Unpacking the Complexity of COVID-19 Fatalities: Adverse Events as Contributing Factors—A Single-Center, Retrospective Analysis of the First Two Years of the Pandemic
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Coinfections
4.2. COVID-19 Complications
4.3. Complications Unrelated to COVID-19
4.4. Causes of Death
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 inealime. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Murray, C.J. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literatureeview and meta-analysis. J. Infect. 2020, 81, 16–25. [Google Scholar]
- Rahman, A.; Sathi, N.J. Risk factors ofhe severity of COVID-19: A meta-analysis. Int. J. Clin. Pract. 2021, 75, e13916. [Google Scholar] [CrossRef] [PubMed]
- Ouchetto, O.; Drissi Bourhanbour, A. Risk Factors of COVID-19 Patients. Disaster Med. Public Health Prep. 2022, 16, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, X.; Liu, G.; Gao, Y. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Tracking SARS-CoV-2 Variants. 2023. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants (accessed on 1 April 2023).
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Brzdęk, M.; Zarębska-Michaluk, D.; Rzymski, P.; Rogalska, M.; Moniuszko-Malinowska, A.; Flisiak, R. Differences between the course of SARS-CoV-2 infections in the periods of the Delta and Omicron variants dominance in Poland. Pol. Arch. Intern. Med. 2023, 2023, 16403. [Google Scholar] [CrossRef]
- Karlinsky, A.; Kobak, D. Tracking excess mortality across countries during the COVID-19 pandemic with the World Mortality Dataset. Elife 2021, 10, e69336. [Google Scholar] [CrossRef]
- Elavarasi, A.; Prasad, M.; Seth, T.; Sahoo, R.K.; Madan, K.; Nischal, N.; Soneja, M.; Sharma, A.; Maulik, S.K.; Shalimar; et al. Chloroquine and Hydroxychloroquine for the Treatment of COVID-19: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2020, 35, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.G. Natural History of COVID-19 and Current Knowledge on Treatment Therapeutic Options. Biomed. Pharmacother. 2020, 129, 110493. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Ryan, H.; Kredo, T.; Chaplin, M.; Fletcher, T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 2, CD013587. [Google Scholar] [CrossRef]
- Panahi, Y.; Gorabi, A.M.; Talaei, S.; Beiraghdar, F.; Akbarzadeh, A.; Tarhriz, V.; Mellatyar, H. An overview on the treatments and prevention against COVID-19. Virol. J. 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiao, B.; Qu, L.; Yang, D.; Liu, R. The development of COVID-19reatment. Front. Immunol. 2023, 14, 1125246. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin. Infect. Dis. 2023, 76, 342–349. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematiceview and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. NextStrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO: Clinical Management of COVID-19; WHO: Geneva, Switzerland, 2020.
- Luft, T.; Benner, A.; Jodele, S.; Dandoy, C.E.; Storb, R.; Gooley, T.; Penack, O. EASIX in patients with acute graft-versus-host disease: Aetrospective cohort analysis. Lancet Haematol. 2017, 4, 414–423. [Google Scholar] [CrossRef]
- Sikora, A.; Zahra, F. Nosocomial Infections. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559312/ (accessed on 10 April 2023).
- Conceptual Framework for the International Classification for Patient Safety Version 1.1: Final Technical Report January 2009. 2023. Available online: http://apps.who.int/iris/handle/10665/70882 (accessed on 10 April 2023).
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Zarębska-Michaluk, D. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists. Pol. Arch. Intern. Med. 2020, 130, 557–558. [Google Scholar] [CrossRef] [Green Version]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, 2022. Pol. Arch. Intern. Med. 2022, 132, 16230. Available online: https://pubmed.ncbi.nlm.nih.gov/35352546/ (accessed on 2 May 2023). [CrossRef]
- Living Guidance for Clinical Management of COVID-19. 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 2 May 2023).
- R Core Team. European Environment Agency. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 12 May 2023).
