Thinking beyond Vaccination: Promising Add-On Strategies to Active Immunization and Vaccination in Pandemics—A Mini-Review
Abstract
:1. Impact of Salt Treatment in Filtering Facepieces in the Time of the Pandemic
2. Salt Impregnation of Filtering Facepieces
3. Mode of Action and Advantages of Filtering Facepieces with Salt Impregnation
4. Effect of Salt Solutions on Yeast, Viruses, and Airborne Microorganisms
5. Strategies and Effects of Passive Immunization in Times of Pandemics
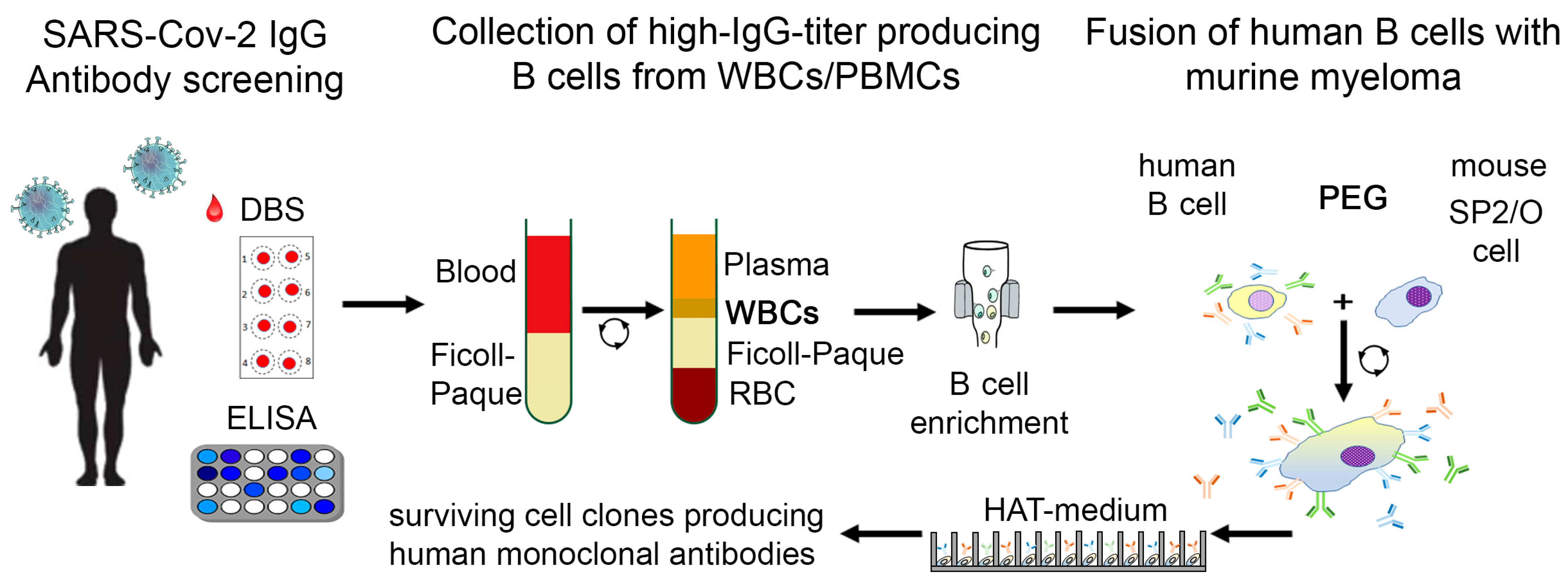
6. Concept for Immortalization of COVID-19 Immunoglobulin-Producing Cells
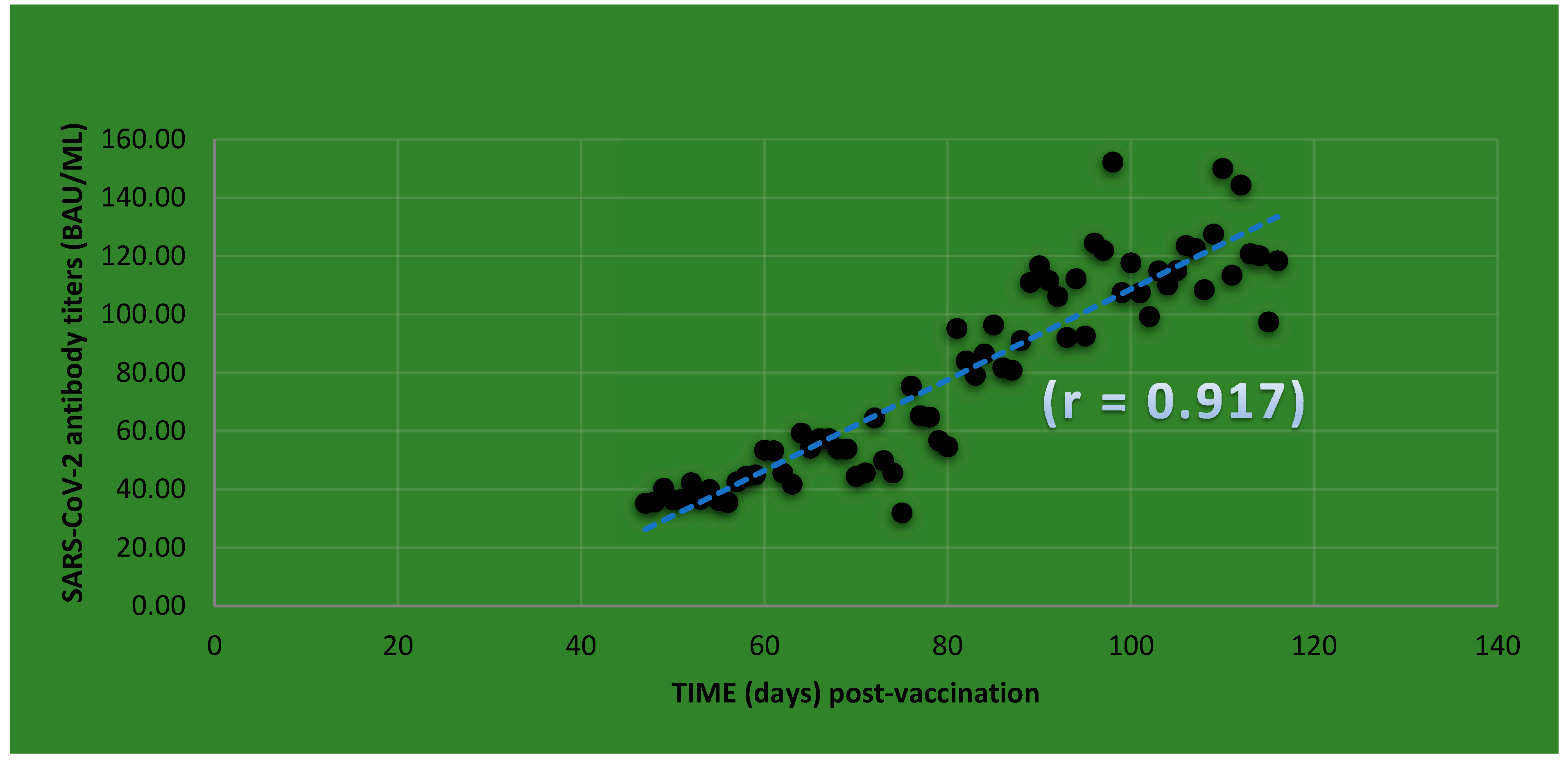
7. Dry Blood Spot (DBS) Screening
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binkerd, E.; Kolari, O. The history and use of nitrate and nitrite in the curing of meat. Food Cosmet. Toxicol. 1975, 13, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hong, H.; Yu, W.; Jiang, X.; Yan, X.; Wu, J. Sodium Chloride Suppresses the Bitterness of Protein Hydrolysates by Decreasing Hydrophobic Interactions. J. Food Sci. 2018, 84, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.D. Microbial Water Stress. Bacteriol. Rev. 1976, 40, 803–846. [Google Scholar] [CrossRef]
- Tyagi, R.; Donaldson, K.; Loftus, C.M.; Jallo, J. Hypertonic saline: A clinical review. Neurosurg. Rev. 2007, 30, 277–290. [Google Scholar] [CrossRef]
- De Oliveira, F.S. Assessing the effectiveness of 30% sodium chloride aqueous solution for the preservation of fixed anatomical specimens: A 5-year follow-up study. J. Anat. 2014, 225, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Gewartowska, M.; Olszewski, W.L.; Buyanowskaya, O.; Frontczak-Baniewicz, M. A novel method for long-lasting preservation of arterial grafts. J. Surg. Res. 2015, 200, 374–386. [Google Scholar] [CrossRef]
- Han, J.; Yang, J.; Wang, Y.; Li, Y. The adequate amount of sodium chloride in Protein A wash buffer for effective host cell protein clearance. Protein Expr. Purif. 2019, 158, 59–64. [Google Scholar] [CrossRef]
