Filter-Free, Harmless, and Single-Wavelength Far UV-C Germicidal Light for Reducing Airborne Pathogenic Viral Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. 207 nm Far UV-C Lamp Development and Characterization
2.2. Cell Lines
2.3. Viral Culture
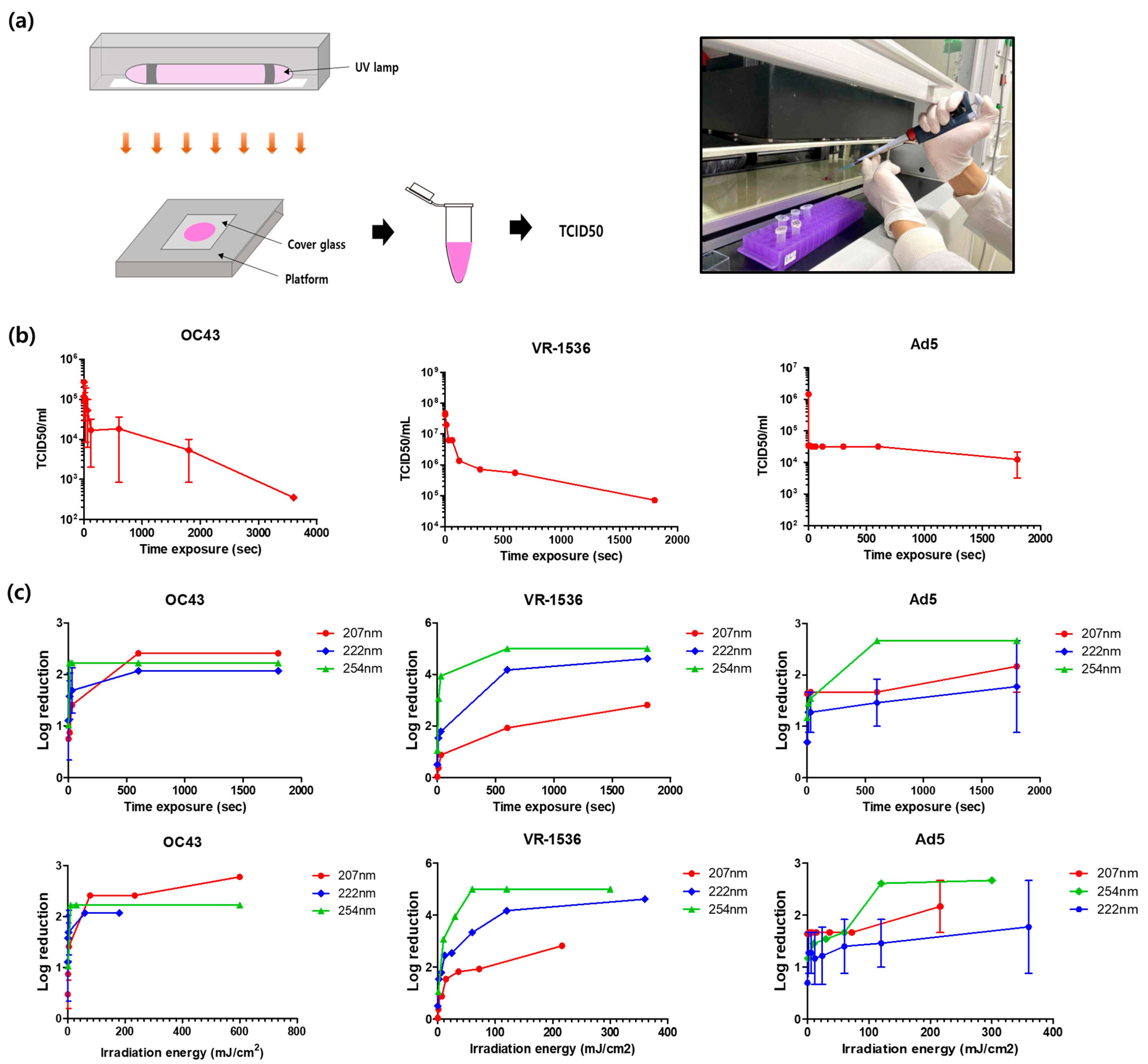
2.4. UV-C Light Exposure to the Virus
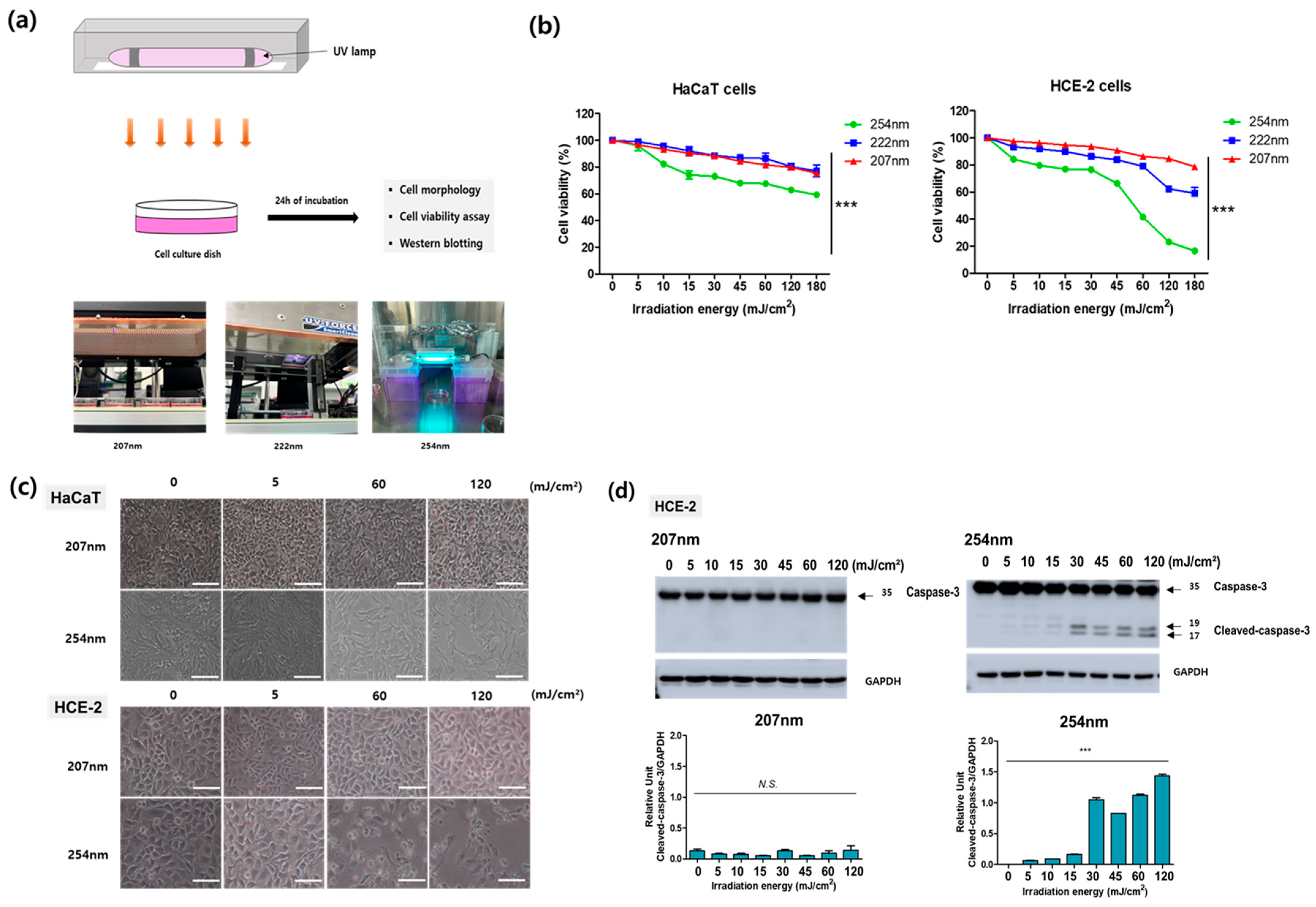
2.5. UV-C Light Exposure to Cells
2.6. Cell-Viability Assay
2.7. Western Blotting
3. Results
3.1. 207 nm Single-Wavelength Far UV-C Lamp Development
3.2. Safety Evaluation of UV-C Light Irradiated onto Human Skin and Corneal Cells
3.3. Evaluation of UV-C Light against Viral Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. Available online: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 29 March 2020).