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.; Franssen, G.H.; Zeegers, M.P. Original research: Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ashcroft, T.; Chung, A.; Dighero, I.; Dozier, M.; Horne, M.; Mc Swiggan, E.; Shamsuddin, A.; Nair, H. Risk factors for poor outcomes in hospitalised COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health 2021, 11, 10001. [Google Scholar] [CrossRef] [PubMed]
- Modes, M.E.; Directo, M.P.; Melgar, M.; Johnson, L.R.; Yang, H.; Chaudhary, P.; Chen, P. Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2nfection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance—One Hospital, California, July 15–September 23, 2021, and December 21, 2021–January 27, 2022. MMWR Recomm. Rep. 2022, 71, 217–223. [Google Scholar]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Thelwall, S. Comparative analysis ofheisks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Alwani, M.; Yassin, A.; Al-Zoubi, R.M.; Aboumarzouk, O.M.; Nettleship, J.; Kelly, D.; Shabsigh, R. Sex-based differences in severity and mortality in COVID-19. Rev. Med. Virol. 2021, 31, 31. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.; Chong, D.S.Y.; Lai, W.Y.Y. Do Men Have a Higher Case Fatality Rate of Severe Acute Respiratory Syndrome than Women Do? Am. J. Epidemiol. 2004, 159, 229. [Google Scholar] [CrossRef] [Green Version]
- Jansen, A.; Chiew, M.; Konings, F.; Lee, C.-K.; Li, A. Sex matters–a preliminary analysis of Middle East respiratory syndrome in the Republic of Korea, 2015. Western. Pac. Surveill. Response J. 2015, 6, 68. [Google Scholar] [CrossRef] [Green Version]
- Kalicińska, E.; Biernat, M.; Rybka, J.; Zińczuk, A.; Janocha-Litwin, J.; Rosiek-Biegus, M.; Morawska, M.; Waszczuk-Gajda, A.; Drozd-Sokołowska, J.; Szukalski; et al. Endothelial Activation and Stressndex (EASIX) as an Early Predictor for Mortality and Overall Survival in Hematological and Non-Hematological Patients with COVID-19: Multicenter Cohort Study. J. Clin. Med. 2021, 10, 4373. [Google Scholar] [CrossRef]
- Zińczuk, A.; Rorat, M.; Simon, K.; Jurek, T. EASIX, Modified EASIX and Simplified EASIX as an Early Predictor for intensive Care Unit Admission and Mortality in Severe COVID-19 Patients. J. Pers. Med. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Luft, T.; Wendtner, C.M.; Kosely, F.; Radujkovic, A.; Benner, A.; Korell, F.; Merle, U. EASIX for Prediction of Outcome in Hospitalized SARS-CoV-2nfected Patients. Front. Immunol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2021, 11, 605908. [Google Scholar] [CrossRef]
- Smadja, D.M.; Guerin, C.L.; Chocron, R.; Yatim, N.; Boussier, J.; Gendron, N.; Khider, L.; Hadjadj, J.; Goudot, G.; Debuc, B.; et al. Angiopoietin-2 as a marker ofndothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020, 23, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Anyfanti, P.; Gavriilaki, M.; Lazaridis, A.; Douma, S.; Gkaliagkousi, E. Endothelial Dysfunction in COVID-19: Lessons Learned from Coronaviruses. Curr. Hypertens. Rep. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Koh, G.; Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Zinkernagel, R.A.A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Faculty Opinions recommendation of Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Epic II Group of Investigators. International study ofhe prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contou, D.; Cally, R.; Sarfati, F.; Desaint, P.; Fraissé, M.; Plantefève, G. Causes and timing of death in critically ill COVID-19 patients. Crit. Care 2021, 25, 1–4. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: Retrospective Cohort Study. Clin. Microbiol. Infect. 2021, 27, 83. [Google Scholar] [CrossRef]
- Soriano, M.C.; Vaquero, C.; Ortiz-Fernández, A.; Caballero, A.; Blandino-Ortiz, A.; de Pablo, R. Low incidence of co-infection, but high incidence of CU-acquired infections in critically ill patients with COVID-19. J. Infect. 2021, 82, 20. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rawson, T.; Moore, L.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Clinical Infectious Diseases Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. 2023. Available online: https://academic.oup.com/cid/article/71/9/2459/5828058 (accessed on 2 May 2023).
