Efficient and Informative Laboratory Testing for Rapid Confirmation of H5N1 (Clade 2.3.4.4) High-Pathogenicity Avian Influenza Outbreaks in the United Kingdom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Disease Suspicion (Report Case) Testing during Late 2021
2.2. Subsequent Report Case Testing (5 December 2021–March 2022)
2.3. AIV RRT-PCR Interpretation and Statistical Analyses
2.4. Surveillance Testing of Commercial Duck Premises Considered to Be at Risk of H5N1 HPAIV Infection: Swab Pooling and AIV RRT-PCR Testing
2.5. Identification of Nucleotide Mismatches in the Primer/Probe Binding Regions of the Four AIV RRT-PCRs
2.6. Serology Testing at Duck IPs
3. Results
3.1. AIV RRT-PCR Testing of 12 IPs (Autumn 2021): Four-Test Strategy
3.2. AIV RRT-PCR Testing of 29 Subsequent IPs (December 2021–March 2022): Three-Test Strategy
3.3. Surveillance by AIV RRT-PCR at Commercial Duck Premises That Represented Further H5N1 HPAIV Infection Risk: IPs 39 and 41
3.4. Assessment of Nucleotide Mismatches at the Primer/Probe Binding Sequences Identified during the H5N1 HPAIV UK Epizootic
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Brown, I.H. History of highly pathogenic avian influenza. OIE Rev. Sci. Tech. 2009, 28, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.T.; Azebi, S.; Balish, A.; Banks, J.; Bhat, N.; Bright, R.A.; Brown, I.; Buchy, P.; Burguiere, A.M.; Chen, H.I.; et al. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 2005, 11, 1515–1521. [Google Scholar] [CrossRef] [Green Version]
- Antigua, K.J.C.; Choi, W.S.; Baek, Y.H.; Song, M.S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohlmann, A.; King, J.; Fusaro, A.; Zecchin, B.; Banyard, A.C.; Brown, I.H.; Byrne, A.M.P.; Beerens, N.; Liang, Y.; Heutink, R.; et al. Has Epizootic Become Enzootic? Evidence for a Fundamental Change in the Infection Dynamics of Highly Pathogenic Avian Influenza in Europe, 2021. mBio 2022, 13, e0060922. [Google Scholar] [CrossRef]
- Alarcon, P.; Brouwer, A.; Venkatesh, D.; Duncan, D.; Dovas, C.I.; Georgiades, G.; Monne, I.; Fusaro, A.; Dan, A.; Śmietanka, K.; et al. Comparison of 2016–17 and previous epizootics of highly pathogenic avian influenza H5 Guangdong lineage in Europe. Emerg. Infect. Dis. 2018, 24, 2270–2283. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Harders, F.; Roose, M.; Pritz-Verschuren, S.B.E.; Germeraad, E.A.; Engelsma, M. Incursion of Novel Highly Pathogenic Avian Influenza A(H5N8) Virus, The Netherlands, October 2020. Emerg. Infect. Dis. 2021, 27, 1750–1753. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Kuiken, T.; Niqueux, E.; Staubach, C.; Terregino, C.; Guajardo, I.M.; Baldinelli, F. Avian influenza overview February–May 2020. EFSA J. 2020, 18, e06194. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Baldinelli, F. Avian influenza overview August–December 2020. EFSA J. 2020, 18, e06379. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Aznar, I.; Muñoz Guajardo, I.; et al. Avian influenza overview September–December 2021. EFSA J. 2021, 19, e07108. [Google Scholar] [CrossRef]
- Poen, M.J.; Venkatesh, D.; Bestebroer, T.M.; Vuong, O.; Scheuer, R.D.; Oude Munnink, B.B.; de Meulder, D.; Richard, M.; Kuiken, T.; Koopmans, M.P.G.; et al. Co-circulation of genetically distinct highly pathogenic avian influenza A clade 2.3.4.4 (H5N6) viruses in wild waterfowl and poultry in Europe and East Asia, 2017–2018. Virus Evol. 2019, 5, vez004. [Google Scholar] [CrossRef] [PubMed]
- Smietanka, K.; Swieton, E.; Kozak, E.; Wyrostek, K.; Tarasiuk, K.; Tomczyk, G.; Konopka, B.; Welz, M.; Domanska-Blicharz, K.; Niemczuk, K. Highly Pathogenic Avian Influenza H5N8 in Poland in 2019-2020. J. Vet. Res. 2020, 64, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Muñoz Guajardo, I.; Lima, E.; et al. Avian influenza overview December 2020–February 2021. EFSA J. 2021, 19, e06497. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, S.; Freath, L.; Brown, I.; Banyard, A.; Byrne, A.; Pacey, A.; Gale, P.; Aegerter, J.; Perrin, L. Highly Pathogenic Avian Influenza (HPAI) in the UK and Europe, Updated Outbreak Assessment #24, 25 April 2022. Available online: https://www.gov.uk/government/publications/avian-influenza-bird-flu-in-europe (accessed on 23 April 2023).