- Ramalingam, S.; Cai, B.; Wong, J.; Twomey, M.; Chen, R.; Fu, R.M.; Boote, T.; McCaughan, H.; Griffiths, S.J.; Haas, J.G. Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels. Sci. Rep. 2018, 8, 13630. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Knobloch, J.K.; Franke, G.; Knobloch, M.J.; Knobling, B.; Kampf, G. Overview of tight fit and infection prevention benefits of respirators (filtering face pieces). J. Hosp. Infect. 2023, 134, 89–96. [Google Scholar] [CrossRef]
- Tatzber, F.; Wonisch, W.; Balka, G.; Marosi, A.; Rusvai, M.; Resch, U.; Lindschinger, M.; Moerkl, S.; Cvirn, G. Coating with Hypertonic Saline Improves Virus Protection of Filtering Facepiece Manyfold—Benefit of Salt Impregnation in Times of Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 7406. [Google Scholar] [CrossRef]
- Picard, J.; Cornec, G.; Baron, R.; Saliou, P. Wearing of face masks by healthcare workers during COVID-19 lockdown: What did the public observe through the French media? J. Hosp. Infect. 2020, 106, 617–620. [Google Scholar] [CrossRef]
- Bundgaard, H.; Bundgaard, J.S.; Raaschou-Pedersen, D.E.T.; von Buchwald, C.; Todsen, T.; Norsk, J.B.; Pries-Heje, M.M.; Vissing, C.R.; Nielsen, P.B.; Winsløw, U.C.; et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers: A randomized controlled trial. Ann. Intern. Med. 2021, 174, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Dooley, L.; Ferroni, E.; Al-Ansary, L.; van Driel, M.L.; Bawazeer, G.; Jones, M.; Hoffmann, T.C.; Clark, J.; Beller, E.M.; et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2023, 2023, CD006207. [Google Scholar] [CrossRef]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef] [PubMed]
- Toomey, E.C.; Conway, Y.; Burton, C.; Smith, S.; Smalle, M.; Chan, X.-H.S.; Adisesh, A.; Tanveer, S.; Ross, L.; Thomson, I.; et al. Extended use or reuse of single-use surgical masks and filtering face-piece respirators during the coronavirus disease 2019 (COVID-19) pandemic: A rapid systematic review. Infect. Control Hosp. Epidemiol. 2020, 42, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.S.; Bulliard, X.; Bonfante, R.; Xiang, Y.; Biselli, S.; Steiner, S.; Constant, S.; Pugin, R.; Laurent, A.; Majeed, S.; et al. In vitro testing of salt coating of fabrics as a potential antiviral agent in reusable face masks. Sci. Rep. 2022, 12, 17041. [Google Scholar] [CrossRef]