- Litwin, A.; Fedorowicz, O.; Duszynska, W. Characteristics of Microbial Factors of Healthcare-Associated Infections Including Multidrug-Resistant Pathogens and Antibiotic Consumption at the University Intensive Care Unit in Poland in the Years 2011–2018. Int. J. Environ. Res. Public Health 2020, 17, 6943. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Taccone, F.S.; Izzi, A.; Yin, N.; Garufi, A.; Hublet, S.; Njimi, H.; Ego, A.; Gorham, J.; Byl, B.; et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms 2020, 8, 1821. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Emerick, M.; Cabunoc, M.K.; Williams, M.H.; Preas, M.A.; Schrank, G.; Rabinowitz, R.; Luethy, P.; Johnson, J.K.; Leekha, S. Rapid Spread and Control of Multidrug-Resistant Gram-Negative Bacteria in COVID-19 Patient Care Units. Emerg. Infect. Dis. 2021, 27, 1234–1237. [Google Scholar] [CrossRef]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L. 15 drugs being tested to treat COVID-19 and how they would work. Nat. Med. 2020; ahead of print. [Google Scholar]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2020, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, D.; Sasaki, K.; Karkar, A.; Sharif, S.; Lewis, K.; Mammen, M.J.; Alexander, P.; Ye, Z.; Lozano, L.E.C.; Munch, M.W.; et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: A systematic review and meta-analysis. Intensive Care Med. 2021, 47, 521–537. [Google Scholar] [CrossRef]
- Cochrane Haematology Group; Wagner, C.; Griesel, M.; Mikolajewska, A.; Mueller, A.; Nothacker, M.; Fichtner, F. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 8, CD014963. [Google Scholar]
- RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef]
- Rochwerg, B.; Einav, S.; Chaudhuri, D.; Mancebo, J.; Mauri, T.; Helviz, Y.; Goligher, E.C.; Jaber, S.; Ricard, J.-D.; Rittayamai, N.; et al. The role for high flow nasal cannula as a respiratory support strategy in adults: A clinical practice guideline. Intensive Care Med. 2020, 46, 2226–2237. [Google Scholar] [CrossRef]
- Rorat, M.; Szymański, W.; Jurek, T.; Karczewski, M.; Zelig, J.; Simon, K. When Conventional Oxygen Therapy Fails-The Effectiveness of High-Flow Nasal Oxygen Therapy in Patients with Respiratory Failure in the Course of COVID-19. J. Clin. Med. 2021, 10, 4751. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis andreatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Hong, H.; Ohana, M.; Bompard, F.; Revel, M.-P.; Valle, C.; Gervaise, A.; Poissy, J.; Susen, S.; Hékimian, G.; et al. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology 2021, 298, E70–E80. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, S.; Guo, H. Acute kidney injury and renal replacement therapy in COVID-19 patients: A systematic review and meta-analysis. Int. Immunopharmacol. 2021, 90, 107159. [Google Scholar] [CrossRef]
- World Health Organization. Patient Safety Incident Reporting and Learning Systems. In Technical Report and Guidance; WHO: Geneva, Switzerland, 2020; pp. 1–51. [Google Scholar]
- Anderson, J.G.; Abrahamson, K. Your Health Care May Kill You: Medical Errors. Stud. Health Technol. Inform. 2017, 234, 13–17. [Google Scholar] [PubMed]
- The Economics of Patient Safety: Strengthening a Value-Based Approach to Reducing Patient Harm at National Level | OECD Health Working Papers | OECD iLibrary. 2023. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/the-economics-of-patient-safety_5a9858cd-en (accessed on 23 May 2023).
- Crossing the Global Quality Chasm: Improving Health Care Worldwide. Crossing the Global Quality Chasm. 2018. Available online: https://pubmed.ncbi.nlm.nih.gov/30605296/ (accessed on 23 May 2023).
- E Kruk, M.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-quality health systems in the Sustainable Development Goals era: Time for a revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef] [Green Version]
- Cohen, G.; Crespo, A.M.; White, D.B. Potential Legal Liability for Withdrawing or Withholding Ventilators During COVID-19: Assessing The Risks and Identifying Needed Reforms. JAMA 2020, 323, 1901–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensuring a Safe Environment for Patients and Staff in COVID-19 Health-Care Facilities: A Module from the Suite of Health Service Capacity Assessments in the Context of the COVID-19 Pandemic. 2023. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-HCF_assessment-Safe_environment-2020.1 (accessed on 23 May 2023).
- Medical Certification, CD Mortality Coding, and Reporting Mortality Associated with COVID-19. 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-mortality-reporting-2020-1 (accessed on 29 April 2023).