- Somsen, G.A.; van Rijn, C.; Kooij, S.; Bem, R.A.; Bonn, D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir. Med. 2020, 8, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.U.; Maqbool, F.; Bhatti, Z.A.; Khan, A.U. Epidemiology of COVID-19 Pandemic: Recovery and mortality ratio around the globe. Pak. J. Med. Sci. 2020, 36, S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, M.; Lee, S.; Lee, Y.J. Discovering spatiotemporal patterns of COVID-19 pandemic in South Korea. Sci. Rep. 2021, 11, 24470. [Google Scholar] [CrossRef]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef]
- Qian, H.; Zheng, X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J. Thorac. Dis. 2018, 10, S2295–S2304. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Nardell, E.A. Air Disinfection for Airborne Infection Control with a Focus on COVID-19: Why Germicidal UV is Essential. Photochem. Photobiol. 2021, 97, 493–497. [Google Scholar] [CrossRef]
- Buchan, A.G.; Yang, L.; Atkinson, K.D. Predicting airborne coronavirus inactivation by far-UVC in populated rooms using a high-fidelity coupled radiation-CFD model. Sci. Rep. 2020, 10, 19659. [Google Scholar] [CrossRef]
- Bergman, R.S. Germicidal UV Sources and Systems. Photochem. Photobiol. 2021, 97, 466–470. [Google Scholar] [CrossRef]
- Eadie, E.; Hiwar, W.; Fletcher, L.; Tidswell, E.; O’Mahoney, P.; Buonanno, M.; Welch, D.; Adamson, C.S.; Brenner, D.J.; Noakes, C.; et al. Far-UVC (222 nm) efficiently inactivates an airborne pathogen in a room-sized chamber. Sci. Rep. 2022, 12, 4373. [Google Scholar] [CrossRef]
- Kitagawa, H.; Nomura, T.; Nazmul, T.; Kawano, R.; Omori, K.; Shigemoto, N.; Sakaguchi, T.; Ohge, H. Effect of intermittent irradiation and fluence-response of 222 nm ultraviolet light on SARS-CoV-2 contamination. Photodiagnosis Photodyn. Ther. 2021, 33, 102184. [Google Scholar] [CrossRef]
- Buonanno, M.; Randers-Pehrson, G.; Bigelow, A.W.; Trivedi, S.; Lowy, F.D.; Spotnitz, H.M.; Hammer, S.M.; Brenner, D.J. 207-nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. I: In Vitro Studies. PLoS ONE 2013, 8, e76968. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Stanislauskas, M.; Ponnaiya, B.; Bigelow, A.W.; Randers-Pehrson, G.; Xu, Y.; Shuryak, I.; Smilenov, L.; Owens, D.M.; Brenner, D.J. 207-nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. II: In-Vivo Safety Studies. PLoS ONE 2016, 11, e0138418. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Welch, D.; Brenner, D.J. Exposure of Human Skin Models to KrCl Excimer Lamps: The Impact of Optical Filtering. Photochem. Photobiol. 2021, 97, 517–523. [Google Scholar] [CrossRef]
