Antiviral Activity of Catechin against Dengue Virus Infection
Abstract
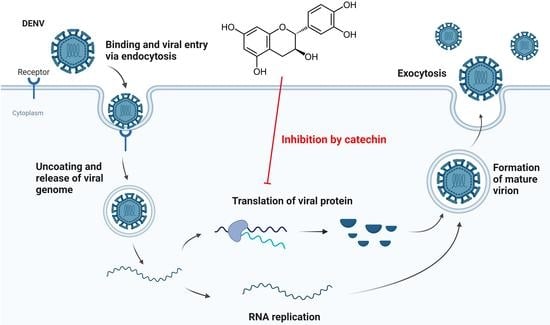
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Compounds
2.3. Cell Viability and Dose-Dependent Inhibition Studies
2.4. Viral Plaque Assay
2.5. Time-of-Addition and Time-of-Removal Studies
2.6. Pre-Treatment, Co-Treatment and Entry Bypass Studies
2.7. SDS-PAGE and Western Blot Analysis
2.8. Translation Reporter Assay
2.9. Statistical Analysis
3. Results
3.1. Antiviral Effects of Catechin against DENV-2 Infection In Vitro
3.2. Inhibitory Effect of Catechin on DENV-2 Replication at a Post-Entry Stage
3.3. Inhibitory Effect of Catechin on DENV-2 Viral Protein Translation
3.4. Antiviral Effects of Catechin against Other Dengue Serotypes and Mosquito-Borne RNA Virus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Rosen, L.; Shroyer, D.A.; Tesh, R.B.; Freier, J.E.; Lien, J.C. Transovarial transmission of dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. Am. J. Trop. Med. Hyg. 1983, 32, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 17 March 2023).
- Nguyen, N.M.; Tran, C.N.; Phung, L.K.; Duong, K.T.; Huynh Hle, A.; Farrar, J.; Nguyen, Q.T.; Tran, H.T.; Nguyen, C.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef]
- Tricou, V.; Minh, N.N.; Van, T.P.; Lee, S.J.; Farrar, J.; Wills, B.; Tran, H.T.; Simmons, C.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl. Trop. Dis. 2010, 4, e785. [Google Scholar] [CrossRef] [Green Version]
- Low, J.G.; Sung, C.; Wijaya, L.; Wei, Y.; Rathore, A.P.S.; Watanabe, S.; Tan, B.H.; Toh, L.; Chua, L.T.; Hou, Y.; et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Whitehorn, J.; Nguyen, C.V.V.; Khanh, L.P.; Kien, D.T.H.; Quyen, N.T.H.; Tran, N.T.T.; Hang, N.T.; Truong, N.T.; Hue Tai, L.T.; Cam Huong, N.T.; et al. Lovastatin for the Treatment of Adult Patients With Dengue: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2016, 62, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Tam, D.T.; Ngoc, T.V.; Tien, N.T.; Kieu, N.T.; Thuy, T.T.; Thanh, L.T.; Tam, C.T.; Truong, N.T.; Dung, N.T.; Qui, P.T.; et al. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: A randomized, placebo-controlled trial. Clin. Infect. Dis. 2012, 55, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharm. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Loe, M.W.C.; Hao, E.; Chen, M.; Li, C.; Lee, R.C.H.; Zhu, I.X.Y.; Teo, Z.Y.; Chin, W.X.; Hou, X.; Deng, J.; et al. Betulinic acid exhibits antiviral effects against dengue virus infection. Antiviral. Res. 2020, 184, 104954. [Google Scholar] [CrossRef]
- Lee, J.K.; Chui, J.L.M.; Lee, R.C.H.; Kong, H.Y.; Chin, W.X.; Chu, J.J.H. Antiviral activity of ST081006 against the dengue virus. Antiviral. Res. 2019, 171, 104589. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; Abubakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [Green Version]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A.; Szymusiak, H.; Tyrakowska, B. The influence of stereochemistry on the antioxidant properties of catechin epimers. Eur. Food Res. Technol. 2012, 235, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef]
- Ogawa, M.; Shimojima, M.; Saijo, M.; Fukasawa, M. Several catechins and flavonols from green tea inhibit severe fever with thrombocytopenia syndrome virus infection in vitro. J. Infect. Chemother. 2021, 27, 32–39. [Google Scholar] [CrossRef]