- Cobos-Siles, M.; Cubero-Morais, P.; Arroyo-Jimenez, I.; Rey-Hernandez, M.; Hernandez-Gomez, L.; Vargas-Parra, D.J.; Corral-Gudino, L. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: More than just acute respiratory failure or thromboembolic events. Intern. Emerg. Med. 2020, 15, 1533. [Google Scholar] [CrossRef]
- Slater, T.A.; Straw, S.; Drozd, M.; Kamalathasan, S.; Cowley, A.; Witte, K.K. Dying ‘dueo’ or ‘with’ COVID-19: A cause of death analysis in hospitalised patients. Clin. Med. 2020, 20, 189. [Google Scholar] [CrossRef]
- Salerno, M.; Sessa, F.; Piscopo, A.; Montana, A.; Torrisi, M.; Patanè, F.; Murabito, P.; Volti, G.L.; Pomara, C. No Autopsies on COVID-19 Deaths: A Missed Opportunity and the Lockdown of Science. J. Clin. Med. 2020, 9, 1472. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Over- and under-estimation of COVID-19 deaths. Eur. J. Epidemiol. 2021, 36, 581. [Google Scholar] [CrossRef] [PubMed]
- Horton, R. Offline: COVID-19 is not a pandemic. Lancet 2020, 396, 874. Available online: http://pmc/articles/PMC7515561/ (accessed on 29 April 2023). [CrossRef] [PubMed]
| Variable | N = 477 |
|---|---|
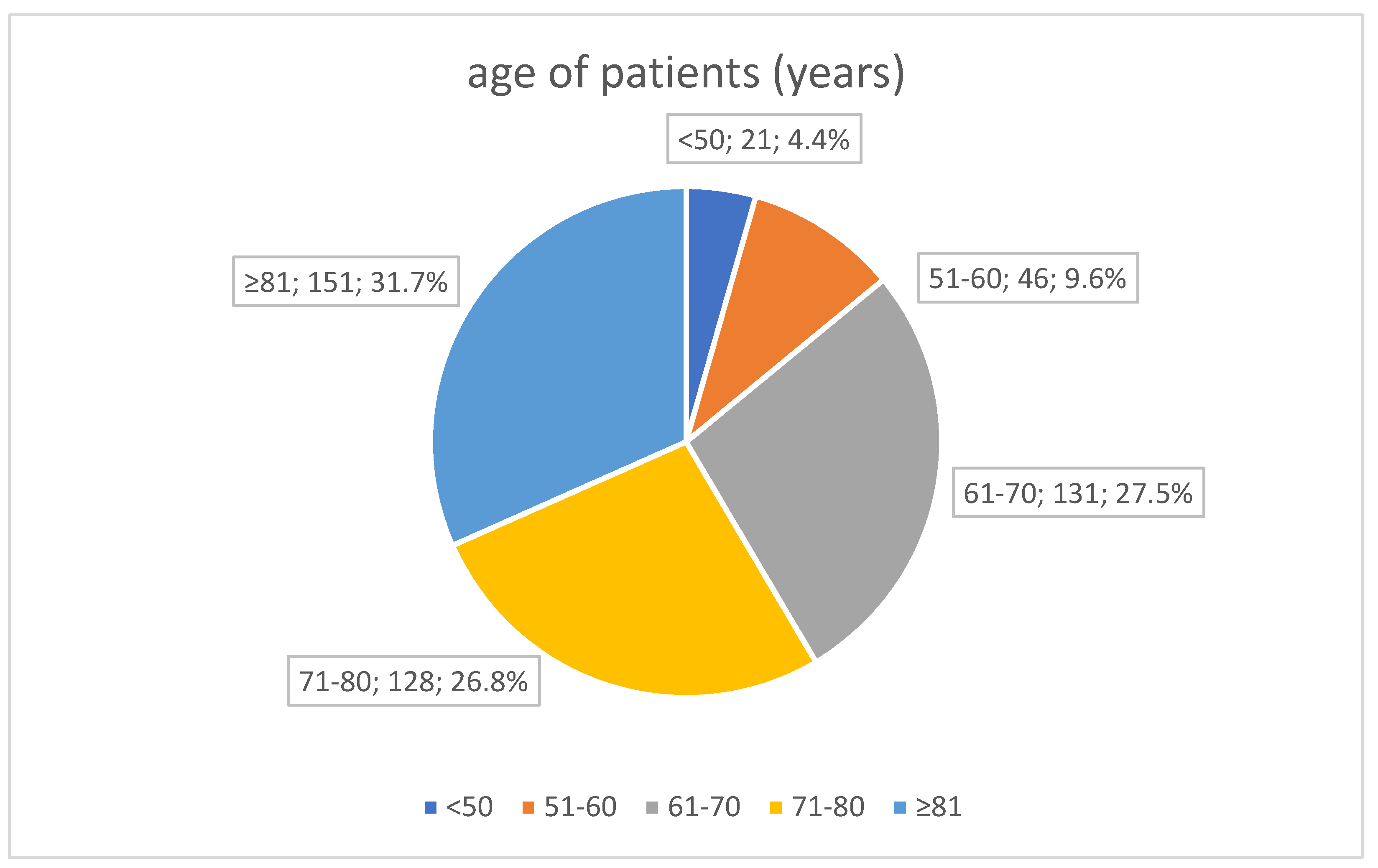
| Age (years) | 73.43 (12.43), 74 [6–83] |
| Sex | female 194 (40.7%) |
| male 283 (59.3%) | |
| Ward of admission | ICU 56 (11.7%) |
| IDU 421 (88.3%) | |
| Ward where the patient died | ICU 264 (55.3%) |
| IDU 213 (44.7%) | |
| Length of hospitalisation (days) | 13.87 (10.97), 11 [6–19] |
| Duration of symptoms before admission (days) | 7.3 (4.62), 7 [5–9] |
| SpO2 at admission (%) | 81.33 (13.5), 85 [74.75–91] |
| Severity of illness at admission acc. to WHO | 1—14 (2.9%) 2—115 (24.1%) 3—236 (49.5%) 4—112 (23.5%) |