- Nunez, I.A.; Ross, T.M. A review of H5Nx avian influenza viruses. Ther. Adv. Vaccines Immunother. 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Banyard, A.C.; Lean, F.Z.X.; Robinson, C.; Howie, F.; Tyler, G.; Nisbet, C.; Seekings, J.; Meyer, S.; Whittard, E.; Ashpitel, H.F.; et al. Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses 2022, 14, 212. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Reid, S.M.; Mertens, P.; Oura, C.A.; van Rijn, P.A.; Slomka, M.J.; Banks, J.; Brown, I.H.; Alexander, D.J.; et al. A review of RT-PCR technologies used in veterinary virology and disease control: Sensitive and specific diagnosis of five livestock diseases notifiable to the World Organisation for Animal Health. Vet. Microbiol. 2009, 139, 1–23. [Google Scholar] [CrossRef] [Green Version]
- WOAH. Chapter 3.3.4, Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses). In Terrestrial Manual; WOAH: Paris, France, 2021; Available online: https://www.woah.org/en/disease/avian-influenza/ (accessed on 23 April 2023).
- Spackman, E.; Senne, D.A.; Myers, T.J.; Bulaga, L.L.; Garber, L.P.; Perdue, M.L.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002, 40, 3256–3260. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A.; Vostinakova, V.; Pirchanova, Z.; Cernikova, L.; Dirbakova, Z.; Mojzis, M.; Jirincova, H.; Havlickova, M.; Dan, A.; Ursu, K.; et al. Development and evaluation of a one-step real-time RT-PCR assay for universal detection of influenza A viruses from avian and mammal species. Arch. Virol. 2010, 155, 665–673. [Google Scholar] [CrossRef]
- Slomka, M.J.; Pavlidis, T.; Banks, J.; Shell, W.; McNally, A.; Essen, S.; Brown, I.H. Validated H5 Eurasian real-time reverse transcriptase-polymerase chain reaction and its application in H5N1 outbreaks in 2005–2006. Avian Dis. 2007, 51, 373–377. [Google Scholar] [CrossRef]
- Slomka, M.J.; Pavlidis, T.; Coward, V.J.; Voermans, J.; Koch, G.; Hanna, A.; Banks, J.; Brown, I.H. Validated RealTime reverse transcriptase PCR methods for the diagnosis and pathotyping of Eurasian H7 avian influenza viruses. Influenza Other Respir. Viruses 2009, 3, 151–164. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Pritz-Verschuren, S.; Germeraad, E.A.; Bergervoet, S.A.; Harders, F.; Bossers, A.; Koch, G. Genetic relationship between poultry and wild bird viruses during the highly pathogenic avian influenza H5N6 epidemic in the Netherlands, 2017–2018. Transbound. Emerg. Dis. 2019, 66, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, R.J.; Koch, G.; Heutink, R.; Harders, F.; van der Spek, A.; Elbers, A.R.; Bossers, A. Phylogenetic analysis of highly pathogenic avian influenza A(H5N8) virus outbreak strains provides evidence for four separate introductions and one between-poultry farm transmission in the Netherlands, November 2014. Euro Surveill. 2015, 20, 21174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, A.; Banks, J.; Marston, D.A.; Ellis, R.J.; Brookes, S.M.; Brown, I.H. Genetic Characterization of Highly Pathogenic Avian Influenza (H5N8) Virus from Domestic Ducks, England, November 2014. Emerg. Infect. Dis. 2015, 21, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.C.; Hansen, R.; Watson, S.; Coward, V.; Russell, C.; Cooper, J.; Essen, S.; Everest, H.; Parag, K.V.; Fiddaman, S.; et al. Comparative micro-epidemiology of pathogenic avian influenza virus outbreaks in a wild bird population. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180259. [Google Scholar] [CrossRef] [Green Version]
- Scoizec, A.; Niqueux, E.; Thomas, R.; Daniel, P.; Schmitz, A.; Le Bouquin, S. Airborne Detection of H5N8 Highly Pathogenic Avian Influenza Virus Genome in Poultry Farms, France. Front. Vet. Sci. 2018, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.D.; Irvine, R.M.; Slomka, M.J.; Pavlidis, T.; Hesterberg, U.; Essen, S.; Cox, B.; Ceeraz, V.; Alexander, D.J.; Manvell, R.; et al. Outbreak of Eurasian lineage H5N1 highly pathogenic avian influenza in turkeys in Great Britain in November 2007. Vet. Rec. 2014, 175, 282. [Google Scholar] [CrossRef]
