ICP4-Associated Activation of Rap1b Facilitates Herpes Simplex Virus Type I (HSV-1) Infection in Human Corneal Epithelial Cells
Abstract
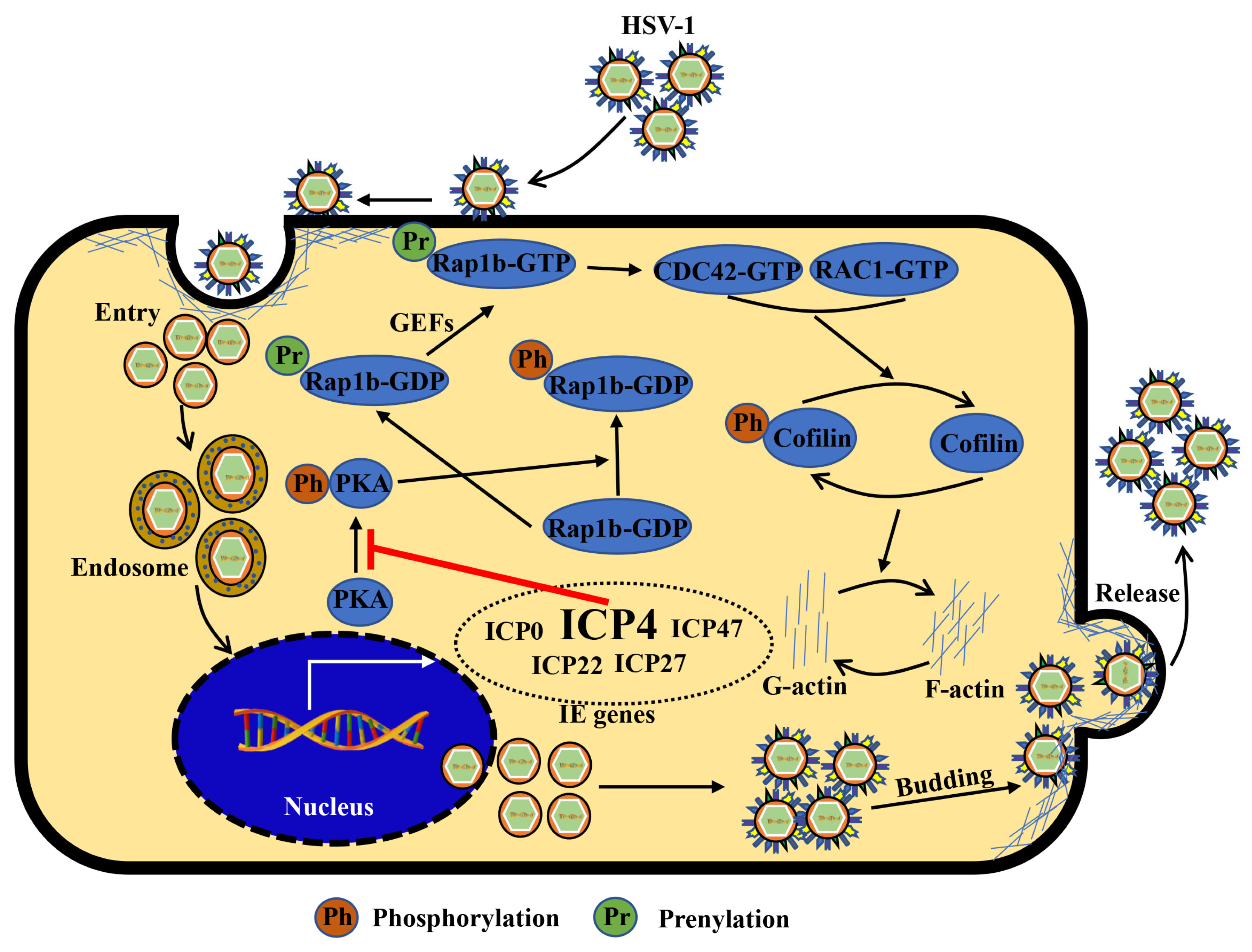
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, Plasmids, Chemicals, and Antibodies
2.2. Construction and Transfection of Recombinant Plasmids
2.3. Virus Infection
2.4. Protein Extraction and Expression Level Evaluation
2.5. Immunofluorescence Assay (IFA)
2.6. Small Interfering RNA (siRNA) Interference
2.7. Virus Titration
2.8. Cell Viability Analysis
2.9. Statistical Analysis
3. Results
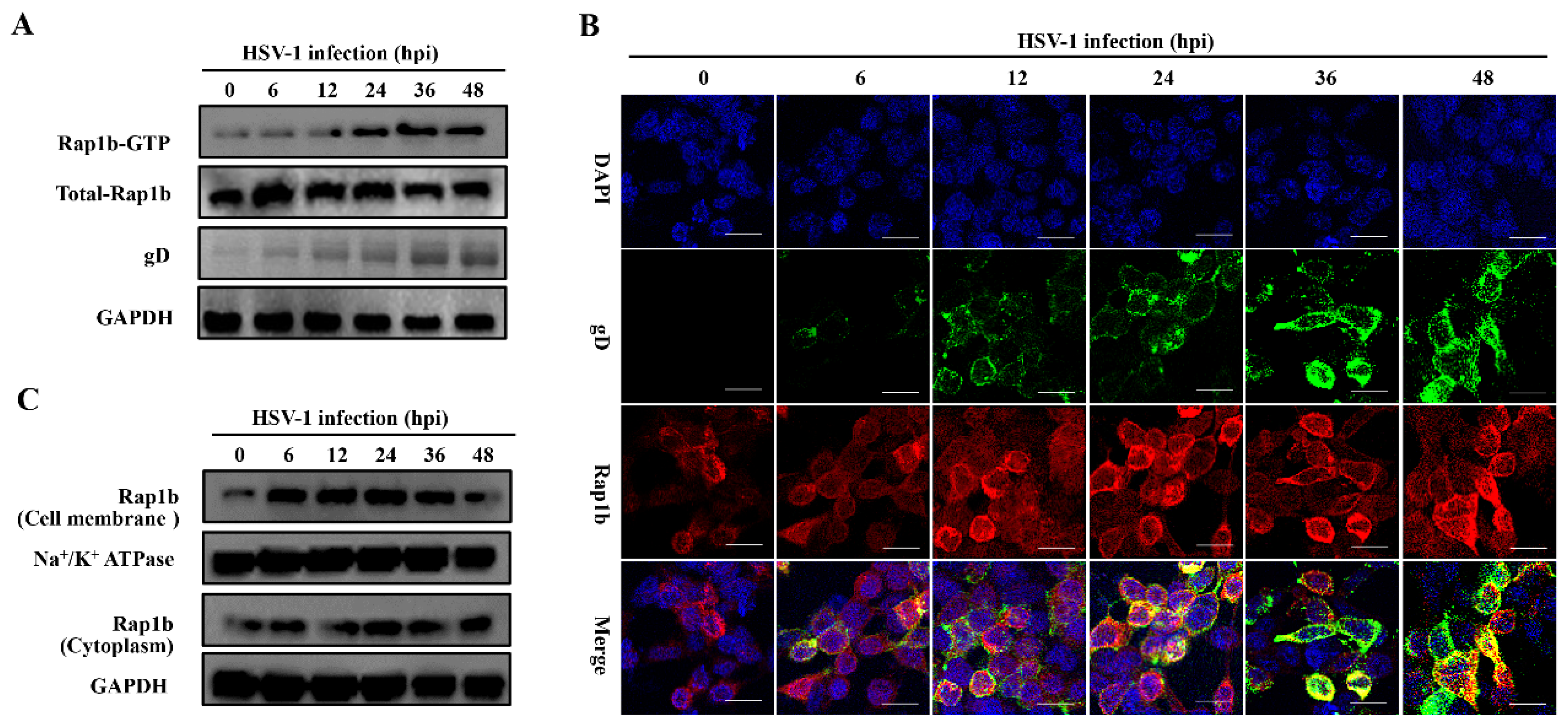
3.1. HSV-1 Infection Induces Rap1b Activation and Membrane Distribution
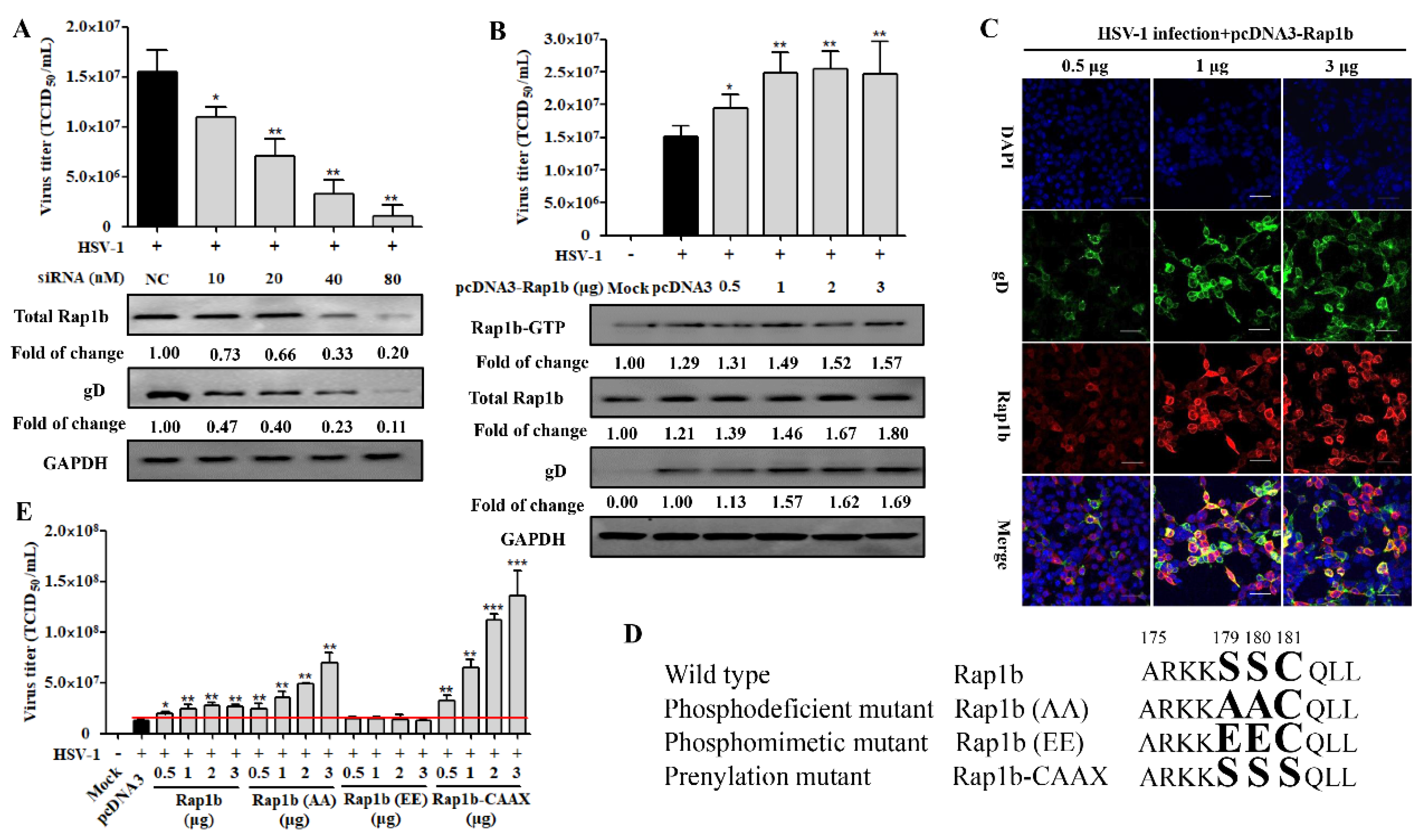
3.2. Rap1b Is Closely Associated with HSV-1 Infection
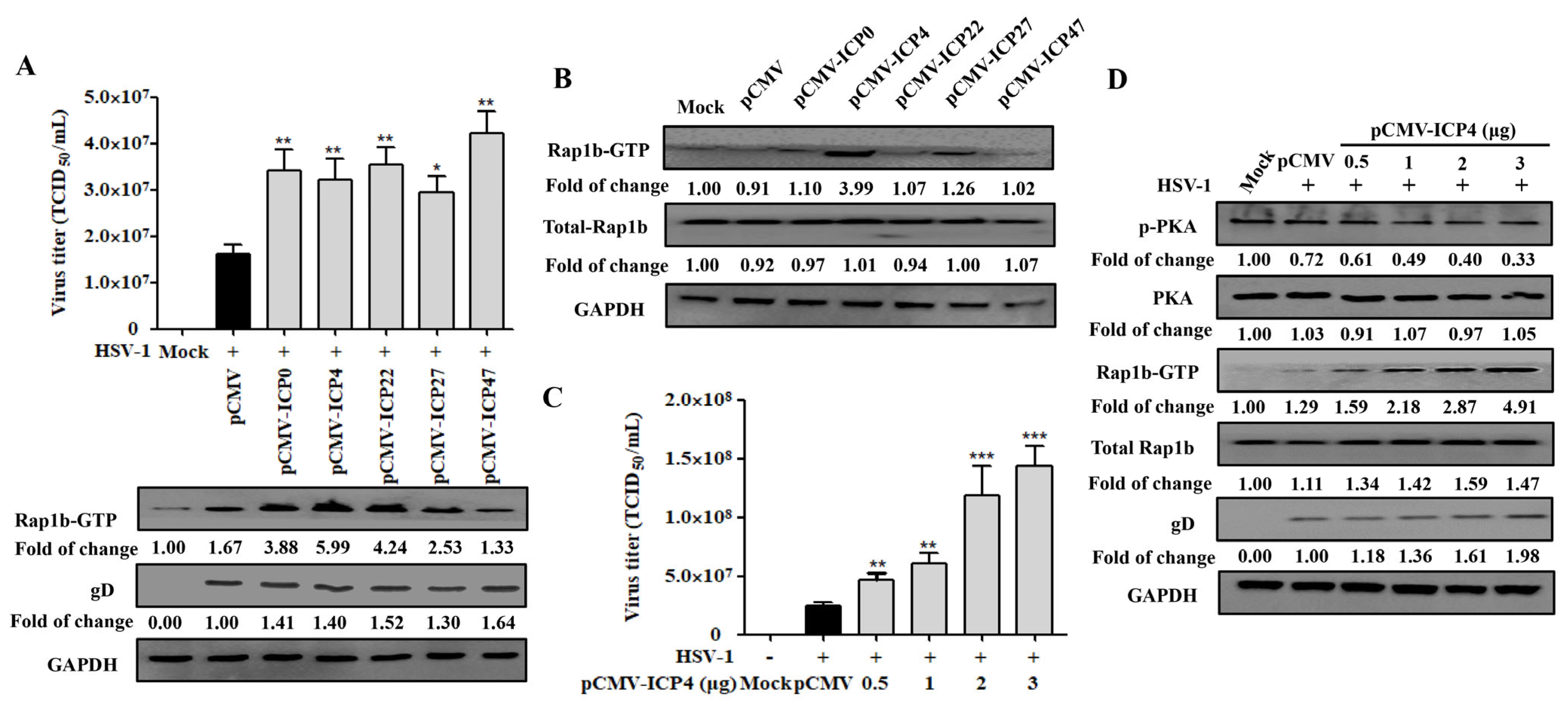
3.3. Dephosphorylation of PKA Is Conducive to HSV-1 Infection
3.4. HSV-1 IE Gene ICP4 Promotes Virus Infection by Inhibiting PKA Activation
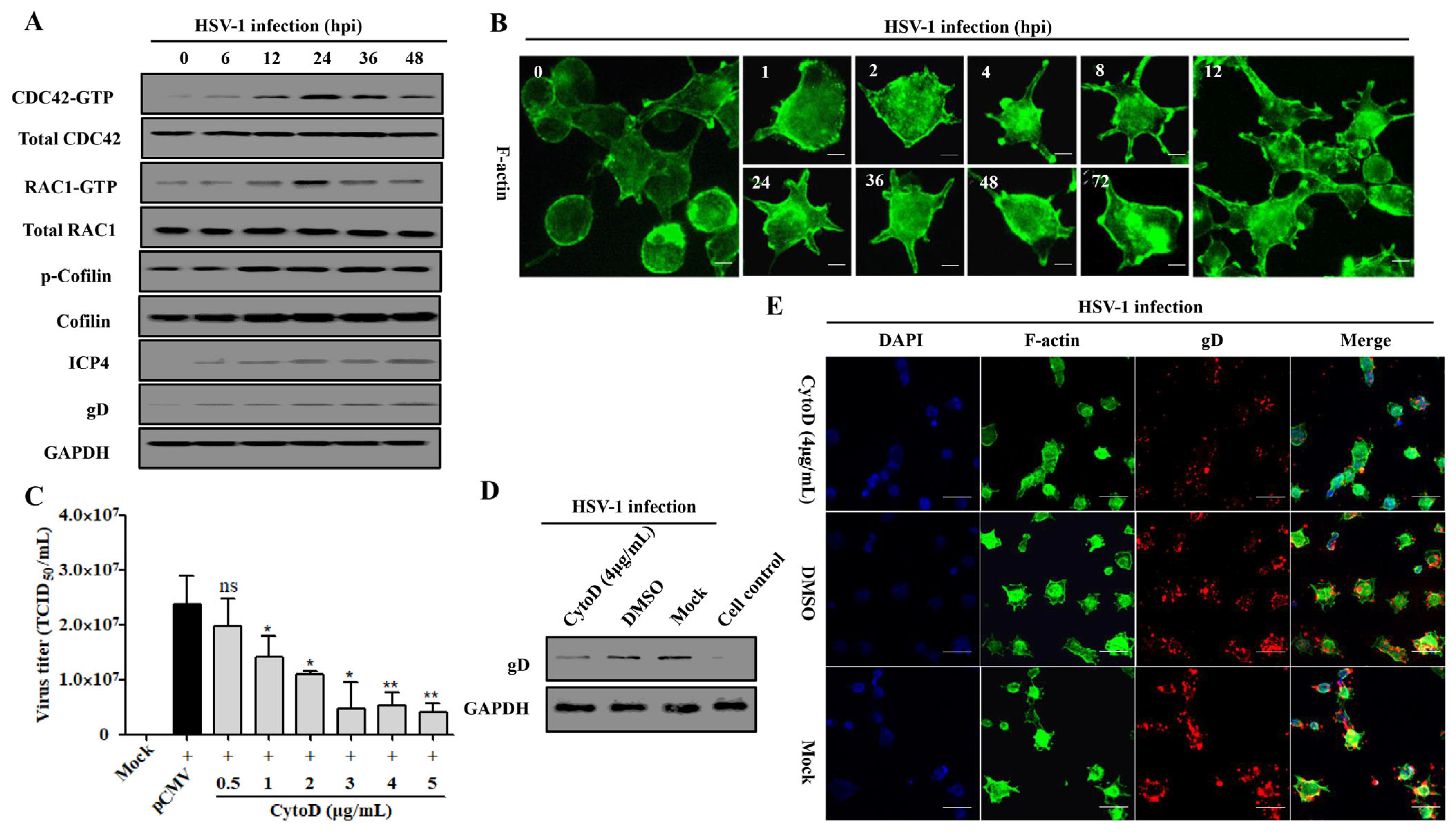
3.5. Rap1b Activation Regulated CDC42 Cascade Signaling Pathway Participates in Viral Infection through Cell Membrane Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caspermeyer, J. Herpes Simplex Viruses: New Relationships between Epidemiology and History. Mol. Biol. Evol. 2020, 37, 1544. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, M.; Kong, M.; Tronstein, E.; Selke, S.; Mikhaylova, A.; Magaret, A.; Huang, M.L.; Johnston, C.; Corey, L.; Wald, A. Herpes Simplex Virus Type 1 Shedding in Tears and Nasal and Oral Mucosa of Healthy Adults. Sex. Transm. Dis. 2016, 43, 756–760. [Google Scholar] [CrossRef]
- Yao, H.W.; Ling, P.; Tung, Y.Y.; Hsu, S.M.; Chen, S.H. In vivo reactivation of latent herpes simplex virus 1 in mice can occur in the brain before occurring in the trigeminal ganglion. J. Virol. 2014, 88, 11264–11270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Bowen, G.N.; Zhou, S.; Cerny, A.; Zacharia, A.; Knipe, D.M.; Finberg, R.W.; Kurt-Jones, E.A. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J. Virol. 2012, 86, 2273–2281. [Google Scholar] [CrossRef]
- Lobo, A.M.; Agelidis, A.M.; Shukla, D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Devilliers, M.J.; Ben Hadj Salah, W.; Barreau, E.; Da Cunha, E.; M’Garrech, M.; Benichou, J.; Labetoulle, M.; Rousseau, A. Ocular manifestations of viral diseases. Rev. Med. Interne 2021, 42, 401–410. [Google Scholar] [CrossRef]