| Comorbidities: Cardiovascular diseases Respiratory diseases Diabetes Malignant neoplasm Chronic kidney disease Obesity | 436 (91.4%) 367 (76.9%) 68 (14.3%) 160 (33.5%) 90 (18.9%) 58 (12.2%) 92 (19.3%) |
| Laboratory results at admission | |
| CRP (>6 mg/L) | 128.2 (95.76), 105.44 [54.07–180.67] |
| D-dimer (>500 ng/mL) | 4221.07 (9138), 1766 [1055–3278] |
| Ferritin (>274.66 ng/mL) | 2279.94 (3887.55), 1292.69 [613.75–2690.47] |
| Creatinine (>1.15 mg/dL) | 1.58 (1.63), 1.10 [0.82–1.67] |
| WBC (4–10 G/L) | 9.58 (10.78), 7.64 [5.25–10.92] |
| PLT (150–420 G/L) | 206 (100), 188 [136–248] |
| PCT (>0.05 ng/mL) | 2.33 (10.18), 0.32 [0.12–0.97] |
| LDH (>220 U/L) | 578.78 (304.16), 510 [368–703] |
| EASIX | 6.22 (11.19), 3.14 [1.89–6.07] |
| Treatment during the entire hospitalisation | |
| Low-dose oxygen therapy > 10 L/min | 431 (90.4%) |
| HFNOT | 265 (55.6%) |
| NIV | 132 (27.7%) |
| IMV | 273 (57.2%) |
| Antiviral treatment (RDV, MPV) | 94 (19.7%) |
| Chloroquine + LPV/r | 29 (6.1%) |
| COVID-19 convalescent plasma | 81 (17%) |
| Dexamethasone | 381 (79.9%) |
| Tocilizumab | 95 (19.9%) |
| Baricitinib | 49 (10.3%) |
| Low-molecular-weight heparin | none—21 (4.4%) prophylactic dose—353 (74%), therapeutic dose—103 (21.6%) |
| Coinfections | 274 (57.4%) |
| Healthcare-associated infections | 159 (33.3%) |
| Predominant cause of death—COVID-19 respiratory failure | 423 (88.7%) |
| Variable | N = 477 |
|---|---|
| Coinfection | 274 (57.4%) |
| Healthcare-associated infection | 159 (33.3%; 58% of all infections) |
| BSIs (bloodstream infections) | 165 (34.6%) |
| VAP (ventilator-associated pneumonia) | 173 (36.3%) |
| UTIs (urinary tract infections) | 121 (25.4%) |
| Use of antibiotics from the beginning of hospitalisation | 452 (94.8%) |
| Inclusion of an antibiotic without medical indication | 215 (47.6%) |
