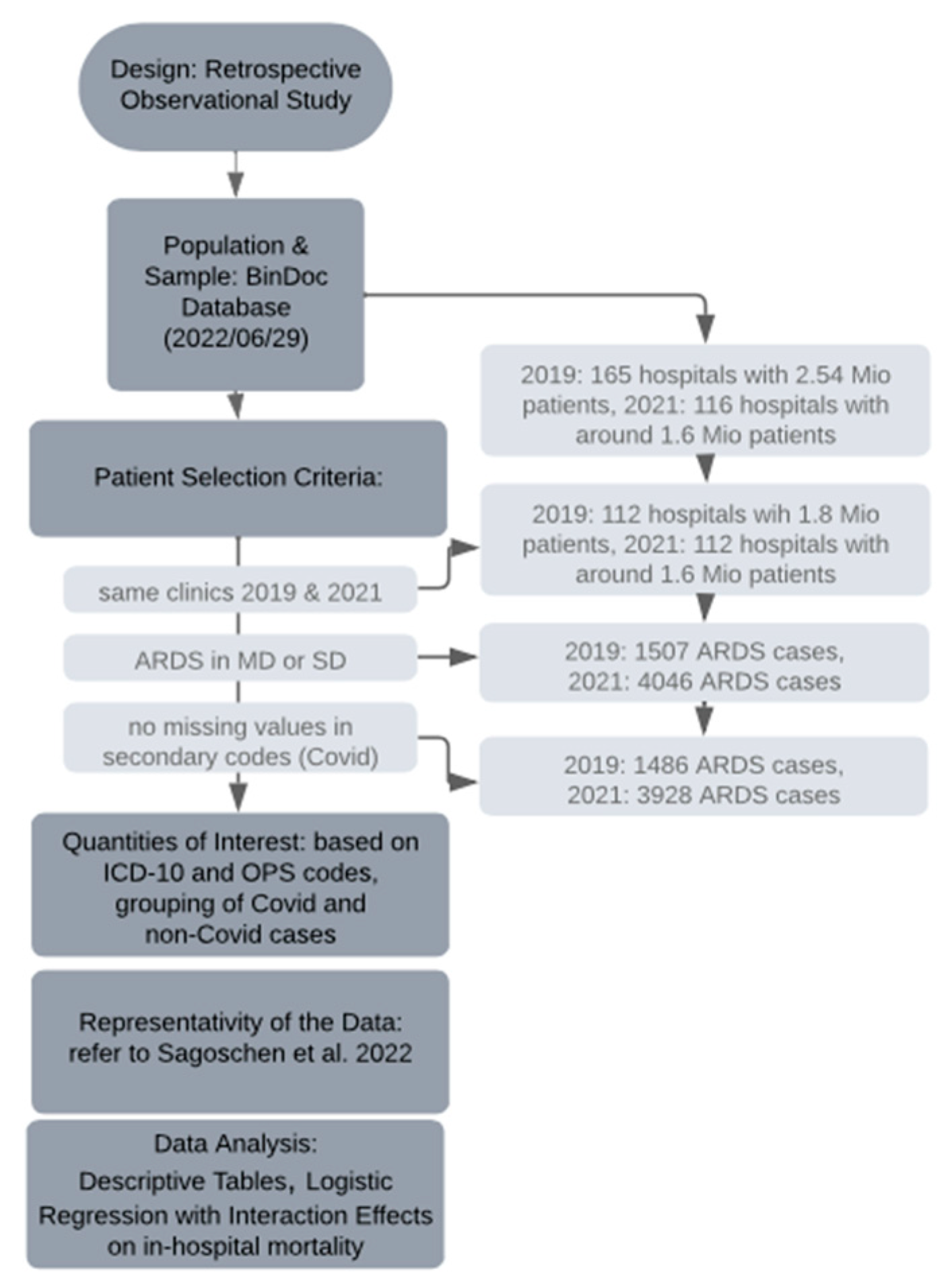
2.4. Research Process and Representativity
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Logistic Regression
4. Discussion
4.1. Epidemiology and Comparison of Comorbidities in COVID-19 and Non-COVID-19 ARDS
4.2. Treatment
4.3. Adverse Events and Outcomes
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Welker, C.; Huang, J.; Gil, I.J.N.; Ramakrishna, H. 2021 Acute Respiratory Distress Syndrome Update, With Coronavirus Disease 2019 Focus. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 516. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit Care 2020, 24, 198. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie—Empfehlungen zur stationären Therapie von Patienten mit COVID-19. Arb. Der Wiss. Med. Fachgesellschaften 2022. [Google Scholar]
- Anesi, G.L. COVID-19: Management of the Intubated Adult; Manaker, S., Ed.; UpToDate: Waltham, MA, USA, 2023. [Google Scholar]
- Panel, C.-T.G. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov (accessed on 21 March 2023).