- Stahl, J.P.; Mailles, A. Herpes simplex virus encephalitis update. Curr. Opin. Infect. Dis. 2019, 32, 239–243. [Google Scholar] [CrossRef]
- Gnann, J.W., Jr.; Whitley, R.J. Herpes Simplex Encephalitis: An Update. Curr. Infect. Dis. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Klysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Drayman, N.; Patel, P.; Vistain, L.; Tay, S. HSV-1 single-cell analysis reveals the activation of anti-viral and developmental programs in distinct sub-populations. eLife 2019, 8, e46339. [Google Scholar] [CrossRef]
- Yan, C.; Luo, Z.; Li, W.; Li, X.; Dallmann, R.; Kurihara, H.; Li, Y.F.; He, R.R. Disturbed Yin-Yang balance: Stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1. Acta Pharm. Sin. B 2020, 10, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Honess, R.W.; Roizman, B. Regulation of herpesvirus macromolecular synthesis: Sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 1975, 72, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, N.A.; Schaffer, P.A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol. 1985, 5, 1997–2008. [Google Scholar] [PubMed]
- DeLuca, N.A.; McCarthy, A.M.; Schaffer, P.A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 1985, 56, 558–570. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Engel, R.; Weinberger, L. The HSV-1 ICP4 Transcriptional Auto-Repression Circuit Functions as a Transcriptional “Accelerator” Circuit. Front. Cell. Infect. Microbiol. 2020, 10, 265. [Google Scholar] [CrossRef]
- Rivas, T.; Goodrich, J.A.; Kugel, J.F. The Herpes Simplex Virus 1 Protein ICP4 Acts as both an Activator and a Repressor of Host Genome Transcription during Infection. Mol. Cell. Biol. 2021, 41, e0017121. [Google Scholar] [CrossRef]
- Alekseev, O.; Donegan, W.E.; Donovan, K.R.; Limonnik, V.; Azizkhan-Clifford, J. HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Seyffert, M.; Georgi, F.; Tobler, K.; Bourqui, L.; Anfossi, M.; Michaelsen, K.; Vogt, B.; Greber, U.F.; Fraefel, C. The HSV-1 Transcription Factor ICP4 Confers Liquid-like Properties to Viral Replication Compartments. Int. J. Mol. Sci. 2021, 22, 4447. [Google Scholar] [CrossRef]
