Immunogenicity of Oral Rabies Vaccine Strain SPBN GASGAS in Local Dogs in Bali, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
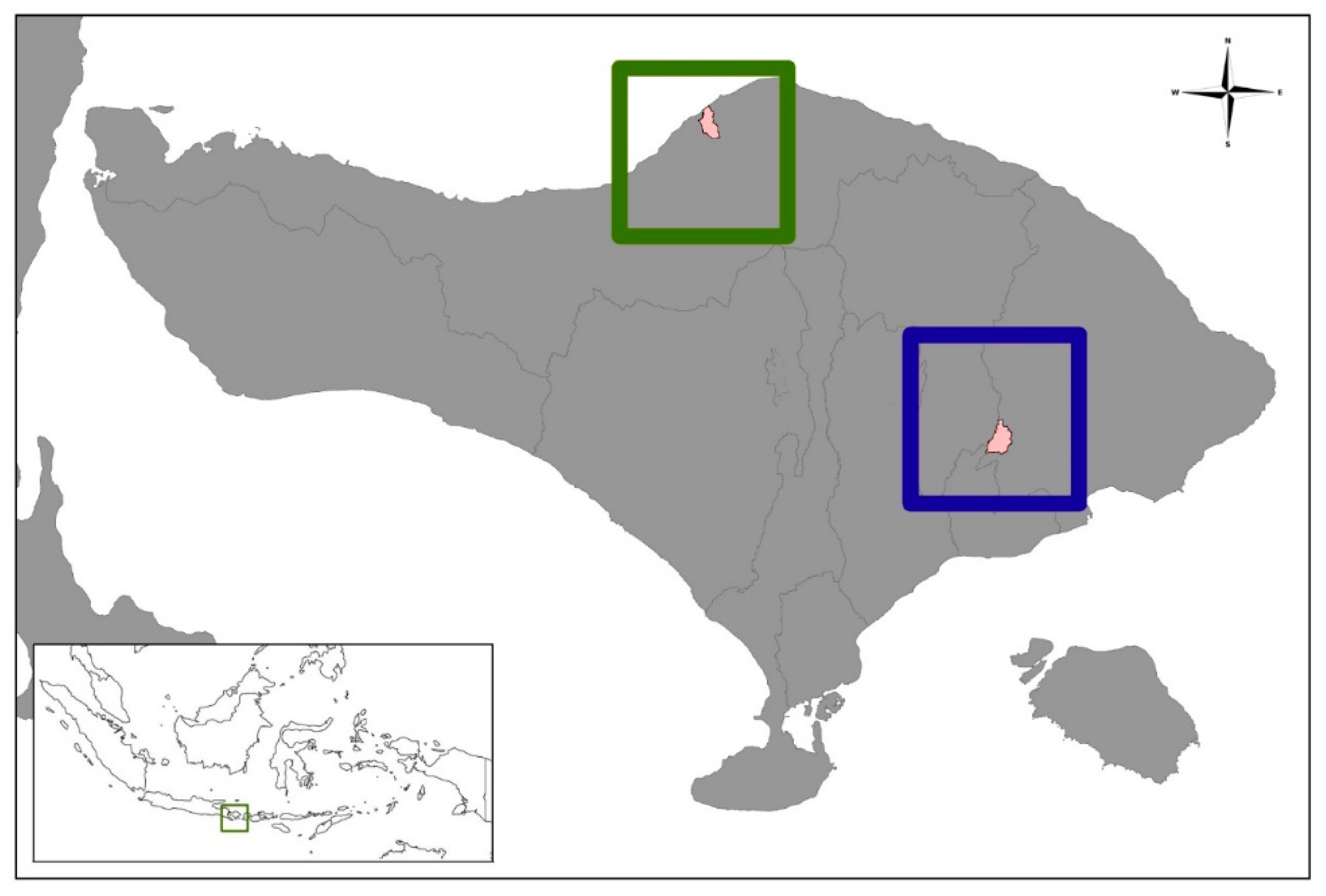
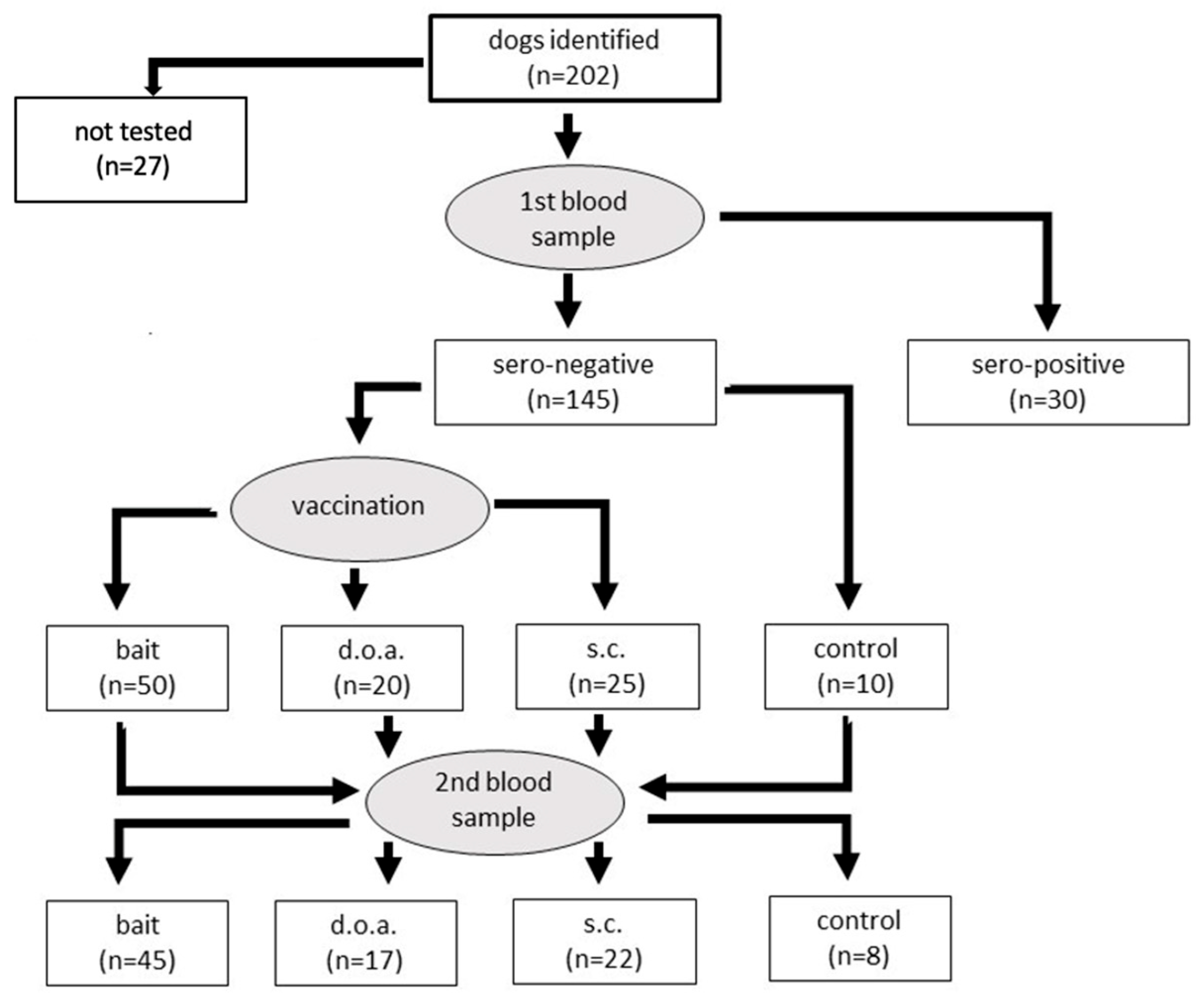
2.1. Study Design
2.2. Assays
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MoA (Ministry of Agriculture); FAO (Food and Agriculture Organization); WAP (World Animal Protection). Masterplan Na-Sional Pemberantasan Rabies di Indonesia; Ministry of Agriculture: Jakarta, Indonesia, 2019; Available online: https://rr-asia.woah.org/wp-content/uploads/2020/03/roadmap-rabies-v05_indonesia.pdf (accessed on 14 February 2023).