- Lu, S.; Huang, X.; Liu, R.; Lan, Y.; Lei, Y.; Zeng, F.; Tang, X.; He, H. Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: Similarities and Differences. Front. Med. 2022, 9, 829771. [Google Scholar] [CrossRef]
- De Vito, E.L. Possible Role of Corollary Discharge in Lack of Dyspnea in Patients With COVID-19 Disease. Front. Physiol. 2021, 12, 719166. [Google Scholar] [CrossRef]
- Brault, C.; Zerbib, Y.; Kontar, L.; Fouquet, U.; Carpentier, M.; Metzelard, M.; Soupison, T.; Cagny, B.D.; Maizel, J.; Slama, M. COVID-19–versus non–COVID-19–related Acute Respiratory Distress Syndrome: Differences and Similarities. Am. J. Respir. Crit. Care Med. 2020, 202, 1301–1304. [Google Scholar] [CrossRef]
- Bain, W.; Yang, H.; Shah, F.A.; Suber, T.; Drohan, C.; Al-Yousif, N.; DeSensi, R.S.; Bensen, N.; Schaefer, C.; Rosborough, B.R.; et al. COVID-19 versus Non-COVID-19 Acute Respiratory Distress Syndrome: Comparison of Demographics, Physiologic Parameters, Inflammatory Biomarkers, and Clinical Outcomes. Ann. Am. Thorac. Soc. 2021, 18, 1202–1210. [Google Scholar] [CrossRef]
- Shah, S.J.; Barish, P.N.; Prasad, P.A.; Kistler, A.; Neff, N.; Kamm, J.; Li, L.M.; Chiu, C.Y.; Babik, J.M.; Fang, M.C.; et al. Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: A retrospective cohort study of patients with and without COVID-19. EClinicalMedicine 2020, 27, 100518. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Ding, D.; Zhang, J.; Xu, L.; Hu, Z.; Xu, W.; Tao, Z. Comparative Study of Acute Lung Injury in COVID-19 and Non-COVID-19 Patients. Front. Med. 2021, 8, 666629. [Google Scholar] [CrossRef]
- Dreher, M.; Kersten, A.; Bickenbach, J.; Balfanz, P.; Hartmann, B.; Cornelissen, C.; Daher, A.; Stöhr, R.; Kleines, M.; Lemmen, S.W.; et al. The Characteristics of 50 Hospitalized COVID-19 Patients With and Without ARDS. Dtsch. Arztebl. Int. 2020, 117, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Adam, E.H.; Notz, Q.; Helmer, P.; Sonntagbauer, M.; Ungemach-Papenberg, P.; Sanns, A.; Zausig, Y.; Steinfeldt, T.; Torje, I.; et al. COVID-19 Induced Acute Respiratory Distress Syndrome—A Multicenter Observational Study. Front. Med. 2020, 7, 599533. [Google Scholar] [CrossRef]
- Sagoschen, I.; Keller, K.; Wild, J.; Münzel, T.; Hobohm, L. Case Fatality of Hospitalized Patients with COVID-19 Infection Suffering from Acute Respiratory Distress Syndrome in Germany. Viruses 2022, 14, 2515. [Google Scholar] [CrossRef] [PubMed]
- Thurau, R.B.J. Chronology: How COVID Has Spread in Germany. Available online: https://www.dw.com/en/covid-how-germany-battles-the-pandemic-a-chronology/a-58026877 (accessed on 21 February 2023).