- Frasson, I.; Solda, P.; Nadai, M.; Lago, S.; Richter, S.N. Parallel G-quadruplexes recruit the HSV-1 transcription factor ICP4 to promote viral transcription in herpes virus-infected human cells. Commun. Biol. 2021, 4, 510. [Google Scholar] [CrossRef]
- Sodeik, B.; Ebersold, M.W.; Helenius, A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell. Biol. 1997, 136, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Prokop, J.W.; Lorimer, E.; Ntantie, E.; Williams, C.L. Differences in the Phosphorylation-Dependent Regulation of Prenylation of Rap1A and Rap1B. J. Mol. Biol. 2016, 428 Pt B, 4929–4945. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, M.; Song, L.; Zhang, M.; Zhang, J. Function, Significance, and Regulation of Rap1b in Malignancy. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.L.; Brown, N.H. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 2002, 295, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Z.; Guan, J.; Hu, S.; Zhang, J.; Lan, Y.; Zhao, K.; Lu, H.; Song, D.; He, H.; et al. Porcine Hemagglutinating Encephalomyelitis Virus Activation of the Integrin alpha5beta1-FAK-Cofilin Pathway Causes Cytoskeletal Rearrangement to Promote Its Invasion of N2a Cells. J. Virol. 2019, 93, e01736-18. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, M.; Fan, J.; Luo, Y.; Wang, J.; Wang, Y.; Liu, B.; Sun, Y.; Zhao, Q.; Hiscox, J.A.; et al. Avian Hepatitis E Virus ORF2 Protein Interacts with Rap1b to Induce Cytoskeleton Rearrangement That Facilitates Virus Internalization. Microbiol. Spectr. 2022, 10, e0226521. [Google Scholar] [CrossRef]
- Kloc, M.; Uosef, A.; Wosik, J.; Kubiak, J.Z.; Ghobrial, R.M. Virus interactions with the actin cytoskeleton-what we know and do not know about SARS-CoV-2. Arch. Virol. 2022, 167, 737–749. [Google Scholar] [CrossRef]
- Miranda-Saksena, M.; Denes, C.E.; Diefenbach, R.J.; Cunningham, A.L. Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton. Viruses 2018, 10, 92. [Google Scholar] [CrossRef]
- Wang, I.H.; Burckhardt, C.J.; Yakimovich, A.; Greber, U.F. Imaging, Tracking and Computational Analyses of Virus Entry and Egress with the Cytoskeleton. Viruses 2018, 10, 166. [Google Scholar] [CrossRef]