- Putra, A.A.G.; Gunata, I.K.; Asrama, I.G. Dog demography in Badung District the Province of Bali and their significance to rabies control. Bul. Vet. Balai Besar Vet. Denpasar 2011, 23, 14–24, (In Bahasa Indonesia). [Google Scholar]
- Widyastuti, M.D.W.; Bardosh, K.L.; Sunandar; Basri, C.; Basuno, E.; Jatikusumah, A.; Arief, R.A.; Putra, A.A.G.; Rukmantara, A.; Estoepangestie, A.T.S.; et al. On dogs, people, and a rabies epidemic: Results from a sociocultural study in Bali, Indonesia. Infect. Dis. Poverty 2015, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arief, R.A.; Hampson, K.; Jatikusumah, A.; Widyastuti, M.D.W.; Sunandar; Basri, C.; Putra, A.A.G.; Willyanto, I.; Estoepangestie, A.T.S.; Mardiana, I.W.; et al. Determinants of Vaccination Coverage and Consequences for Rabies Control in Bali, Indonesia. Front. Veter. Sci. 2017, 3, 123. [Google Scholar] [CrossRef] [Green Version]
- Coleman, P.G.; Dye, C. Immunization coverage required to prevent outbreaks of dog rabies. Vaccine 1996, 14, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Dushoff, J.; Cleaveland, S.; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009, 7, e53. [Google Scholar] [CrossRef]
- Putra, A.A.G.; Hampson, K.; Girardi, J.; Hiby, E.; Knobel, D.; Mardiana, W.; Townsend, S.; Scott-Orr, H. Response to a Rabies Epidemic, Bali, Indonesia, 2008–2011. Emerg. Infect. Dis. 2013, 19, 648–651. [Google Scholar] [CrossRef]
- Suseno, P.P.; Rysava, K.; Brum, E.; De Balogh, K.; Diarmita, I.K.; Husein, W.F.; McGrane, J.; Rasa, F.S.T.; Schoonman, L.; Crafter, S.; et al. Lessons for abies control and elimination programmes: A Decade of One Health ex-perience from Bali, Indonesia. Rev. Sci. Tech. Off. Int. Epiz. 2019, 38, 213–224. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Abela-Ridder, B.; Abila, R.; Amparo, A.C.; Banyard, A.; Blanton, J.; Chanachai, K.; Dallmeier, K.; De Balogh, K.; Del Rio Vilas, V.; et al. Towards rabies elimination in the Asia-Pacific region: From theory to practice. Biologicals 2020, 64, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Slate, D.; Algeo, T.P.; Nelson, K.M.; Chipman, R.B.; Donovan, D.; Blanton, J.D.; Niezgoda, M.; Rupprecht, C.E. Oral Rabies Vaccination in North America: Opportunities, Complexities, and Challenges. PLoS Negl. Trop. Dis. 2009, 3, e549. [Google Scholar] [CrossRef] [Green Version]
- Freuling, C.M.; Hampson, K.; Selhorst, T.; Schröder, R.; Meslin, F.X.; Mettenleiter, T.C.; Müller, T. The elimination of fox rabies from Europe: Determinants of success and lessons for the future. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120142. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.M.; Cliquet, F.; Fehlner-Gardiner, C.; Fooks, A.R.; Sabeta, C.T.; Setién, A.A.; Tu, C.; Vuta, V.; Yakobson, B.; Yang, D.-K.; et al. Role of Oral Rabies Vaccines in the Elimination of Dog-Mediated Human Rabies Deaths. Emerg. Infect. Dis. 2020, 26, e201266. [Google Scholar] [CrossRef] [PubMed]