- Robert Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19); Robert Koch-Institut: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Thakur, V.; Bhola, S.; Thakur, P.; Patel, S.K.S.; Kulshrestha, S.; Ratho, R.K.; Kumar, P. Waves and variants of SARS-CoV-2: Understanding the causes and effect of the COVID-19 catastrophe. Infection 2022, 50, 309–325. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Gasparini, A. Comorbidity: An R package for computing comorbidity scores. J. Open Source Softw. 2018, 3, 648. [Google Scholar] [CrossRef] [Green Version]
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Ahlström, B.; Frithiof, R.; Larsson, I.-M.; Strandberg, G.; Lipcsey, M.; Hultström, M. A comparison of impact of comorbidities and demographics on 60-day mortality in ICU patients with COVID-19, sepsis and acute respiratory distress syndrome. Sci. Rep. 2022, 12, 15703. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Norton, E.C. Interaction terms in logit and probit models. Econ. Lett. 2003, 1, 123–129. [Google Scholar] [CrossRef]
- Gordon, M.; Lumley, T.; Gordon, M.M. Package ‘Forestplot’. Advanced Forest Plot Using ‘Grid’graphics; The Comprehensive R Archive Network: Vienna, Austria, 2019. [Google Scholar]
- Armstrong, D. Package ‘DAMisc’. 2022. Available online: https://cran.r-project.org/web/packages/DAMisc/DAMisc.pdf (accessed on 14 February 2023).
- Ldecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Jdiaa, S.S.; Mansour, R.; El Alayli, A.; Gautam, A.; Thomas, P.; Mustafa, R.A. COVID–19 and chronic kidney disease: An updated overview of reviews. J. Nephrol. 2022, 35, 69–85. [Google Scholar] [CrossRef]
- Brewster, D.J.; Chrimes, N.; Do, T.B.; Fraser, K.; Groombridge, C.J.; Higgs, A.; Humar, M.J.; Leeuwenburg, T.J.; McGloughlin, S.; Newman, F.G.; et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med. J. Aust. 2020, 212, 472–481. [Google Scholar] [CrossRef]
- Brown, C.A., 3rd; Mosier, J.M.; Carlson, J.N.; Gibbs, M.A. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J. Am. Coll. Emerg. Physicians Open 2020, 1, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.M.; El-Boghdadly, K.; McGuire, B.; McNarry, A.F.; Patel, A.; Higgs, A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020, 75, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.Z.; Huang, Y.G.; Ma, W.H.; Xue, Z.G.; Zhang, J.Q.; Gong, Y.H.; Che, L.; Chinese Society of Anesthesiology Task Force on Airway Management. Expert Recommendations for Tracheal Intubation in Critically ill Patients with Noval Coronavirus Disease 2019. Chin. Med. Sci. J. 2020, 35, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papoutsi, E.; Giannakoulis, V.G.; Xourgia, E.; Routsi, C.; Kotanidou, A.; Siempos, I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. Crit. Care 2021, 25, 121. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Barbieri, C.; Fontana, M.; Scelfo, C.; Castagnetti, C.; Ghidoni, G.; Ruggiero, P.; Livrieri, F.; Piro, R.; Ghidorsi, L.; et al. Effectiveness of noninvasive ventilation in COVID-19 related-acute respiratory distress syndrome. Clin. Respir. J. 2021, 15, 779–787. [Google Scholar] [CrossRef]