- Hickerson, R.P.; Conneely, M.J.; Hirata Tsutsumi, S.K.; Wood, K.; Jackson, D.N.; Ibbotson, S.H.; Eadie, E. Minimal, superficial DNA damage in human skin from filtered far-ultraviolet C. Br. J. Dermatol. 2021, 184, 1197–1199. [Google Scholar] [CrossRef]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID50 for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012, 11, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Kowalczuk, C.; Priestner, M.; Pearson, A.; Saunders, R.; Bouffler, S. Wavelength dependence of cellular responses in human melanocytes and melanoma cells following exposure to ultraviolet radiation. Int. J. Radiat. Biol. 2006, 82, 781–792. [Google Scholar] [CrossRef]
- Lindenbach, B.D. Measuring HCV infectivity produced in cell culture and in vivo. Methods Mol. Biol. 2009, 510, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yang, M.; Marabella, I.A.; McGee, D.A.J.; Aboubakr, H.; Goyal, S.; Hogan, C.J.J.; Olson, B.A.; Torremorell, M. Greater than 3-Log Reduction in Viable Coronavirus Aerosol Concentration in Ducted Ultraviolet-C (UV-C) Systems. Environ. Sci. Technol. 2021, 55, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Smith, L.M.; Li, X.; Yancey, O.; Franzblau, A.; Dvonch, J.T.; Xi, C.; Neitzel, R.L. Monitoring SARS-CoV-2 in air and on surfaces and estimating infection risk in buildings and buses on a university campus. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Heßling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—Review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control. 2020, 15, Doc08. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.C.; Fisher, D.; Hing, E.C.H.; Hanjing, L.; Lin, Y.Y.; Lim, J.; Chen, O.W.; Chye, L.T. Disinfection capabilities of a 222 nm wavelength ultraviolet lighting device: A pilot study. J. Wound Care 2021, 30, 96–104. [Google Scholar] [CrossRef]
- Ramos, C.C.R.; Roque, J.L.A.; Sarmiento, D.B.; Suarez, L.E.G.; Sunio, J.T.P.; Tabungar, K.I.B.; Tengco, G.S.C.; Rio, P.C.; Hilario, A.L. Use of ultraviolet-C in environmental sterilization in hospitals: A systematic review on efficacy and safety. Int. J. Health Sci. 2020, 14, 52–65. [Google Scholar]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Biasin, M.; Strizzi, S.; Bianco, A.; Macchi, A.; Utyro, O.; Pareschi, G.; Loffreda, A.; Cavalleri, A.; Lualdi, M.; Trabattoni, D.; et al. UV and violet light can Neutralize SARS-CoV-2 Infectivity. J. Photochem. Photobiol. 2022, 10, 100107. [Google Scholar] [CrossRef]