- Cliquet, F.; Guiot, A.L.; Aubert, M.; Robardet, E.; Rupprecht, C.E.; Meslin, F.X. Oral vaccination of dogs: A well-studied and undervalued tool for achieving human and dog rabies elimination. Vet. Res. 2018, 49, 61. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Expert Consultation on Rabies, Third Report; WHO Technical Report Series; WHO: Geneva, Switzerland, 2018; ISSN 0512-3054. [Google Scholar]
- Yale, G.; Lopes, M.; Isloor, S.; Head, J.R.; Mazeri, S.; Gamble, L.; Dukpa, K.; Gongal, G.; Gibson, A.D. Review of oral rabies vaccination of dogs and its ap-plication in India. Viruses 2022, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Millien, M.; Vos, A.; Fracciterne, F.A.; Crowdis, K.; Chirodea, C.; Medley, A.; Chipman, R.; Qin, Y.; Blanton, J.; et al. Evaluation of immune responses in dogs to oral rabies vaccine under field conditions. Vaccine 2019, 37, 4743–4749. [Google Scholar] [CrossRef]
- Leelahapongsathon, K.; Kasemsuwan, S.; Pinyopummintr, T.; Boodde, O.; Phawaphutayanchai, P.; Aiyara, N.; Bobe, K.; Vos, A.; Friedrichs, V.; Müller, T.; et al. Humoral Immune Response of Thai Dogs after Oral Vaccination against Rabies with the SPBN GASGAS Vaccine Strain. Vaccines 2020, 8, 573. [Google Scholar] [CrossRef]
- Molini, U.; Hassel, R.; Ortmann, S.; Vos, A.; Loschke, M.; Shilongo, A.; Freuling, C.M.; Müller, T. Immunogenicity of the Oral Rabies Vaccine Strain SPBN GASGAS in Dogs Under Field Settings in Namibia. Front. Veter. Sci. 2021, 8, 737250. [Google Scholar] [CrossRef] [PubMed]
- Bobe, K.; Ortmann, S.; Kaiser, C.; Perez-Bravo, D.; Gethmann, J.; Kliemt, J.; Körner, S.; Theuß, T.; Lindner, T.; Freuling, C.; et al. Efficacy of Oral Rabies Vaccine Baits Containing SPBN GASGAS in Domestic Dogs According to International Standards. Vaccines 2023, 11, 307. [Google Scholar] [CrossRef]
- Kasemsuwan, S.; Chanachai, K.; Pinyopummintr, T.; Leelalapongsathon, K.; Sujit, K.; Vos, A. Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand. Veter. Sci. 2018, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Bender, S.; Bergman, D.; Vos, A.; Martin, A.; Chipman, R. Field Studies Evaluating Bait Acceptance and Handling by Dogs in Navajo Nation, USA. Trop. Med. Infect. Dis. 2017, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.D.; Mazeri, S.; Yale, G.; Desai, S.; Naik, V.; Corfmat, J.; Ortmann, S.; King, A.; Müller, T.; Handel, I.; et al. Development of a Non-Meat-Based, Mass Producible and Effective Bait for Oral Vaccination of Dogs against Rabies in Goa State, India. Trop. Med. Infect. Dis. 2019, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- OIE (World Organisation for Animal Health). Manual Diagnostic Tests and Vaccines for Terrestrial Animals 2018; OIE: Paris, France, 2018; Chapter 3.1.18; Available online: https://www.woah.org/en/produit/manual-of-diagnostic-tests-and-vaccines-for-terrestrial-animals-2018/ (accessed on 17 January 2023).