- Demoule, A.; Vieillard Baron, A.; Darmon, M.; Beurton, A.; Géri, G.; Voiriot, G.; Dupont, T.; Zafrani, L.; Girodias, L.; Labbé, V.; et al. High-Flow Nasal Cannula in Critically III Patients with Severe COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.; Schönhofer, B.; Neumann, P.; Bickenbach, J.; Barchfeld, T.; Becker, H.; Dubb, R.; Fuchs, H.; Heppner, H.; Janssens, U. Nicht-invasive Beatmung als Therapie der akuten respiratorischen Insuffizienz. Pneumo 2015, 69, 719–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fichtner, F.; Mörer, O.; Laudi, S.; Weber-Carstens, S.; Kaisers, U. S3-Leitlinie “Invasive Beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz”. DIVI 2017, 4, 154–163. [Google Scholar]
- Karagiannidis, C.; Hentschker, C.; Westhoff, M.; Weber-Carstens, S.; Janssens, U.; Kluge, S.; Pfeifer, M.; Spies, C.; Welte, T.; Rossaint, R.; et al. Observational study of changes in utilization and outcomes in mechanical ventilation in COVID-19. PLoS ONE 2022, 17, e0262315. [Google Scholar] [CrossRef]
- Irwin, R.S.; Rippe, J.M. Irwin and Rippe’s Intensive Care Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- McNicholas, B.A.; Rezoagli, E.; Simpkin, A.J.; Khanna, S.; Suen, J.Y.; Yeung, P.; Brodie, D.; Li Bassi, G.; Pham, T.; Bellani, G.; et al. Epidemiology and outcomes of early-onset AKI in COVID-19-related ARDS in comparison with non-COVID-19-related ARDS: Insights from two prospective global cohort studies. Crit. Care 2023, 27, 3. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Admon, A.J.; Saha, A.K.; Kay, S.G.; Brown, C.A.; Co, I.; Claar, D.; McSparron, J.I.; Dickson, R.P. Comparing Clinical Features and Outcomes in Mechanically Ventilated Patients with COVID-19 and Acute Respiratory Distress Syndrome. Ann. Am. Thorac. Soc. 2021, 18, 1876–1885. [Google Scholar] [CrossRef]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.A.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Blanco, J.; Añón, J.M.; Santos-Bouza, A.; Blanch, L.; Ambrós, A.; Gandía, F.; Carriedo, D.; Mosteiro, F.; Basaldúa, S.; et al. The ALIEN study: Incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011, 37, 1932–1941. [Google Scholar] [CrossRef] [Green Version]
- Dmytriw, A.A.; Chibbar, R.; Chen, P.P.Y.; Traynor, M.D.; Kim, D.W.; Bruno, F.P.; Cheung, C.C.; Pareek, A.; Chou, A.C.C.; Graham, J.; et al. Outcomes of acute respiratory distress syndrome in COVID-19 patients compared to the general population: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2021, 15, 1347–1354. [Google Scholar] [CrossRef]


| Characteristic | 10% Sample of COVID-19 ARDS Cases in 2020 2 | Population Data of COVID-19 ARDS Cases in 2020 1 |
|---|---|---|
| age group/age | 65–70 (55–60, 75–80) | 69.0 (59.0, 78.0) |
| female | 298 (26.94%) | 3379 (29.1%) |
| adipositas/obesity | 142 (12.79%) | 1464 (12.6%) |
| infectious pneumonia or abscess of thorax | 1071 (96.49%) | 11,214 (96.7%) |
| mechanical ventilation | 446 (40.18%) | 4747 (40.9%) |
| ECMO | 113 (10.18%) | 1307 (11.3%) |
| LOS | 16 (8, 27) | 16 (8, 29) |
| mortality | 538 (48.47%) | 5604 (48.3%) |
| Parameters | COVID-19 ARDS 2021 (N = 2654) | Non-COVID-19 ARDS 2021 (N = 1274) | Non-COVID-19 ARDS 2019 (N = 1486) | p-Value (1) 1 | p-Value (2) 2 |
|---|---|---|---|---|---|
| age group | 60–65 (55–75) | 60–65 (50–75) | 60–65 (50–75) | 0.7 | 0.3 |