- Sliney, D.H.; Stuck, B.E. A Need to Revise Human Exposure Limits for Ultraviolet UV-C Radiation. Photochem. Photobiol. 2021, 97, 485–492. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Lai, K.M.; Rudnick, S.N.; Houseman, E.A.; First, M.W.; Milton, D.K. Characterization of UVC light sensitivity of vaccinia virus. Appl. Environ. Microbiol. 2007, 73, 5760–5766. [Google Scholar] [CrossRef] [Green Version]
- Gerba, C.P.; Gramos, D.M.; Nwachuku, N. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 2002, 68, 5167–5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blatchley, E.R., III; Brenner, D.J.; Claus, H.; Cowan, T.E.; Linden, K.G.; Liu, Y.; Mao, T.; Park, S.-J.; Piper, P.J.; Simons, R.M. Far UV-C radiation: An emerging tool for pandemic control. Crit. Rev. Environ. Sci. Technol. 2022, 53, 733–753. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation). Health Phys. 2004, 87, 171–186. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Preiss, J.W.; Setlow, R. Spectra of Some Amino Acids, Peptides, Nucleic Acids, and Protein in the Vacuum Ultraviolet. J. Chem. Phys. 1956, 25, 138–141. [Google Scholar] [CrossRef]
- Alhasaniah, A.; Sherratt, J.M.; O’Neill, A.C. The Impact of Ultraviolet Radiation on Barrier Function in Human Skin: Molecular Mechanisms and Topical Therapeutics. Curr. Med. Chem. 2018, 25, 5503–5511. [Google Scholar] [CrossRef]
- Green, H.; Boll, J.; Parrish, J.A.; Kochevar, I.E.; Oseroff, A.R. Cytotoxicity and Mutagenicity of Low Intensity, 248 and 193 nm Excimer Laser Radiation in Mammalian Cells1. Cancer Res. 1987, 47, 410–413. [Google Scholar]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brenner, D.J. Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light. Radiat. Res. 2017, 187, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Narita, K.; Asano, K.; Morimoto, Y.; Igarashi, T.; Nakane, A. Chronic irradiation with 222-nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses. PLoS ONE 2018, 13, e0201259. [Google Scholar] [CrossRef]
- Fukui, T.; Niikura, T.; Oda, T.; Kumabe, Y.; Ohashi, H.; Sasaki, M.; Igarashi, T.; Kunisada, M.; Yamano, N.; Oe, K.; et al. Exploratory clinical trial on the safety and bactericidal effect of 222-nm ultraviolet C irradiation in healthy humans. PLoS ONE 2020, 15, e0235948. [Google Scholar] [CrossRef]