- Freuling, C.M.; Eggerbauer, E.; Finke, S.; Kaiser, C.; Kretzschmar, A.; Nolden, T.; Ortmann, S.; Schröder, C.; Teifke, J.P.; Schuster, P.; et al. Efficacy of the oral rabies virus vaccine strain SPBN GASGAS in foxes and raccoon dogs. Vaccine 2019, 37, 4750–4757. [Google Scholar] [CrossRef]
- Freuling, C.M.; Te Kamp, V.; Klein, A.; Günther, M.; Zaeck, L.; Potratz, M.; Eggerbauer, E.; Bobe, K.; Kaiser, C.; Kretzschmar, A.; et al. Long-Term Immunogenicity and Efficacy of the Oral Rabies Virus Vaccine Strain SPBN GASGAS in Foxes. Viruses 2019, 11, 790. [Google Scholar] [CrossRef] [Green Version]
- Ortmann, S.; Kretzschmar, A.; Kaiser, C.; Lindner, T.; Freuling, C.; Schuster, P.; Mueller, T.; Vos, A. In Vivo Safety Studies With SPBN GASGAS in the Frame of Oral Vaccination of Foxes and Raccoon Dogs Against Rabies. Front. Veter. Sci. 2018, 5, 91. [Google Scholar] [CrossRef] [Green Version]
- Bedeković, T.; Šimić, I.; Krešić, N.; Lojkić, I.; Mihaljević, Ž.; Sučec, I.; Janković, I.L.; Hostnik, P. Evaluation of ELISA for the detection of rabies virus anti-bodies from the thoracic liquid and muscle extract samples in the monitoring of fox oral vaccination campaigns. BMC Vet. Res. 2016, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Wasniewski, M.; Guiot, A.L.; Schereffer, J.L.; Tribout, L.; Mähar, K.; Cliquet, F. Evaluation of an ELISA to detect rabies anti-bodies in orally vaccinated foxes and raccoon dogs sampled in the field. J. Virol. Methods 2013, 187, 264–270. [Google Scholar] [CrossRef]
- Wasniewski, M.; Cliquet, F. Evaluation of ELISA for detection of rabies antibodies in domestic carnivores. J. Virol. Methods 2011, 179, 166–175. [Google Scholar] [CrossRef]
- Cliquet, F.; Verdier, Y.; Sagné, L.; Aubert, M.; Schereffer, J.L.; Selve, M.; Wasniewski, M.; Servat, A. Neutralising antibody titration in 25,000 sera of dogs and cats vaccinated against rabies in France, in the framework of the new regulations that offer an alternative to quar-antine. Rev. Sci. Tech. 2003, 22, 857–866. [Google Scholar] [CrossRef]
- Vos, A. Oral vaccination of dogs against rabies. Int. Anim. Health J. 2019, 6, 25–29. [Google Scholar]
- Vos, A.; Nokireki, T.; Isomursu, M.; Gadd, T.; Kovacs, F. Oral vaccination of foxes and raccoon dogs against rabies with the 3rd generation oral rabies virus vaccine, SPBN GASGAS, in Finland. BMC Acta Vet. Scand. 2021, 63, 40. [Google Scholar] [CrossRef]
- Husein, W.F.; Megawati-Saputra, I.L.; Suseno, P.P.; Arthawan, I.M.; Prayoga, I.M.A.; Daryono, J.; Vos, A.; Wicaksono, A.; Schoonman, L.; Weaver, J.; et al. Assessing Bait Ac-ceptance of Local Dogs, Oral Vaccination Success and Human Contact Risk in Bali, Indonesia. One Health Implement. Res. 2023, 3, 16–29. [Google Scholar] [CrossRef]
- Langguth, A.; Leelahapongsathon, K.; Wannapong, N.; Kasemsuwan, S.; Ortmann, S.; Vos, A.; Böer, M. Comparative Study of Optical Markers to Assess Bait System Efficiency Concerning Vaccine Release in the Oral Cavity of Dogs. Viruses 2021, 13, 1382. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, M.; Nesby, S.; Shaddock, J.; Schumacher, C.; Linhart, S.; Sanderlin, D. Immunogenicity, efficacy and safety of an oral rabies vaccine (SAG-2) in dogs. Vaccine 1996, 14, 465–468. [Google Scholar] [CrossRef]