| female | 819 (31.0%) | 435 (34.2%) | 484 (32.8%) | 0.046 | 0.2 |
| adipositas | 462 (17.4%) | 197 (15.5%) | 183 (12.3%) | 0.13 | <0.001 |
| emergency | 1922 (72.4%) | 826 (64.8%) | 869 (58.5%) | <0.001 | <0.001 |
| Comorbidities | |||||
| Myocardial infarction (mi) | 144 (5.4%) | 98 (7.7%) | 150 (10.1%) | 0.006 | <0.001 |
| Congestive heart failure (chf) | 583 (22.0%) | 441 (34.6%) | 573 (38.6%) | <0.001 | <0.001 |
| Peripheral vascular disease (pvd) | 160 (6.0%) | 134 (10.5%) | 169 (11.4%) | <0.001 | <0.001 |
| Cerebrovascular disease (cevd) | 169 (6.4%) | 122 (9.6%) | 171 (11.5%) | <0.001 | <0.001 |
| Dementia | 47 (1.8%) | 25 (2.0%) | 33 (2.2%) | 0.7 | 0.3 |
| Chronic pulmonary disease (cpd) | 400 (15.1%) | 218 (17.1%) | 267 (18.0%) | 0.10 | 0.015 |
| Rheumatic disease (rheumd) | 49 (1.9%) | 32 (2.5%) | 28 (1.9%) | 0.2 | >0.9 |
| Peptic ulcer disease (pud) | 32 (1.2%) | 20 (1.6%) | 44 (3.0%) | 0.3 | <0.001 |
| Liver disease, mild (mld) | 108 (4.1%) | 99 (7.8%) | 128 (8.6%) | <0.001 | <0.001 |
| Diabetes w/o chronic compl. (diab) | 738 (27.8%) | 291 (22.8%) | 276 (18.6%) | <0.001 | <0.001 |
| Diabetes w chronic compl. (diabwc) | 141 (5.3%) | 72 (5.7%) | 95 (6.4%) | 0.7 | 0.2 |
| Hemiplegia/paraplegia (hp) | 112 (4.2%) | 83 (6.5%) | 124 (8.3%) | 0.002 | <0.001 |
| Renal disease (rend) | 452 (17.0%) | 219 (17.2%) | 357 (24.0%) | >0.9 | <0.001 |
| Any malignancy (canc) | 102 (3.8%) | 95 (7.5%) | 138 (9.3%) | <0.001 | <0.001 |
| Liver disease, moderate/severe (msld) | 18 (0.7%) | 39 (3.1%) | 46 (3.1%) | <0.001 | <0.001 |
| Metastatic solid tumor (metacanc) | 17 (0.6%) | 52 (4.1%) | 84 (5.7%) | <0.001 | <0.001 |
| AIDS | 1 (0.04%) | 3 (0.2%) | 7 (0.5%) | 0.10 | 0.004 |
| Charlson Score | 0.0 (0.0, 2.0) | 2.0 (0.0, 3.0) | 2.0 (0.0, 3.0) | <0.001 | <0.001 |
| Risk Factors | |||||
| Pneumonia | 2639 (99.43%) | 1028 (80.7%) | 1125 (75.7%) | <0.001 | <0.001 |
| Sepsis | 1195 (45.0%) | 753 (59.1%) | 859 (57.8%) | <0.001 | <0.001 |
| Trauma | 160 (6.0%) | 182 (14.3%) | 212 (14.3%) | <0.001 | <0.001 |
| Aspiration | 39 (1.5%) | 195 (15.3%) | 190 (12.8%) | <0.001 | <0.001 |
| Cancer | 118 (4.5%) | 172 (13.5%) | 254 (17.1%) | <0.001 | <0.001 |
| Thoracic surgery | 37 (1.4%) | 78 (6.1%) | 115 (7.7%) | <0.001 | <0.001 |
| Acute pancreatitis | 26 (1.0%) | 50 (3.9%) | 73 (4.9%) | <0.001 | <0.001 |
| Parameters | COVID-19 ARDS 2021 (N = 2654) | Non-COVID-19 ARDS 2021 (N = 1274) | Non-COVID-19 ARDS 2019 (N = 1486) | p-Value (1) 1 | p-Value (2) 2 |
|---|---|---|---|---|---|
| Treatments | |||||
| NIV at all (OPS 8-706) | 1567 (59.0%) | 529 (41.5%) | 523 (35.2%) | <0.001 | <0.001 |
| NHF at all (OPS 8-713) | 892 (33.6%) | 279 (21.9%) | 211 (14.2%) | <0.001 | <0.001 |
| Invasive (tube/tracheostomy) at all | 1865 (70.2%) | 943 (74.0%) | 1185 (79.7%) | 0.015 | <0.001 |
| NIV maximum | 220 (8.3%) | 86 (6.8%) | 64 (4.3%) | 0.092 | <0.001 |
| NHF maximum | 291 (11.0%) | 68 (5.3%) | 31 (2.1%) | <0.001 | <0.001 |
| Tube maximum (8-701, 8-704) | 1087 (41.0%) | 550 (43.2%) | 744 (50.1%) | 0.2 | <0.001 |
| Tracheostomy maximum (5-311, 5-312) | 776 (29.2%) | 391 (30.7%) | 439 (29.5%) | 0.4 | 0.8 |
| Ventilation started prior | 209 (7.9%) | 134 (10.5%) | 142 (9.6%) | 0.006 | 0.063 |