- Sheng, Y.; Ding, S.; Chen, K.; Chen, J.; Wang, S.; Zou, C.; Zhang, J.; Cao, Y.; Huang, A.; Tang, H. Functional analysis of miR-101-3p and Rap1b involved in hepatitis B virus-related hepatocellular carcinoma pathogenesis. Biochem. Cell Biol. 2014, 92, 152–162. [Google Scholar] [CrossRef]
- Fan, M.; Luo, Y.; Zhang, B.; Wang, J.; Chen, T.; Liu, B.; Sun, Y.; Nan, Y.; Hiscox, J.A.; Zhao, Q.; et al. Cell Division Control Protein 42 Interacts with Hepatitis E Virus Capsid Protein and Participates in Hepatitis E Virus Infection. Front. Microbiol. 2021, 12, 775083. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.M.; Grzanka, A.; Zuryn, A.; Stepien, A. The Rho protein family and its role in the cellular cytoskeleton. Postępy Hig. Med. Doświadczalnej 2008, 62, 110–117. [Google Scholar]
- Wang, J.L.; Zhang, J.L.; Chen, W.; Xu, X.F.; Gao, N.; Fan, D.Y.; An, J. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLOS Negl. Trop. Dis. 2010, 4, e809. [Google Scholar] [CrossRef] [PubMed]
- Quetglas, J.I.; Hernaez, B.; Galindo, I.; Munoz-Moreno, R.; Cuesta-Geijo, M.A.; Alonso, C. Small rho GTPases and cholesterol biosynthetic pathway intermediates in African swine fever virus infection. J. Virol. 2012, 86, 1758–1767. [Google Scholar] [CrossRef]
- Jacob, T.; Broeke, C.V.D.; Waesberghe, C.V.; Troys, L.V.; Favoreel, H.W. Pseudorabies virus US3 triggers RhoA phosphorylation to reorganize the actin cytoskeleton. J. Gen. Virol. 2015, 96, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeke, C.; Favoreel, H.W. Actin’ up: Herpesvirus interactions with Rho GTPase signaling. Viruses 2011, 3, 278–292. [Google Scholar] [CrossRef]
- Hoppe, S.; Schelhaas, M.; Jaeger, V.; Liebig, T.; Petermann, P.; Knebel-Morsdorf, D. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J. Gen. Virol. 2006, 87 Pt 12, 3483–3494. [Google Scholar] [CrossRef]
- Sasivimolrattana, T.; Hansasuta, P.; Maneesri Le Grand, S.; Bhattarakosol, P. Actin Polymerization Is Required for Filopodia Formation Supporting HSV-1 Entry into Activated T Cells. Curr. Microbiol. 2021, 79, 23. [Google Scholar] [CrossRef]
- Xiang, Y.; Zheng, K.; Ju, H.; Wang, S.; Pei, Y.; Ding, W.; Chen, Z.; Wang, Q.; Qiu, X.; Zhong, M.; et al. Cofilin 1-mediated biphasic F-actin dynamics of neuronal cells affect herpes simplex virus 1 infection and replication. J. Virol. 2012, 86, 8440–8451. [Google Scholar] [CrossRef]