| COVID-19 Therapy | Hospital-Acquired Infection [N (%), p-Value] |
|---|---|
| Anti-viral treatment (RDV, MPV) (N = 94) | 27 (28.7%), p = 0.3 |
| Chloroquine + LPV/r (N = 29) | 12 (41.4%), p = 0.4 |
| Dexamethasone (N = 381) | 117 (30.7%), p = 0.052 |
| Tocilizumab (N = 95) | 32 (33.7%), p > 0.9 |
| Baricitinib (N = 49) | 33 (67.3%), p < 0.001 |
| Variable | N = 477 |
|---|---|
| Thrombotic complications: | 112 (23.5%) |
| Venous thromboembolism | 46 (9.6%) |
| Ischaemic stroke | 29 (6.1%) |
| Myocardial infarction | 22 (4.6%) |
| Limb ischaemia | 16 (3.4%) |
| Bleeding complications | 71 (14.9%) |
| Other: | 261 (54.7%) |
| Acute kidney injury (AKI) | 149 (31.2%) |
| Exacerbation of chronic heart disease | 100 (21%) |
| Decompensation of liver function | 12 (2.5%) |
| Variable | N = 477 |
|---|---|
| Complications of hospitalisation | 220 (46.1%), including more than one complication in 22 patients (4.6%) |
| Healthcare-associated infection | 159 (33.3%) |
| Emphysema and other complications of ventilation | 36 (7.5%) |
| Trauma, fall | 25 (5.2%) |
| Personnel malpractice | 237 (49.7%) |
| Lack of specialist treatment | 81 (17.0%) |
| Delay in specialist treatment | 156 (32.7%) |
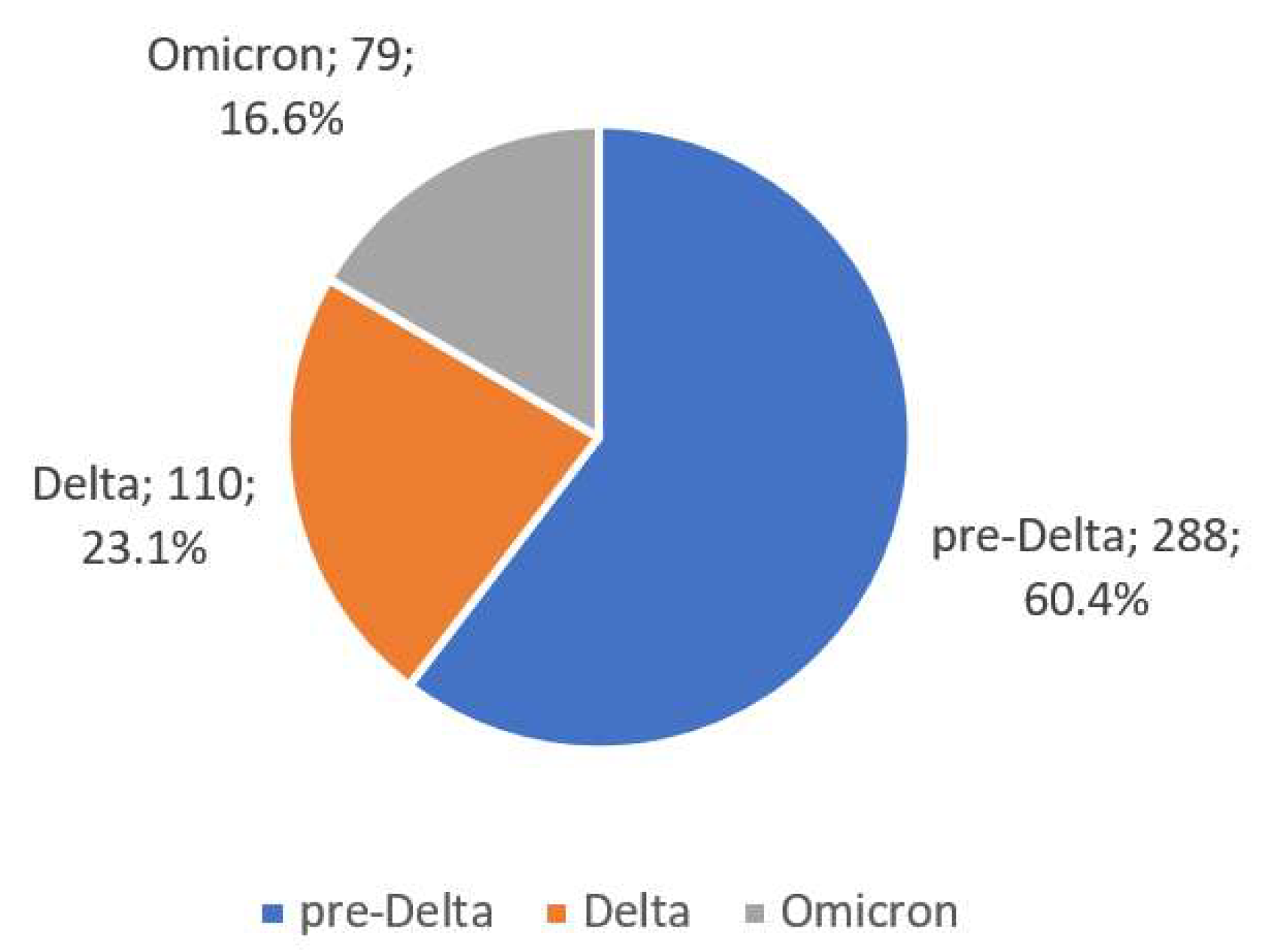
| Variable | Pre-Delta (N = 288) | Delta (N = 110) | Omicron (N = 79) | p-Value |
|---|---|---|---|---|
| Age (years) | 72.34 (11.9), 72 [65–82] | 74.71 (13.23), 76 [66–85.75] | 75.63 (12.86), 77 [69–85.5] | 0.015 |
| Sex | 0.065 | |||
| Men Women | 183 (63.5%) 105 (36.5%) | 57 (51.8%) 53 (48.2%) | 43 (54.4%) 36 (45.6%) | |
| Duration of symptoms before admission (days) | 6.94 (4.44), 7 [4–8] | 7.44 (3.53), 7 [5–9] | 8.83 (6.32), 7 [4.25–14] | 0.093 |