- Reid, S.M.; Shell, W.M.; Barboi, G.; Onita, I.; Turcitu, M.; Cioranu, R.; Marinova-Petkova, A.; Goujgoulova, G.; Webby, R.J.; Webster, R.G.; et al. First reported incursion of highly pathogenic notifiable avian influenza A H5N1 viruses from clade 2.3.2 into European poultry. Transbound. Emerg. Dis. 2011, 58, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Smietanka, K.; Minta, Z. Avian influenza in Poland. Acta Biochim. Pol. 2014, 61, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Slomka, M.J.; To, T.L.; Tong, H.H.; Coward, V.J.; Hanna, A.; Shell, W.; Pavlidis, T.; Densham, A.L.; Kargiolakis, G.; Arnold, M.E.; et al. Challenges for accurate and prompt molecular diagnosis of clades of highly pathogenic avian influenza H5N1 viruses emerging in Vietnam. Avian Pathol. 2012, 41, 177–193. [Google Scholar] [CrossRef]
- James, J.; Slomka, M.J.; Reid, S.M.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Cooper, J.; Russell, C.; Mollett, B.C.; Agyeman-Dua, E.; et al. Development and Application of Real-Time PCR Assays for Specific Detection of Contemporary Avian Influenza Virus Subtypes N5, N6, N7, N8, and N9. Avian Dis. 2019, 63, 209–218. [Google Scholar] [CrossRef]
- Nagy, A.; Cernikova, L.; Kunteova, K.; Dirbakova, Z.; Thomas, S.S.; Slomka, M.J.; Dan, A.; Varga, T.; Mate, M.; Jirincova, H.; et al. A universal RT-qPCR assay for “One Health” detection of influenza A viruses. PLoS ONE 2021, 16, e0244669. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Seekings, A.H.; Skinner, P.; Purchase, K.; Mahmood, S.; Brown, I.H.; Hansen, R.D.E.; Banyard, A.C.; Reid, S.M. Rapid and sensitive detection of high pathogenicity Eurasian clade 2.3.4.4b avian influenza viruses in wild birds and poultry. J. Virol. Methods 2022, 301, 114454. [Google Scholar] [CrossRef]
- Irvine, R.M. Recognising avian notifiable diseases 1. Avian influenza. In Pract. 2013, 35, 426–437. [Google Scholar] [CrossRef]
- Slomka, M.J.; Puranik, A.; Mahmood, S.; Thomas, S.; Seekings, A.H.; Byrne, A.M.P.; Núñez, A.; Bianco, C.; Mollett, B.C.; Watson, S.; et al. Ducks are susceptible to infection with a range of doses of h5n8 highly pathogenic avian influenza virus (2016, Clade 2.3.4.4b) and are largely resistant to virus-specific mortality, but efficiently transmit infection to contact Turkeys. Avian Dis. 2019, 63, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.A.; Allen, D.P.; Fuller, C.M.; Mayers, J.; Mollett, B.C.; Londt, B.Z.; Reid, S.M.; Mansfield, K.L.; Brown, I.H. Development of an avian avulavirus 1 (AAvV-1) L-gene real-time RT-PCR assay using minor groove binding probes for application as a routine diagnostic tool. J. Virol. Methods 2019, 265, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Irvine, R.M.; Pavlidis, T.; Banks, J.; Brown, I.H. Role of real-time RT-PCR platform technology in the diagnosis and management of notifiable avian influenza outbreaks: Experiences in Great Britain. Avian Dis. 2010, 54, 591–596. [Google Scholar] [CrossRef]
- Arnold, M.E.; Slomka, M.J.; Coward, V.J.; Mahmood, S.; Raleigh, P.J.; Brown, I.H. Evaluation of the pooling of swabs for real-time PCR detection of low titre shedding of low pathogenicity avian influenza in turkeys. Epidemiol. Infect. 2013, 141, 1286–1297. [Google Scholar] [CrossRef]
- Byrne, A.M.; James, J.; Mollett, B.C.; Meyer, S.M.; Czepiel, M.; Seekings, A.H.; Mahmood, S.; Thomas, S.S.; Ross, C.S.; Byrne, D.J.; et al. Investigating the genetic diversity of H5 avian influenza in the UK 2020–2022. Microbiol. Spectr. 2023; in press. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Arnold, M.E.; Slomka, M.J.; Breed, A.C.; Hjulsager, C.K.; Pritz-Verschuren, S.; Venema-Kemper, S.; Bouwstra, R.J.; Trebbien, R.; Zohari, S.; Ceeraz, V.; et al. Evaluation of ELISA and haemagglutination inhibition as screening tests in serosurveillance for H5/H7 avian influenza in commercial chicken flocks. Epidemiol. Infect. 2018, 146, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Bowen, J.; Gale, P.; Perrin, L.; Pacey, T.; Stephan, L. Highly Pathogenic Avian Influenza (HPAI) in the UK and Europe, Updated Outbreak Assessment #5, 6 December 2021. Available online: https://www.gov.uk/government/publications/avian-influenza-bird-flu-in-europe (accessed on 24 April 2023).