- Aylan, O.; Vos, A. Efficacy of oral rabies vaccine baits in indigenous Turkish dogs. Infect Dis Rev. 2000, 2, 74–77. [Google Scholar]
- Shuai, L.; Feng, N.; Wang, X.; Ge, J.; Wen, Z.; Chen, W.; Qin, L.; Xia, X.; Bu, Z. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antivir. Res. 2015, 121, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Fooks, A.R.; Zhang, F.; Hu, R. Oral vaccination of dogs (Canis familiaris) with baits containing the re-combinant rabies-canine adenovirus type-2 vaccine confers long-lasting immunity against rabies. Vaccine 2008, 26, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhugunissov, K.; Bulatov, Y.; Taranov, D.; Yershebulov, Z.; Koshemetov, Z.; Abduraimov, Y.; Kondibayeva, Z.; Samoltyrova, A.; Amanova, Z.; Khairullin, B.; et al. Protective immune response of oral rabies vaccine in stray dogs, corsacs and steppe wolves after a single immunization. Arch. Virol. 2017, 162, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Cliquet, F.; Gurbuxani, J.P.; Pradhan, H.K.; Pattnaik, B.; Patil, S.S.; Regnault, A.; Begouen, H.; Guiot, A.L.; Sood, R.; Mahl, P.; et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine 2007, 25, 3409–3418. [Google Scholar] [CrossRef]
- Hammami, S.; Schumacher, C.; Cliquet, F.; Tlatli, A.; Aubert, A.; Aubert, M. Vaccination of Tunisian dogs with the lyophilised SAG2 oral rabies vac- cine incorporated into the DBL2 dog bait. Vet. Res. 1999, 30, 607–613. [Google Scholar]
- Faizah, F.; Mantik-Astawa, I.N.; Putra, A.A.; Suwarno, S. The humoral immunity response of dog vaccinated with oral SAG2 and parenteral Rabisin and Rabivet SUPRA92. Indones. J. Biomed. Sci. 2012, 6, 26–29. [Google Scholar]
- França, T.; Ishikawa, L.; Zorzella-Pezavento, S.; Chiuso-Minicucci, F.; Da Cunha, M.; Sartori, A. Impact of malnutrition on immunity and infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 374–390. [Google Scholar] [CrossRef] [Green Version]
- Akakpo, A.J.; Mbou, G.; Bornarel, P.; Sarradin, P.; Bada Alambjedi, R. Serologic response in dogs after a mass primary anti-rabies vaccination (inactivated vaccine) at Pikine Dakar (Senegal). Dakar Med. 1993, 38, 123–128. [Google Scholar]
- Wait, L.F.; Dobson, A.P.; Graham, A.L. Do parasite infections interfere with immunisation? A review and meta-analysis. Vaccine 2020, 38, 5582–5590. [Google Scholar] [CrossRef]
- Morters, M.K.; McKinley, T.J.; Horton, D.L.; Cleaveland, S.; Schoeman, J.P.; Restif, O.; Whay, H.R.; Goddard, A.; Fooks, A.R.; Damriyasa, I.M.; et al. Achieving Population-Level Immunity to Rabies in Free-Roaming Dogs in Africa and Asia. PLoS Negl. Trop. Dis. 2014, 8, e3160. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.M.; Gilbert, A.; Vos, A.; Freuling, C.M.; Ellis, C.; Kliemt, J.; Müller, T. Review of rabies virus antibodies from oral vaccination as a correlate of protection against lethal infection in animals. Trop. Med. Infect. Dis. 2017, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Chanachai, K.; Wongphruksasoong, V.; Vos, A.; Leelahapongsathon, K.; Tangwangvivat, R.; Sagarasaeranee, O.; Lekcharoen, P.; Trinuson, P.; Kasemsuwan, S. Feasibility