- Tatzber, F.; Resch, U.; Lindschinger, M.; Cvirn, G.; Wonisch, W. Improved protection of filtering facepiece through inactivation of pathogens by hypertonic salt solutions—A possible COVID-19 prevention device. Prev. Med. Rep. 2020, 20, 101270. [Google Scholar] [CrossRef]
- Rubino, I.; Oh, E.; Han, S.; Kaleem, S.; Hornig, A.; Lee, S.-H.; Kang, H.-J.; Lee, D.-H.; Chu, K.-B.; Kumaran, S.; et al. Salt coatings functionalize inert membranes into high-performing filters against infectious respiratory diseases. Sci. Rep. 2020, 10, 13875. [Google Scholar] [CrossRef]
- Tatzber, F.; Pursch, E.; Resch, U.; Pfragner, R.; Holasek, S.; Lindschinger, M.; Cvirn, G.; Wonisch, W. Cultivation and Immortalization of Human B-Cells Producing a Human Monoclonal IgM Antibody Binding to MDA-LDL: Further Evidence for Formation of Atherogenic MDA-LDL Adducts in Humans In Vivo. Oxidative Med. Cell. Longev. 2017, 2017, 6047142. [Google Scholar] [CrossRef] [Green Version]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Östberg, L.; Pursch, E. Human X (Mouse X Human) Hybridomas Stably Producing Human Antibodies. Hybridoma 1983, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Tatzber, F.; Resch, U.; Kuper, M.; Bhaduri, C.; Wonisch, W.; Cvirn, G. Dry Blood Spots for Monitoring SARS-CoV-2 IgG Anti-body Titres—A Pilot Study. COJ Biomed. Sci. Res. 2022, 2. [Google Scholar] [CrossRef]
- Itell, H.L.; Weight, H.; Fish, C.S.; Logue, J.K.; Franko, N.; Wolf, C.R.; McCulloch, D.J.; Galloway, J.; Matsen, F.A.; Chu, H.Y.; et al. SARS-CoV-2 Antibody Binding and Neutralization in Dried Blood Spot Eluates and Paired Plasma. Microbiol. Spectr. 2021, 9, e0129821. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Markwalter, C.F.; Nyunt, M.H.; Han, Z.Y.; Henao, R.; Jain, A.; Taghavian, O.; Felgner, P.L.; Han, K.T.; Nyunt, M.M.; Plowe, C.V. Antibody signatures of asymptomatic Plasmodium falciparum malaria infections measured from dried blood spots. Malar. J. 2021, 20, 378. [Google Scholar] [CrossRef]