- OFFLU Influenza A Cleavage Sites Background. Available online: https://www.offlu.org/wp-content/uploads/2022/01/Influenza-A-Cleavage-Sites-Final-04-01-2022.pdf (accessed on 24 April 2023).
- Senne, D.A.; Pedersen, J.C.; Suarez, D.L.; Panigrahy, B. Rapid diagnosis of avian influenza (AI) and assessment of pathogenicity of avian H5 and H7 subtypes by molecular methods. Dev. Biol. 2006, 126, 171–177; discussion 326–327. [Google Scholar]
- Slomka, M.J.; Coward, V.J.; Banks, J.; Londt, B.Z.; Brown, I.H.; Voermans, J.; Koch, G.; Handberg, K.J.; Jorgensen, P.H.; Cherbonnel-Pansart, M.; et al. Identification of sensitive and specific avian influenza polymerase chain reaction methods through blind ring trials organized in the European Union. Avian Dis. 2007, 51, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Beerens, N.; Germeraad, E.A.; Venema, S.; Verheij, E.; Pritz-Verschuren, S.B.E.; Gonzales, J.L. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg. Microbes Infect. 2021, 10, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.; Slomka, M.J.; Warren, C.J.W.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Ramsay, A.M.; Skinner, P.; Watson, S.; Everett, H.E.; et al. Transmission dynamics between infected waterfowl and terrestrial poultry: Differences between the transmission and tropism of H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4a) among ducks, chickens and turkeys. Virology 2020, 541, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M.; Nunez, A.; Seekings, A.H.; Thomas, S.S.; Slomka, M.J.; Mahmood, S.; Clark, J.R.; Banks, J.; Brookes, S.M.; Brown, I.H. Two Single Incursions of H7N7 and H5N1 Low Pathogenicity Avian Influenza in U.K. Broiler Breeders During 2015 and 2016. Avian Dis. 2019, 63, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.; Koch, G.; Bouma, A. Performance of clinical signs in poultry for the detection of outbreaks during the avian influenza A (H7N7) epidemic in The Netherlands in 2003. Avian Pathol. 2005, 34, 181–187. [Google Scholar] [CrossRef]
- Leyson, C.; Youk, S.S.; Smith, D.; Dimitrov, K.; Lee, D.H.; Larsen, L.E.; Swayne, D.E.; Pantin-Jackwood, M.J. Pathogenicity and genomic changes of a 2016 European H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4) in experimentally infected mallards and chickens. Virology 2019, 537, 172–185. [Google Scholar] [CrossRef]
- Spackman, E.; Pantin-Jackwood, M.J.; Kapczynski, D.R.; Swayne, D.E.; Suarez, D.L. H5N2 Highly Pathogenic Avian Influenza Viruses from the US 2014-2015 outbreak have an unusually long pre-clinical period in turkeys. BMC Vet. Res. 2016, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Grund, C.; Hoffmann, D.; Ulrich, R.; Naguib, M.; Schinkothe, J.; Hoffmann, B.; Harder, T.; Saenger, S.; Zscheppang, K.; Tonnies, M.; et al. A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg. Microbes Infect. 2018, 7, 132. [Google Scholar] [CrossRef]
- Nunez, A.; Brookes, S.M.; Reid, S.M.; Garcia-Rueda, C.; Hicks, D.J.; Seekings, J.M.; Spencer, Y.I.; Brown, I.H. Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4 Virus: Equivocal Pathogenicity and Implications for Surveillance Following Natural Infection in Breeder Ducks in the United Kingdom. Transbound. Emerg. Dis. 2016, 63, 5–9. [Google Scholar] [CrossRef]