- Banerjee, A.; Kulkarni, S.; Mukherjee, A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020, 11, 733. [Google Scholar] [CrossRef]
- Heredia, L.; Helguera, P.; de Olmos, S.; Kedikian, G.; Sola Vigo, F.; LaFerla, F.; Staufenbiel, M.; de Olmos, J.; Busciglio, J.; Caceres, A.; et al. Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: A potential mechanism of neuronal dystrophy in Alzheimer’s disease. J. Neurosci. 2006, 26, 6533–6542. [Google Scholar] [CrossRef] [PubMed]
- Kopra, K.; Harma, H. Methods to Monitor Ras Activation State. Methods Mol. Biol. 2021, 2262, 137–167. [Google Scholar] [PubMed]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID50 for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Fezio, W.L. A computer program for estimating LD50 and its confidence limits using modified Behrens-Reed-Muench cumulant method. Drug Chem. Toxicol. 1981, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, S.; Barila, G.; Edreira, M.M.; Hong, K.; Wankhede, M.; Naim, N.; Buck, M.; Altschuler, D.L. Cyclase-associated protein 1 (CAP1) is a prenyl-binding partner of Rap1 GTPase. J. Biol. Chem. 2018, 293, 7659–7673. [Google Scholar] [CrossRef]
- Subramanian, H.; Zahedi, R.P.; Sickmann, A.; Walter, U.; Gambaryan, S. Phosphorylation of CalDAG-GEFI by protein kinase A prevents Rap1b activation. J. Thromb. Haemost. 2013, 11, 1574–1582. [Google Scholar] [CrossRef]
- Petermann, P.; Haase, I.; Knebel-Morsdorf, D. Impact of Rac1 and Cdc42 signaling during early herpes simplex virus type 1 infection of keratinocytes. J. Virol. 2009, 83, 9759–9772. [Google Scholar] [CrossRef]
- Narita, A. ADF/cofilin regulation from a structural viewpoint. J. Muscle Res. Cell Motil. 2020, 41, 141–151. [Google Scholar] [CrossRef]
- Pires, R.H.; Shree, N.; Manu, E.; Guzniczak, E.; Otto, O. Cardiomyocyte mechanodynamics under conditions of actin remodelling. Philos. Trans. R. Soc. B 2019, 374, 20190081. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Perez-Pedrero Sanchez-Belmonte, M.J.; Sanchez-Casado, M.; Moran Gallego, F.J.; Piza Pinilla, R.; Gomez Hernando, C.; Paredes Borrachero, I. Herpes simplex virus type 1 (HSV-1) over-infection in patients with acute respiratory distress syndrome secondary to COVID-19 pneumonia: Impact on mortality. Med. Clin. 2022, 160, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Sufiawati, I.; Tugizov, S.M. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J. Gen. Virol. 2018, 99, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Seessle, J.; Hippchen, T.; Schnitzler, P.; Gsenger, J.; Giese, T.; Merle, U. High rate of HSV-1 reactivation in invasively ventilated COVID-19 patients: Immunological findings. PLoS ONE 2021, 16, e0254129. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, T.; Zeng, M.; Peng, T. Herpes simplex virus type 1 infection activates the Epstein-Barr virus replicative cycle via a CREB-dependent mechanism. Cell. Microbiol. 2012, 14, 546–559. [Google Scholar] [CrossRef]
- Franzoso, F.D.; Seyffert, M.; Vogel, R.; Yakimovich, A.; de Andrade Pereira, B.; Meier, A.F.; Sutter, S.O.; Tobler, K.; Vogt, B.; Greber, U.F.; et al. Cell Cycle-Dependent Expression of Adeno-Associated Virus 2 (AAV2) Rep in Coinfections with Herpes Simplex Virus 1 (HSV-1) Gives Rise to a Mosaic of Cells Replicating either AAV2 or HSV-1. J. Virol. 2017, 91, e00357-17. [Google Scholar] [CrossRef]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef]
- Matthews, J.D.; Morgan, R.; Sleigher, C.; Frey, T.K. Do viruses require the cytoskeleton? Virol. J. 2013, 10, 121. [Google Scholar] [CrossRef]
- Stefanini, L.; Bergmeier, W. RAP GTPases and platelet integrin signaling. Platelets 2019, 30, 41–47. [Google Scholar] [CrossRef]
- Brandt, A.C.; Koehn, O.J.; Williams, C.L. SmgGDS: An Emerging Master Regulator of Prenylation and Trafficking by Small GTPases in the Ras and Rho Families. Front. Mol. Biosci. 2021, 8, 685135. [Google Scholar] [CrossRef]
- Lova, P.; Campus, F.; Lombardi, R.; Cattaneo, M.; Sinigaglia, F.; Balduini, C.; Torti, M. Contribution of protease-activated receptors 1 and 4 and glycoprotein Ib-IX-V in the Gi-independent activation of platelet Rap1B by thrombin. J. Biol. Chem. 2004, 279, 25299–25306. [Google Scholar] [CrossRef]
- Lanfranca, M.P.; Mostafa, H.H.; Davido, D.J. HSV-1 ICP0: An E3 Ubiquitin Ligase That Counteracts Host Intrinsic and Innate Immunity. Cells 2014, 3, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Prod’hon, C.; Machuca, I.; Berthomme, H.; Epstein, A.; Jacquemont, B. Characterization of regulatory functions of the HSV-1 immediate-early protein ICP22. Virology 1996, 226, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lalli, J.; Sedlackova-Slavikova, L.; Rice, S.A. Functional comparison of herpes simplex virus 1 (HSV-1) and HSV-2 ICP27 homologs reveals a role for ICP27 in virion release. J. Virol. 