- Yamano, N.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaki-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term Effects of 222-nm ultraviolet radiation C Sterilizing Lamps on Mice Susceptible to Ultraviolet Radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Welch, D.; Buonanno, M.; Grilj, V.; Shuryak, I.; Crickmore, C.; Bigelow, A.W.; Randers-Pehrson, G.; Johnson, G.W.; Brenner, D.J. Far-UVC light: A new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018, 8, 2752. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 10285. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; You, Y.H.; Besaratinia, A. Mutations induced by ultraviolet light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef]
- Eadie, E.; Barnard, I.M.R.; Ibbotson, S.H.; Wood, K. Extreme Exposure to Filtered Far-UVC: A Case Study. Photochem. Photobiol. 2021, 97, 527–531. [Google Scholar] [CrossRef]
- Kaidzu, S.; Sugihara, K.; Sasaki, M.; Nishiaki, A.; Ohashi, H.; Igarashi, T.; Tanito, M. Re-Evaluation of Rat Corneal Damage by Short-Wavelength UV Revealed Extremely Less Hazardous Property of Far-UV-C. Photochem. Photobiol. 2021, 97, 505–516. [Google Scholar] [CrossRef]

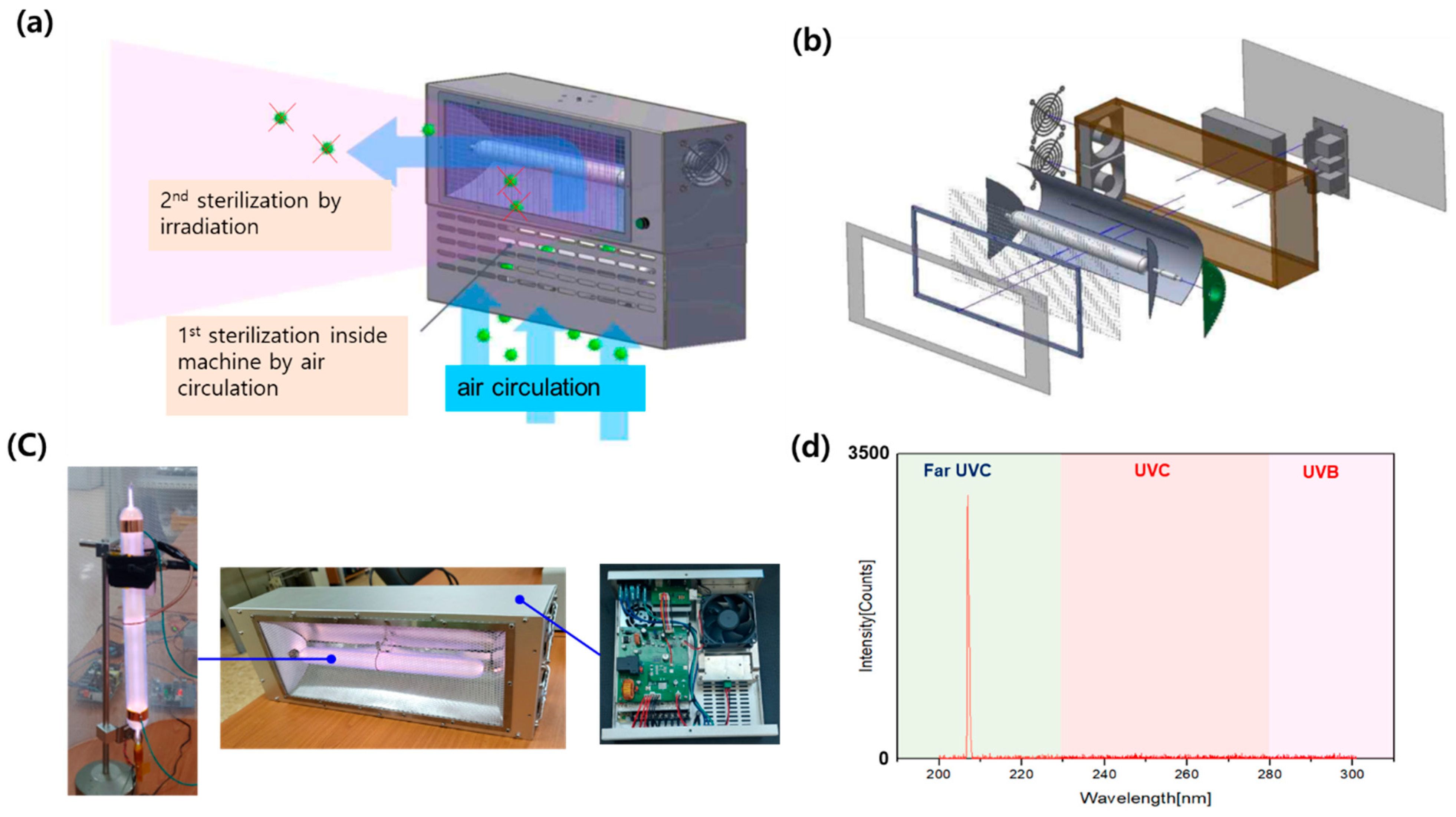
| Aperture Size | Peak Wavelength | Input Power | Consumption Power | Weight | Lamp Lifer Time |
|---|---|---|---|---|---|
| 368 × 150 (mm) | Far UVC 207 nm | AC100~AC240 V | 80 w/h | 3.8 kg | 3000 h |
| Time Exposure | Control | 1 s | 10 s | 30 s | 10 min | 30 min |
|---|---|---|---|---|---|---|
| Energy (mJ/cm2) | 0 | 0.13 | 1.3 | 3.9 | 78 | 234 |
| TCID50/mL | 2.15 × 105 | 3.86 × 104 | 2.94 × 104 | 8.43 × 103 | 8.43 × 102 * | 8.43 × 102 * |
| Log reduction | 0 | 0.75 | 0.87 | 1.41 | 2.41 | 2.41 |
| Inactivation (%) | 0 | 82 | 86 | 96 | 99 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, C.-S.; Muthukutty, P.; Jang, H.K.; Kim, Y.-H.; Lee, D.H.; Yoo, S.Y. Filter-Free, Harmless, and Single-Wavelength Far UV-C Germicidal Light for Reducing Airborne Pathogenic Viral Infection. Viruses 2023, 15, 1463. https://doi.org/10.3390/v15071463
Truong C-S, Muthukutty P, Jang HK, Kim Y-H, Lee DH, Yoo SY. Filter-Free, Harmless, and Single-Wavelength Far UV-C Germicidal Light for Reducing Airborne Pathogenic Viral Infection. Viruses. 2023; 15(7):1463. https://doi.org/10.3390/v15071463
Chicago/Turabian StyleTruong, Cao-Sang, Palaniyandi Muthukutty, Ho Kyung Jang, Young-Ho Kim, Dong Hoon Lee, and So Young Yoo. 2023. "Filter-Free, Harmless, and Single-Wavelength Far UV-C Germicidal Light for Reducing Airborne Pathogenic Viral Infection" Viruses 15, no. 7: 1463. https://doi.org/10.3390/v15071463