- Comeau, A.M.; Pitt, J.; Hillyer, G.V.; Landesman, S.; Bremer, J.; Chang, B.-H.; Lew, J.; Moye, J.; Grady, G.F.; McIntosh, K. Early detection of human immunodeficiency virus on dried blood spot specimens: Sensitivity across serial specimens. J. Pediatr. 1996, 129, 111–118. [Google Scholar] [CrossRef]
| Titer | Untreated | Post-Treated * | Pre-Treated * | |
| Initial | (TCID50/mL) | 106.5 | 106.5 | 106.5 |
| Mean | (TCID50/mL) | 105.86 | 102.36 | 101.74 |
| SD | 101.05 | 100.36 | 100.18 | |
| Min | (TCID50/mL) | 105.12 | 102.11 | 101.62 |
| Max | (TCID50/mL) | 106.61 | 102.62 | 101.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatzber, F.; Wonisch, W.; Resch, U.; Strohmaier, W.; Lindschinger, M.; Mörkl, S.; Cvirn, G. Thinking beyond Vaccination: Promising Add-On Strategies to Active Immunization and Vaccination in Pandemics—A Mini-Review. Viruses 2023, 15, 1372. https://doi.org/10.3390/v15061372
Tatzber F, Wonisch W, Resch U, Strohmaier W, Lindschinger M, Mörkl S, Cvirn G. Thinking beyond Vaccination: Promising Add-On Strategies to Active Immunization and Vaccination in Pandemics—A Mini-Review. Viruses. 2023; 15(6):1372. https://doi.org/10.3390/v15061372
Chicago/Turabian StyleTatzber, Franz, Willibald Wonisch, Ulrike Resch, Wolfgang Strohmaier, Meinrad Lindschinger, Sabrina Mörkl, and Gerhard Cvirn. 2023. "Thinking beyond Vaccination: Promising Add-On Strategies to Active Immunization and Vaccination in Pandemics—A Mini-Review" Viruses 15, no. 6: 1372. https://doi.org/10.3390/v15061372
APA StyleTatzber, F., Wonisch, W., Resch, U., Strohmaier, W., Lindschinger, M., Mörkl, S., & Cvirn, G. (2023). Thinking beyond Vaccination: Promising Add-On Strategies to Active Immunization and Vaccination in Pandemics—A Mini-Review. Viruses, 15(6), 1372. https://doi.org/10.3390/v15061372