| Length of hospitalisations (days) | 14.01 (11.17), 12 [6–20] | 13.99 (9.90), 11.5 [7–19] | 13.16 (11.17), 11 [5–16] | 0.5 |
| Comorbidities | 266 (92.4%) | 97 (88.2%) | 73 (92.4%) | 0.4 |
| Cardiovascular diseases | 219 (76.0%) | 81 (73.6%) | 67 (84.8%) | 0.15 |
| Respiratory diseases | 42 (14.6%) | 9 (8.2%) | 17 (21.5%) | 0.031 |
| Diabetes | 113 (39.2%) | 29 (26.4%) | 18 (22.8%) | 0.004 |
| Malignant neoplasm | 57 (19.8%) | 15 (13.6%) | 18 (22.8%) | 0.2 |
| Chronic kidney disease | 41 (14.2%) | 9 (8.2%) | 8 (10.1%) | 0.2 |
| Obesity | 71(24.7%) | 16 (14.5%) | 5 (6.3%) | <0.001 |
| EASIX | 6.67 (12.63), 3.17 [1.91–6.23] | 5.14 (5.28), 3.28 [2.05–6] | 5.64 (9.97), 2.69 [1.66–4.45] | 0.3 |
| Treatment | ||||
| Antiviral treatment (RDV, MPV) | 67 (23.3%) | 16 (14.5%) | 11(13.9%) | 0.054 |
| Dexamethasone | 208 (72.2%) | 107 (97.3%) | 66 (83.5%) | <0.001 |
| Tocilizumab | 51 (17.7%) | 29 (26.4%) | 15 (19.0%) | 0.2 |
| Baricitinib | 2 (0.7%) | 30 (27.3%) | 17 (21.5%) | <0.001 |
| Antibiotic use | 281 (97.6%) | 105 (95.5%) | 66 (83.5%) | <0.001 |
| Inclusion of an antibiotic without medical indication | 133 (47.0%) | 58 (55.2%) | 24 (36.4%) | 0.051 |
| Coinfection | 166 (57.6%) | 65 (59.1%) | 43 (54.4%) | 0.8 |
| COVID-19 complications | ||||
| Pulmonary embolism | 22 (7.6%) | 14 (12.7%) | 10 (12.7%) | 0.2 |
| Ischaemic stroke | 17 (5.9%) | 5 (4.5%) | 6 (7.6%) | 0.7 |
| Myocardial infarction | 13 (4.5%) | 5 (4.5%) | 4 (5.1%) | >0.9 |
| Limb ischaemia | 7 (2.4%) | 2 (1.8%) | 7 (8.9%) | 0.028 |
| Bleeding complications | 39 (13.5%) | 17 (15.5%) | 15 (19.0%) | 0.5 |
| Acute kidney injury | 86 (29.9%) | 33 (30.0%) | 30 (38.0%) | 0.4 |
| Exacerbation of chronic heart disease | 60 (20.8%) | 29 (26.4%) | 11 (13.9%) | 0.11 |
| Decompensation of liver function | 5 (1.7%) | 1 (0.9%) | 6 (7.6%) | 0.011 |