2015, 89, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Raafat, N.; Sadowski-Cron, C.; Mengus, C.; Heberer, M.; Spagnoli, G.C.; Zajac, P. Preventing vaccinia virus class-I epitopes presentation by HSV-ICP47 enhances the immunogenicity of a TAP-independent cancer vaccine epitope. Int. J. Cancer 2012, 131, E659–E669. [Google Scholar] [CrossRef] [PubMed]
- Rajcani, J.; Andrea, V.; Ingeborg, R. Peculiarities of herpes simplex virus (HSV) transcription: An overview. Virus Genes 2004, 28, 293–310. [Google Scholar] [CrossRef]
- Bigalke, J.M.; Heuser, T.; Nicastro, D.; Heldwein, E.E. Membrane deformation and scission by the HSV-1 nuclear egress complex. Nat. Commun. 2014, 5, 4131. [Google Scholar] [CrossRef]
- Kurokawa, K.; Itoh, R.E.; Yoshizaki, H.; Nakamura, Y.O.; Matsuda, M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol. Biol. Cell 2004, 15, 1003–1010. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Wu, J.Q.; Ni, B.; Wen, L.B.; Huang, L.; Liao, Y.; Tong, G.Z.; Ding, C.; Mao, X. Efficient porcine reproductive and respiratory syndrome virus entry in MARC-145 cells requires EGFR-PI3K-AKT-LIMK1-COFILIN signaling pathway. Virus Res. 2016, 225, 23–32. [Google Scholar] [CrossRef]
- Stolp, B.; Fackler, O.T. How HIV takes advantage of the cytoskeleton in entry and replication. Viruses 2011, 3, 293–311. [Google Scholar] [CrossRef]
- Zheng, K.; Xiang, Y.; Wang, X.; Wang, Q.; Zhong, M.; Wang, S.; Wang, X.; Fan, J.; Kitazato, K.; Wang, Y. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio 2014, 5, e00958-13. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Li, F.; Cao, W.; Zeng, Q.; Qin, S.; Wang, Z.; Jia, J.; Xiao, J.; Hu, X.; et al. Hsp90 Inhibitors Inhibit the Entry of Herpes Simplex Virus 1 Into Neuron Cells by Regulating Cofilin-Mediated F-Actin Reorganization. Front. Microbiol. 2021, 12, 799890. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Al-Sammarraie, N.; DiPette, D.J.; Singh, U.S. Metformin impairs Rho GTPase signaling to induce apoptosis in neuroblastoma cells and inhibits growth of tumors in the xenograft mouse model of neuroblastoma. Oncotarget 2014, 5, 11709–11722. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequences (5′–3′) |