- Ide, K.; Kawasaki, Y.; Kawakami, K.; Yamada, H. Anti-influenza Virus Effects of Catechins: A Molecular and Clinical Review. Curr. Med. Chem. 2016, 23, 4773–4783. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; von Rhein, C.; Kummerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, H.; Gong, J.; Liu, Q.; Xiao, H.; Chen, X.W.; Sun, B.L.; Yang, R.G. (-)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch. Virol. 2012, 157, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Weng, S.F.; Leu, Y.L.; Chiu, D.T. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 2009, 57, 6140–6147. [Google Scholar] [CrossRef] [PubMed]
- Obi, J.O.; Gutierrez-Barbosa, H.; Chua, J.V.; Deredge, D.J. Current Trends and Limitations in Dengue Antiviral Research. Trop. Med. Infect. Dis. 2021, 6, 180. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeanurit, N.; Tuong, T.L.; Nguyen, V.K.; Vibulakhaophan, V.; Hengphasatporn, K.; Shigeta, Y.; Ho, S.X.; Chu, J.J.H.; Rungrotmongkol, T.; Chavasiri, W.; et al. Lichen-Derived Diffractaic Acid Inhibited Dengue Virus Replication in a Cell-Based System. Molecules 2023, 28, 974. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Sreelatha, L.; Dechtawewat, T.; Noisakran, S.; Yenchitsomanus, P.T.; Chu, J.J.H.; Limjindaporn, T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus. Res. 2018, 255, 171–178. [Google Scholar] [CrossRef]
- Lee, J.L.; Loe, M.W.C.; Lee, R.C.H.; Chu, J.J.H. Antiviral activity of pinocembrin against Zika virus replication. Antiviral. Res. 2019, 167, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Liu, C.; Qu, Z.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Role and mechanism of catechin in skeletal muscle cell differentiation. J. Nutr. Biochem. 2019, 74, 108225. [Google Scholar] [CrossRef]
- Cheng, A.W.; Tan, X.; Sun, J.Y.; Gu, C.M.; Liu, C.; Guo, X. Catechin attenuates TNF-alpha induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS ONE 2019, 14, e0217090. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Lee, Y.N.; Youn, H.N.; Lee, D.H.; Kwak, J.H.; Seong, B.L.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult. Sci. 2012, 91, 66–73. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quinones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 298. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Vibhuti, A.; Raj, S. Molecular docking NS4B of DENV 1-4 with known bioactive phyto-chemicals. Bioinformation 2016, 12, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raekiansyah, M.; Buerano, C.C.; Luz, M.A.D.; Morita, K. Inhibitory effect of the green tea molecule EGCG against dengue virus infection. Arch. Virol. 2018, 163, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Calvo, A.; Jimenez de Oya, N.; Martin-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef] [Green Version]
- Ciesek, S.; von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef] [PubMed]





| Cell Line | CC50 (μM) | IC50 (μM) | Selectivity Index |
|---|---|---|---|
| HUH 7 | >100 | 6.422 | >15.57 |
| K562 | >100 | 50.28 | >1.99 |
| Virus | CC50 (μM) | IC50 (μM) | Selectivity Index |
|---|---|---|---|
| DENV-1 | >100 | 37.94 | >2.64 |
| DENV-3 | >100 | 38.95 | >2.57 |
| DENV-4 | >100 | 16.83 | >5.94 |
| CHIKV | >100 | 47.14 | >2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, B.; Chew, B.X.Z.; Chen, H.; Lee, R.C.H.; Fong, Y.D.; Chin, W.X.; Mok, C.K.; Chu, J.J.H. Antiviral Activity of Catechin against Dengue Virus Infection. Viruses 2023, 15, 1377. https://doi.org/10.3390/v15061377
Yi B, Chew BXZ, Chen H, Lee RCH, Fong YD, Chin WX, Mok CK, Chu JJH. Antiviral Activity of Catechin against Dengue Virus Infection. Viruses. 2023; 15(6):1377. https://doi.org/10.3390/v15061377
Chicago/Turabian StyleYi, Bowen, Benjamin Xuan Zheng Chew, Huixin Chen, Regina Ching Hua Lee, Yuhui Deborah Fong, Wei Xin Chin, Chee Keng Mok, and Justin Jang Hann Chu. 2023. "Antiviral Activity of Catechin against Dengue Virus Infection" Viruses 15, no. 6: 1377. https://doi.org/10.3390/v15061377