- Kim, J.K.; Negovetich, N.J.; Forrest, H.L.; Webster, R.G. Ducks: The “Trojan horses” of H5N1 influenza. Influenza Other Respir. Viruses 2009, 3, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Seekings, A.H.; Warren, C.J.; Thomas, S.S.; Mahmood, S.; James, J.; Byrne, A.M.P.; Watson, S.; Bianco, C.; Nunez, A.; Brown, I.H.; et al. Highly pathogenic avian influenza virus H5N6 (clade 2.3.4.4b) has a preferable host tropism for waterfowl reflected in its inefficient transmission to terrestrial poultry. Virology 2021, 559, 74–85. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Billington, E.; Warren, C.J.; De Sliva, D.; Di Genova, C.; Airey, M.; Meyer, S.M.; Lewis, T.; Peers-Dent, J.; Thomas, S.S.; et al. Clade 2.3.4.4b H5N1 high pathogenicity avian influenza virus (HPAIV) from the 2021/22 epizootic is highly duck adapted and poorly adapted to chickens. J. Gen. Virol. 2023, 104, 1852. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Miller, P.J.; Afonso, C.L.; Spackman, E.R.; Kapczynski, D.; Shepherd, E.; Smith, D.E.; Swayne, D. Experimental co-infections of domestic ducks with a virulent Newcastle disease virus and low or highly pathogenic avian influenza viruses. Vet. Microbiol. 2015, 177, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Krog, J.S.; Ryt-Hansen, P.; Pedersen, A.G.; Kvisgaard, L.K.; Holm, E.; Nielsen, P.D.; Hammer, A.S.; Madsen, J.J.; Thorup, K.; et al. Molecular Characterization of Highly Pathogenic Avian Influenza Viruses H5N6 Detected in Denmark in 2018–2019. Viruses 2021, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.M.; Mansfield, K.L.; Reid, S.M.; Coward, V.; Warren, C.; Seekings, J.; Brough, T.; Gray, D.; Núñez, A.; Brown, I.H. Incursion of H5N8 high pathogenicity avian influenza virus (HPAIV) into gamebirds in England. Epidemiol. Infect. 2022, 150, E51. [Google Scholar] [CrossRef]
- Stoimenov, G.M.; Goujgoulova, G.V.; Nikolov, B.; Hristov, K.; Teneva, A. Pathological Changes in Natural Infection of Pheasants with Highly Pathogenic Avian Influenza A (H5N8) in Bulgaria. J. Vet. Res. 2019, 63, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Hjulsager, C.K.; Seekings, A.H.; Warren, C.J.; Lean, F.Z.X.; Núñez, A.; James, J.; Thomas, S.S.; Banyard, A.C.; Slomka, M.J.; et al. Pathogenesis and infection dynamics of high pathogenicity avian influenza virus (HPAIV) H5N6 (clade 2.3.4.4b) in pheasants and onward transmission to chickens. Virology 2022, 577, 138–148. [Google Scholar] [CrossRef]
- Bertran, K.; Dolz, R.; Majo, N. Pathobiology of avian influenza virus infection in minor gallinaceous species: A review. Avian Pathol. 2014, 43, 9–25. [Google Scholar] [CrossRef]
- WOAH. Chapter 1.1.2, Principles and methods of validation of diagnostic assays for infectious diseases. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; WOAH: Paris, France, 2019; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/chapitre_validation_diagnostics_assays.pdf (accessed on 24 April 2023).
- Brown, I.H.; Slomka, M.J.; Cassar, C.A.; Mcelhinney, L.M. The role of national and international veterinary laboratories. Rev. Sci. Tech. Off. Int. Epiz 2021, 40, 159–172. [Google Scholar] [CrossRef]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.; Freath, L.; Perrin, L. Highly Pathogenic Avian Influenza (HPAI) in the UK and Europe; Updated Outbreak Assessment #41, 29 March 2023. Available online: https://www.gov.uk/government/publications/avian-influenza-bird-flu-in-europe (accessed on 24 April 2023).