and Effectiveness Studies with Oral Vaccination of Free-Roaming Dogs against Rabies in Thailand. Viruses 2021, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.D.; Yale, G.; Vos, A.; Corfmat, J.; Airikkala-Otter, I.; King, A.; Wallace, R.M.; Gamble, L.; Handel, I.G.; Mellanby, R.J.; et al. Oral bait handout as a method to access roaming dogs for rabies vaccination in Goa, India: A proof of principle study. Vaccine X 2019, 1, 100015. [Google Scholar] [CrossRef] [PubMed]
- Lembo, T.; Hampson, K.; Kaare, M.T.; Ernest, E.; Knobel, D.; Kazwala, R.R.; Haydon, D.T.; Cleaveland, S. The Feasibility of Canine Rabies Elimination in Africa: Dispelling Doubts with Data. PLoS Negl. Trop. Dis. 2010, 4, e626. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Treatment | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bait | d.o.a. | Parenteral | Control | (excl. Control) | ||||||
| Area | n/N | % | n/N | % | n/N | % | n/N | % | n/N | % |
| 26/29 | 89.7 | 10/11 | 90.9 | 16/18 | 88.9 | 0/4 | 0 | 52/58 | 89.7 |
| 14/16 | 87.5 | 6/6 | 100 | 4/4 | 100 | 0/4 | 0 | 24/26 | 92.3 |
| Size | ||||||||||
| 18/22 | 81.8 | 9/10 | 90.0 | 12/13 | 92.3 | 0/0 | 0 | 39/45 | 86.7 |
| 22/23 | 95.7 | 7/7 | 100 | 8/9 | 88.9 | 0/7 | 0 | 37/39 | 94.9 |
| - | - | - | - | - | - | 0/1 | 0 | - | - |
| Sex | ||||||||||
| 24/26 | 92.3 | 11/12 | 91.7 | 11/13 | 84.6 | 0/6 | 0 | 46/51 | 90.2 |
| 16/19 | 84.2 | 5/5 | 100 | 9/9 | 100 | 0/2 | 0 | 30/33 | 90.9 |
| Age | ||||||||||
| 20/24 | 83.3 | 9/10 | 90.0 | 9/9 | 100 | 0/3 | 0 | 38/43 | 88.4 |
| 20/24 | 83.3 | 7/7 | 100 | 11/13 | 84.6 | 0/5 | 0 | 38/44 | 86.4 |
| Supervision | ||||||||||
| 13/14 | 92.9 | 6/6 | 100 | 8/9 | 88.9 | 0/3 | 0 | 27/29 | 93.1 |
| 27/31 | 87.1 | 10/11 | 90.9 | 12/13 | 92.3 | 0/5 | 0 | 49/55 | 89.1 |
| Total | 40/45 | 88.9 | 16/17 | 94.1 | 20/22 | 90.9 | 0/8 | 0 | 76/84 | 90.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megawati Saputra, I.L.; Suwarno, S.; Husein, W.F.; Suseno, P.P.; Prayoga, I.M.A.; Vos, A.; Arthawan, I.M.; Schoonman, L.; Weaver, J.; Zainuddin, N. Immunogenicity of Oral Rabies Vaccine Strain SPBN GASGAS in Local Dogs in Bali, Indonesia. Viruses 2023, 15, 1405. https://doi.org/10.3390/v15061405
Megawati Saputra IL, Suwarno S, Husein WF, Suseno PP, Prayoga IMA, Vos A, Arthawan IM, Schoonman L, Weaver J, Zainuddin N. Immunogenicity of Oral Rabies Vaccine Strain SPBN GASGAS in Local Dogs in Bali, Indonesia. Viruses. 2023; 15(6):1405. https://doi.org/10.3390/v15061405
Chicago/Turabian StyleMegawati Saputra, Irene Linda, Suwarno Suwarno, Wahid Fakhri Husein, Pebi Purwo Suseno, I Made Angga Prayoga, Ad Vos, I Made Arthawan, Luuk Schoonman, John Weaver, and Nuryani Zainuddin. 2023. "Immunogenicity of Oral Rabies Vaccine Strain SPBN GASGAS in Local Dogs in Bali, Indonesia" Viruses 15, no. 6: 1405. https://doi.org/10.3390/v15061405
APA StyleMegawati Saputra, I. L., Suwarno, S., Husein, W. F., Suseno, P. P., Prayoga, I. M. A., Vos, A., Arthawan, I. M., Schoonman, L., Weaver, J., & Zainuddin, N. (2023). Immunogenicity of Oral Rabies Vaccine Strain SPBN GASGAS in Local Dogs in Bali, Indonesia. Viruses, 15(6), 1405. https://doi.org/10.3390/v15061405