| ECMO treatment (8-852.0/.3/.5/.6) | 371 (14.0%) | 168 (13.2%) | 245 (16.5%) | 0.5 | 0.03 |
| Dialysis (8-853, 8-854, 8-855) | 619 (23.3%) | 413 (32.4%) | 546 (36.7%) | <0.001 | <0.001 |
| Blood transfusion (8-800) | 949 (35.8%) | 704 (55.3%) | 890 (59.9%) | <0.001 | <0.001 |
| Cardioversion (8-640.0) | 171 (6.4%) | 124 (9.7%) | 148 (10.0%) | <0.001 | <0.001 |
| Defibrillation (8-640.1) | 23 (0.9%) | 27 (2.1%) | 46 (3.1%) | 0.001 | <0.001 |
| Reanimation (8-771, 8-779) | 247 (9.3%) | 190 (14.9%) | 260 (17.5%) | <0.001 | <0.001 |
| Adverse Events and Outcomes | |||||
| Septic shock | 523 (19.7%) | 409 (32.1%) | 419 (28.2%) | <0.001 | <0.001 |
| Cardiogenic shock | 62 (2.3%) | 117 (9.2%) | 152 (10.2%) | <0.001 | <0.001 |
| Hypovolemic shock | 87 (3.3%) | 92 (7.2%) | 126 (8.5%) | <0.001 | <0.001 |
| Acute kidney failure (Stad 2) | 221 (8.3%) | 122 (9.6%) | 142 (9.6%) | 0.2 | 0.2 |
| Acute kidney failure (Stad 3) | 696 (26.2%) | 447 (35.1%) | 561 (37.8%) | <0.001 | <0.001 |
| Myocardial infarction | 62 (2.3%) | 39 (3.1%) | 78 (5.3%) | 0.2 | <0.001 |
| Cardiac arrest | 216 (8.1%) | 214 (16.8%) | 277 (18.6%) | <0.001 | <0.001 |
| Acute liver failure | 185 (7.0%) | 124 (9.7%) | 190 (12.8%) | 0.003 | <0.001 |
| Acute ulcer/gastrointest. bleeding | 82 (3.1%) | 58 (4.6%) | 90 (6.1%) | 0.021 | <0.001 |
| DIC | 118 (4.5%) | 47 (3.7%) | 58 (3.9%) | 0.3 | 0.4 |
| Pulmonary embolism | 205 (7.7%) | 78 (6.1%) | 58 (3.9%) | 0.069 | <0.001 |
| Pneumothorax and lung collapse | 349 (13.2%) | 315 (24.7%) | 342 (23.0%) | <0.001 | <0.001 |
| Pleural effusion | 191 (7.2%) | 181 (14.2%) | 260 (17.5%) | <0.001 | <0.001 |
| Stroke/Cerebral hemorrhage | 82 (3.1%) | 51 (4.0%) | 85 (5.7%) | 0.14 | <0.001 |
| Delirium | 528 (19.0%) | 278 (21.8%) | 328 (22.1%) | 0.2 | 0.10 |
| ARDS severity | <0.001 | <0.001 | |||
| Mild ARDS | 56 (2.1%) | 70 (5.5%) | 115 (7.7%) | ||
| Moderate ARDS | 517 (19.5%) | 247 (19.4%) | 310 (20.9%) | ||
| Severe ARDS | 2019 (76.1%) | 886 (69.5%) | 918 (61.8%) | ||
| Indeterminate ARDS | 62 (2.3%) | 71 (5.6%) | 143 (9.6%) | ||
| LOS | 17 (10, 29) | 19 (9, 34) | 21 (10, 37) | 0.027 | <0.001 |
| LOS ICU | 12 (6, 23) | 13 (6, 26) | 13 (4, 26) | 0.5 | 0.2 |
| Mortality | 1219 (45.9%) | 652 (51.2%) | 722 (48.6%) | 0.002 | 0.10 |
| Transfer to other hospital | 678 (25.6%) | 231 (18.1%) | 327 (22.0%) | <0.001 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernauer, E.; Alebrand, F.; Heurich, M. Same but Different? Comparing the Epidemiology, Treatments and Outcomes of COVID-19 and Non-COVID-19 ARDS Cases in Germany Using a Sample of Claims Data from 2021 and 2019. Viruses 2023, 15, 1324. https://doi.org/10.3390/v15061324
Bernauer E, Alebrand F, Heurich M. Same but Different? Comparing the Epidemiology, Treatments and Outcomes of COVID-19 and Non-COVID-19 ARDS Cases in Germany Using a Sample of Claims Data from 2021 and 2019. Viruses. 2023; 15(6):1324. https://doi.org/10.3390/v15061324
Chicago/Turabian StyleBernauer, Eva, Felix Alebrand, and Manuel Heurich. 2023. "Same but Different? Comparing the Epidemiology, Treatments and Outcomes of COVID-19 and Non-COVID-19 ARDS Cases in Germany Using a Sample of Claims Data from 2021 and 2019" Viruses 15, no. 6: 1324. https://doi.org/10.3390/v15061324