|---|---|
| Rap1b-F | GGAATTCATGCGTGAGTATAAGCTAGTCGTTCTTGGC |
| Rap1b-R | CGCTCGAGAAGCAGCTGACATGATGACTTT |
| Rap1b(AA)-F | GAATTCATGCGTGAGATAAAGCTAGTCGTTCTTGGCTCAGGAGGCGTTGG |
| Rap1b(AA)-R | GCTCGAGAAGCAGCTGACAAGCAGCCTTTTTGCGAGCCTTCCCA |
| Rap1b(EE)-F | CCGGAATTCATGCGTGAGTATAAGCTAGTCGTTCTTGGCTAAGGAGGCGTT |
| Rap1b(EE)-R | CCGCTCGAGAAGCAGCTGACACTCCTCCTTTTTGCGAGCCTTCCC |
| Rap1b-CAAX-F | CCGGAATTCATGCGTGAGTATAAGCTAGTCGTTCTTGGCTCAGGAGGCGT |
| Rap1b-CAAX-R | CCGCTCGAGAAGCGAATGTGATGATGACTTTTTGCGAGCCTTCCC |
| ICP0-F | GGAATTCATGGAGCCCCGCCCCGGAG |
| ICP0-R | GCTCTAGATTGTTTTCCCTCGTCCCGGGTCGACGC |
| ICP4-F | GGAATTCATGGCGTCGGAGAACAAGCAGCGC |
| ICP4-R | GCTCTAGACAGCACCCCGTCCCCCTCGAAC |
| ICP22-F | GGAATTCATGGCCGACATTTCCCCAGGCG |
| ICP22-R | GCTCTAGACGGCCGGACCAACGTGTCGCTG |
| ICP27-F | GGAATTCATGGCGACTGACATTGAT |
| ICP27-R | GCTCTAGAAAACAGGGAGTTGCAATAAAAATATTTGC |
| ICP47-F | GGAATTCATGTCAACGGGTTACCGGATT |
| ICP47-R | GCTCTAGAATGTCGTGGGCCCTGGAA |
| siRNA | Sequences (5′–3′) |
|---|---|
| siRap1b(sense) | CCACAUUUAACGAUUUACATT |
| siRap1b(antisense) | UUCUGUUAAUUUGCCGCACTT |
| siRNA(sense)-negative control | UUCUCCGAACGUGUCACGUTT |
| siRNA(antisense)-negative control | ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Ding, J.; Ma, Z. ICP4-Associated Activation of Rap1b Facilitates Herpes Simplex Virus Type I (HSV-1) Infection in Human Corneal Epithelial Cells. Viruses 2023, 15, 1457. https://doi.org/10.3390/v15071457
Zhang B, Ding J, Ma Z. ICP4-Associated Activation of Rap1b Facilitates Herpes Simplex Virus Type I (HSV-1) Infection in Human Corneal Epithelial Cells. Viruses. 2023; 15(7):1457. https://doi.org/10.3390/v15071457
Chicago/Turabian StyleZhang, Beibei, Juntao Ding, and Zhenghai Ma. 2023. "ICP4-Associated Activation of Rap1b Facilitates Herpes Simplex Virus Type I (HSV-1) Infection in Human Corneal Epithelial Cells" Viruses 15, no. 7: 1457. https://doi.org/10.3390/v15071457
APA StyleZhang, B., Ding, J., & Ma, Z. (2023). ICP4-Associated Activation of Rap1b Facilitates Herpes Simplex Virus Type I (HSV-1) Infection in Human Corneal Epithelial Cells. Viruses, 15(7), 1457. https://doi.org/10.3390/v15071457