| Complications of hospitalisation | ||||
| Hospital-acquired infection | 91 (31.6%) | 42 (38.2%) | 26 (32.9%) | 0.5 |
| Emphysema and other complications of ventilation | 21 (7.3%) | 12 (10.9%) | 3 (3.8%) | 0.2 |
| Trauma, fall | 8 (2.8%) | 9 (8.2%) | 8 (10.1%) | 0.005 |
| Predominant cause of death—COVID-19 respiratory failure | 252 (87.5%) | 103 (93.6%) | 68 (86.1%) | 0.15 |
| Other causes of death | ||||
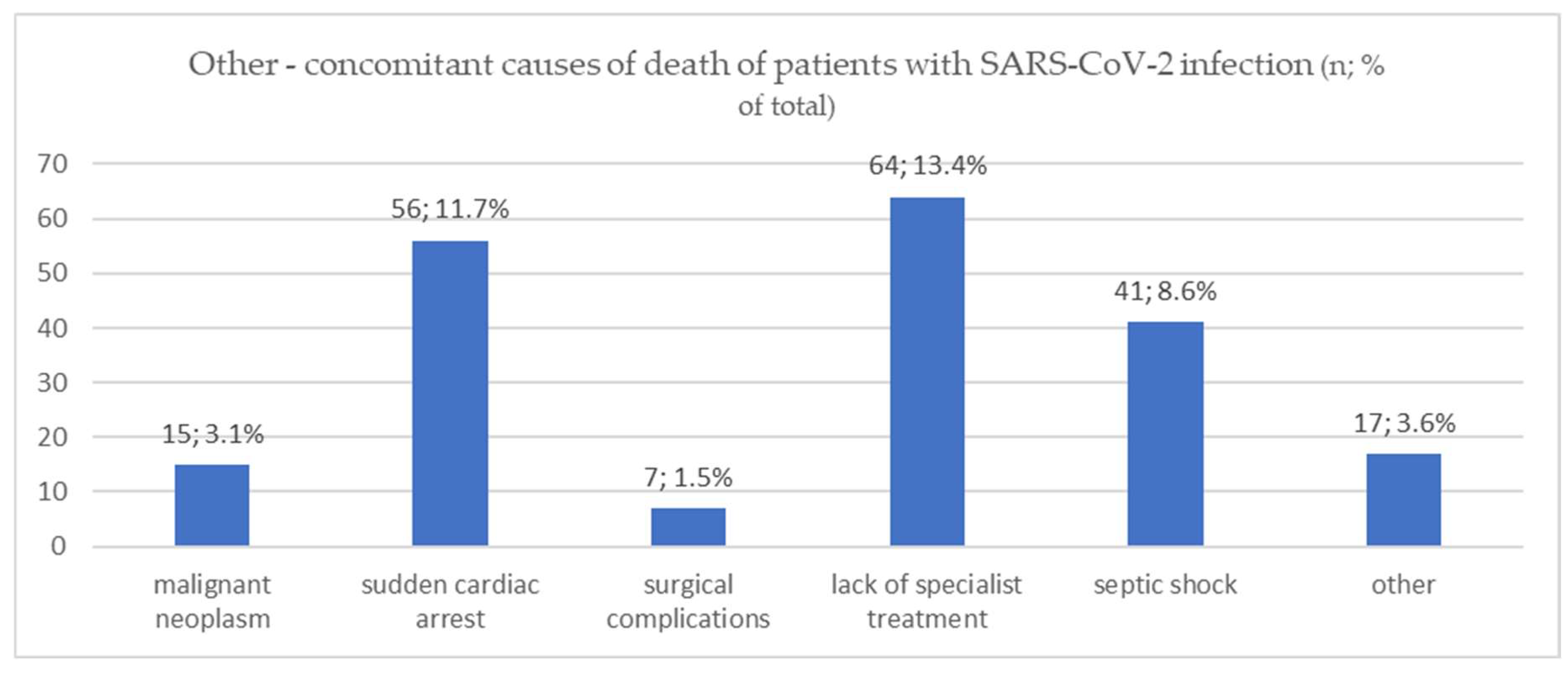
| Malignant neoplasm | 10 (3.5%) | 2 (1.8%) | 3 (3.8%) | 0.7 |
| Sudden cardiac arrest | 33 (11.5%) | 10 (9.1%) | 13 (16.5%) | 0.3 |
| Surgical complications | 5 (1.7%) | 2 (1.8%) | 0 (0%) | 0.7 |
| Lack of specialist treatment | 38 (13.2%) | 14 (12.7%) | 12 (15.2%) | 0.9 |
| Septic shock | 14 (4.9%) | 16 (14.5%) | 11 (13.9%) | 0.002 |
| other | 8 (2.8%) | 3 (2.7%) | 6 (7.6%) | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zińczuk, A.; Rorat, M.; Simon, K.; Jurek, T. Unpacking the Complexity of COVID-19 Fatalities: Adverse Events as Contributing Factors—A Single-Center, Retrospective Analysis of the First Two Years of the Pandemic. Viruses 2023, 15, 1430. https://doi.org/10.3390/v15071430
Zińczuk A, Rorat M, Simon K, Jurek T. Unpacking the Complexity of COVID-19 Fatalities: Adverse Events as Contributing Factors—A Single-Center, Retrospective Analysis of the First Two Years of the Pandemic. Viruses. 2023; 15(7):1430. https://doi.org/10.3390/v15071430
Chicago/Turabian StyleZińczuk, Aleksander, Marta Rorat, Krzysztof Simon, and Tomasz Jurek. 2023. "Unpacking the Complexity of COVID-19 Fatalities: Adverse Events as Contributing Factors—A Single-Center, Retrospective Analysis of the First Two Years of the Pandemic" Viruses 15, no. 7: 1430. https://doi.org/10.3390/v15071430