- Oliver, I.; Roberts, J.; Brown, C.S.; Byrne, A.M.P.; Mellon, D.; Hansen, R.D.E.; Banyard, A.C.; James, J.; Donati, M.; Porter, R.; et al. A case of avian influenza A(H5N1) in England, January 2022. Eurosurveillance 2022, 27, 2200061. [Google Scholar] [CrossRef] [PubMed]
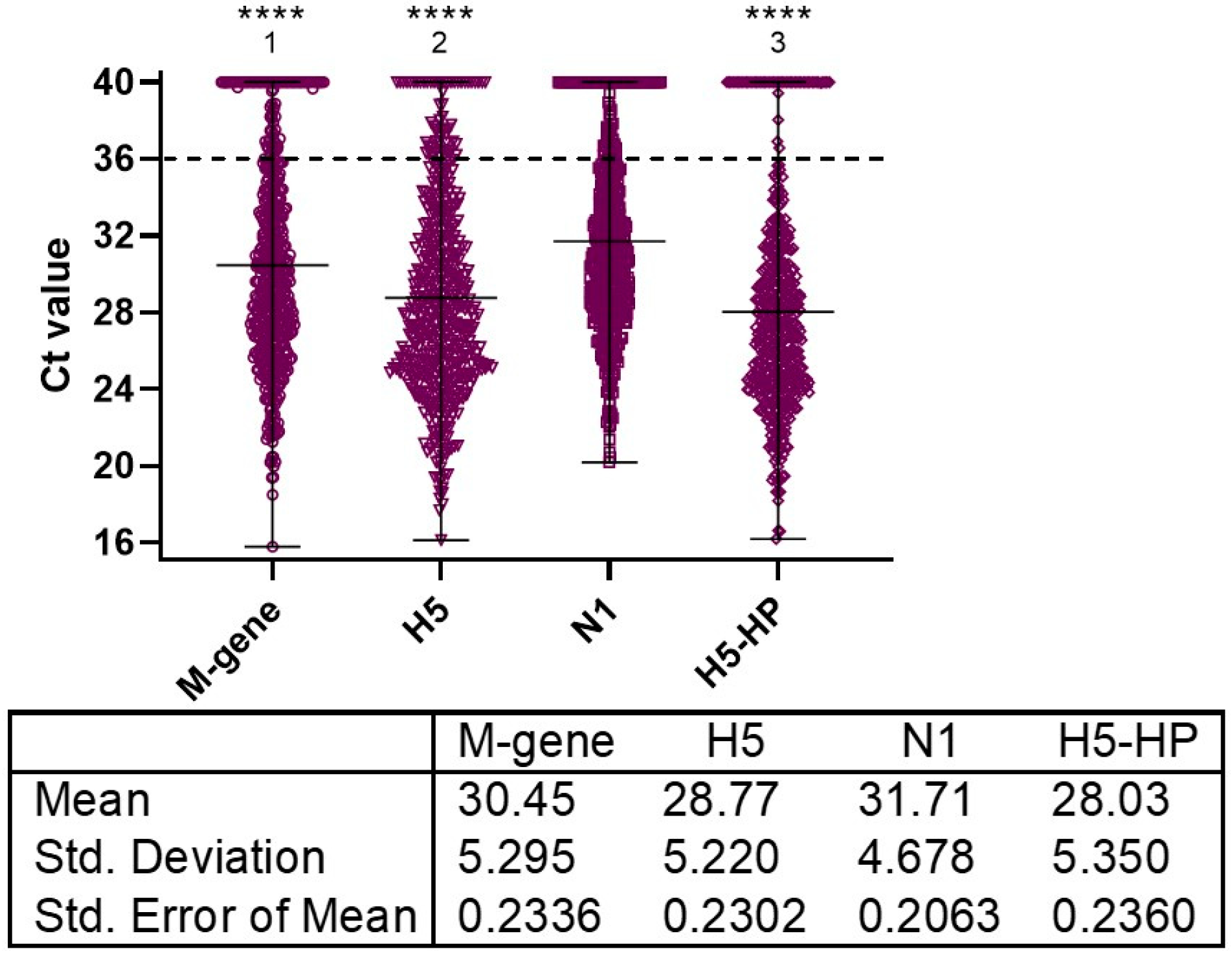




| Infected Premises (IP) ID in Chrono-Logical Order and Report Case Date | Poultry Sector: Numbers of Birds Kept across the Whole IP | Number of Epidemiological Units Sampled Per IP; Total Numbers of Swabbed (OP and C Swabs) Birds; Proportion of Infected Birds among Those Sampled (Percentage in Parentheses), with Proportions Per Unit in Square Parentheses, Where Applicable | Significant Difference between OP and C Shedding for the AIV RRT-PCRs among the Swabbed Birds (Figure Reference in Parentheses) | H5N1 Genotype |
|---|---|---|---|---|
| 1 5/11/21 | Small free-range turkey flock: 51 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: **** H5: **** N1: **** H5-HP: **** (Figure 3a) | B2 |
| 2 11/11/21 | Commercial turkey rearers: 1400 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: ns H5: ns N1: ns H5-HP: ns (Figure 3b) | B2 |
| 3 11/11/21 | Free-range layers: 120,000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: * H5: ** N1: ** H5-HP: ** (Figure 2a) | B2 |
| 4 16/11/21 | Commercial turkey rearers: 17,100 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: *** H5: **** N1: * H5-HP: **** (Figure 3c) | B2 |
| 5 17/11/21 | Domestic ducks: 40 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: **** H5: **** N1: **** H5-HP: **** (Figure 3d) | B2 |
| 6 17/11/21 | Commercial free-range layers: 12,000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: ns H5: ns N1: ns H5-HP: ns (Figure 2b) | B2 |
| 7 29/11/21 | Free-range layer chickens: 32,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: * H5: * N1: * H5-HP: * (Figure 2c) | B2 |
| 8 1/12/21 | Commercial free-range layers: 16,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: *** H5: **** N1: **** H5-HP: **** (Figure 2d) | B2 |
| 9 1/12/21 | Commercial broiler breeders: 26,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: ns H5: ns N1: ns H5-HP: ns (Figure 2h) | B1 |
| 10 2/12/21 | Commercial free-range layers: 22,000 | One; 25 pairs of swabs; 24/25 (96%) | M-gene: ** H5:*** N1: *** H5-HP: *** (Figure 2e) | B2 |
| 11 4/12/21 | Commercial layers: 70,000 | One; 24 pairs of swabs; 23/24 (96%) | M-gene: ** H5: ** N1: ** H5-HP: ** (Figure 2f) | B2 |
| 12 5/12/21 | Indoor layers: 30,000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: ns H5: ns N1: ns H5-HP: ns (Figure 2g) | B2 |
| 13 5/12/21 | Commercial free-range layers: 96,000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: ns H5-HP: ns N1: ns (Figure S2a) | B2 |
| 14 5/12/21 | Indoor commercial duck rearers: 40,000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: ** H5-HP: *** N1: ** (Figure S5a) | B1 |
| 15 6/12/21 | Indoor layers: 7000 | One; 22 pairs of swabs; 20/22 (91%) | M-gene: **** H5-HP: **** N1: **** (Figure S2b) | B2 |
| 16 7/12/21 | Commercial turkey breeders: 4000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: *** H5-HP: **** N1: *** (Figure S4a) | B2 |
| 17 8/12/21 | Indoor commercial layers: 32,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: ns H5-HP: ns N1: ns (Figure S2c) | B2 |
| 18 9/12/21 | Commercial turkey rearers: 8400 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S4b) | B2 |
| 19 10/12/21 | Indoor commercial pullets: 393,600 | Two; 40 pairs of swabs; 24/40 (60%) [9/20 [45%] and 15/20 [75%]] | M-gene: **** H5-HP: **** N1: **** (Figure S2d) | No WGS obtained |
| 20 11/12/21 | Indoor commercial layers: 22,730 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: * H5-HP: * N1: * (Figure S2e) | B2 |
| 21 11/12/21 | Indoor commercial layers: 36,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: ns H5-HP: ns N1: ns (Figure S2f) | B2 |
| 22 13/12/21 | Indoor commercial ducks: 39,900 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S5b) | B1 |
| 23 13/12/21 | Indoor commercial layers: 15,500 | One; 20 pairs of swabs; 18/20 (90%) | M-gene: *** H5-HP: *** N1: *** (Figure S2g) | B2 |
| 24 13/12/21 | Commercial broiler breeders (indoor): 14,800 | One; 22 pairs of swabs; 21/22 (96%) | M-gene: ns H5-HP: ns N1: ns (Figure S3a) | B2 |
| 25 14/12/21 | Indoor commercial layers: 700,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: ns H5-HP: ns N1: ns (Figure S2h) | B2 |
| 26 14/12/21 | Indoor commercial broilers: 114,000 | One; 22 pairs of swabs; 5/22 (23%) | M-gene: ns H5-HP: ns N1: ns (Figure S3b) | B2 |
| 27 21/12/21 | Indoor hobby ducks (Muscovy ducks): 100 | One; 22 pairs of swabs; 20/22 (91%) | M-gene: **** H5-HP: **** N1: **** (Figure S5c) | B1 |
| 28 27/12/21 | Fattening turkeys (indoor): 30,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S4c) | B2 |
| 29 27/12/21 | Turkey stags: 3900 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S4d) | B2 |
| 30 29/12/21 | Finishing turkeys (in finishing houses): 3600 | One; 20 pairs of swabs; 19/20 (95%) | M-gene: ** H5-HP: * N1: * (Figure S4e) | B2 |
| 31 4/1/22 | Fattening turkeys: 4240 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S4f) | B2 |
| 32 12/1/22 | Grandparent turkey breeders: 9500 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: *** N1: *** (Figure S4g) | B2 |
| 33 12/1/22 | Turkey breeders: 4700 | One; 22 pairs of swabs; 21/22 (96%) | M-gene: **** H5-HP: **** N1: **** (Figure S4h) | B2 |
| 34 3/2/22 | Commercial broiler breeders: 15,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S3c) | B1 |
| 35 20/2/22 | Turkey rearers: 7500 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S4i) | B2 |
| 36 20/2/22 | Pheasant breeders: 6000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: **** H5-HP: **** N1: *** (Figure S6a) | B2 |
| 37 20/2/22 | Pheasant breeders: 5000 | One; 20 pairs of swabs; 20/20 (100%) | M-gene: ns H5-HP: ns N1: ns (Figure S6b) | B2 |
| 38 28/2/22 | Commercial fattening ducks: 60,000 | One; 22 pairs of swabs; 22/22 (100%) | M-gene: **** H5-HP: **** N1: **** (Figure S5d) | B2 |
| 39 10/3/22 | Indoor fattening ducks: 19,200 | Two; 60 pairs of swabs; 44/60 (73%) [20/30 [67%] and 24/30 [80%]] | M-gene: **** H5-HP: **** N1: * (Figure S5e) | B2 |
| 40 10/3/22 | Indoor fattening ducks: 700 | Two; 44 pairs of swabs; 32/44 (73%) [24/24 [100%] and 8/20 [40%]] | M-gene: *** H5-HP: * N1: ** (Figure S5f) | B2 |
| 41 24/3/22 | Indoor layer ducks: 13,500. | Two; 60 pairs of swabs; 44/60 (73%) [18/28 [64%] and 26/32 [81%]] | M-gene: **** H5-HP: **** N1: **** (Figure S5g) | B2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slomka, M.J.; Reid, S.M.; Byrne, A.M.P.; Coward, V.J.; Seekings, J.; Cooper, J.L.; Peers-Dent, J.; Agyeman-Dua, E.; de Silva, D.; Hansen, R.D.E.; et al. Efficient and Informative Laboratory Testing for Rapid Confirmation of H5N1 (Clade 2.3.4.4) High-Pathogenicity Avian Influenza Outbreaks in the United Kingdom. Viruses 2023, 15, 1344. https://doi.org/10.3390/v15061344
Slomka MJ, Reid SM, Byrne AMP, Coward VJ, Seekings J, Cooper JL, Peers-Dent J, Agyeman-Dua E, de Silva D, Hansen RDE, et al. Efficient and Informative Laboratory Testing for Rapid Confirmation of H5N1 (Clade 2.3.4.4) High-Pathogenicity Avian Influenza Outbreaks in the United Kingdom. Viruses. 2023; 15(6):1344. https://doi.org/10.3390/v15061344
Chicago/Turabian StyleSlomka, Marek J., Scott M. Reid, Alexander M. P. Byrne, Vivien J. Coward, James Seekings, Jayne L. Cooper, Jacob Peers-Dent, Eric Agyeman-Dua, Dilhani de Silva, Rowena D. E. Hansen, and et al. 2023. "Efficient and Informative Laboratory Testing for Rapid Confirmation of H5N1 (Clade 2.3.4.4) High-Pathogenicity Avian Influenza Outbreaks in the United Kingdom" Viruses 15, no. 6: 1344. https://doi.org/10.3390/v15061344
APA StyleSlomka, M. J., Reid, S. M., Byrne, A. M. P., Coward, V. J., Seekings, J., Cooper, J. L., Peers-Dent, J., Agyeman-Dua, E., de Silva, D., Hansen, R. D. E., Banyard, A. C., & Brown, I. H. (2023). Efficient and Informative Laboratory Testing for Rapid Confirmation of H5N1 (Clade 2.3.4.4) High-Pathogenicity Avian Influenza Outbreaks in the United Kingdom. Viruses, 15(6), 1344. https://doi.org/10.3390/v15061344






