In Silico Screening of Inhibitors of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid Constructs of FRET Substrates
2.3. Plasmid Constructs of VEEV nsP2 Protease
2.4. Expression and Purification of the VEEV nsP2 Cysteine Protease
2.5. Expression and Purification of His-Tag Free FRET Protein Substrates
2.6. Continuous FRET Assay
2.7. Discontinuous Gel-Based Assay
2.8. Time-Dependent Inhibition of VEEV nsP2 by CA074 and Its Derivatives
2.9. Crystallization
2.10. Cell-Based ELISA Assays
2.11. Virus Yield Reduction Assay
2.12. Virtual Screening and Focused Set
3. Results
3.1. In Silico and In Vitro Screening
3.2. CA074me, but Not E64d, Effectively Reduces Virus Yield in A549 Cells
3.3. CA074me Effectively Reduces Virus Yield in Primary Neuronal Cells
3.4. X-ray Crystal Structure of CA074 Bound to the VEEV nsP2 Cysteine Protease
3.5. Effects of Divalent Metal Ions on Covalent Inhibition
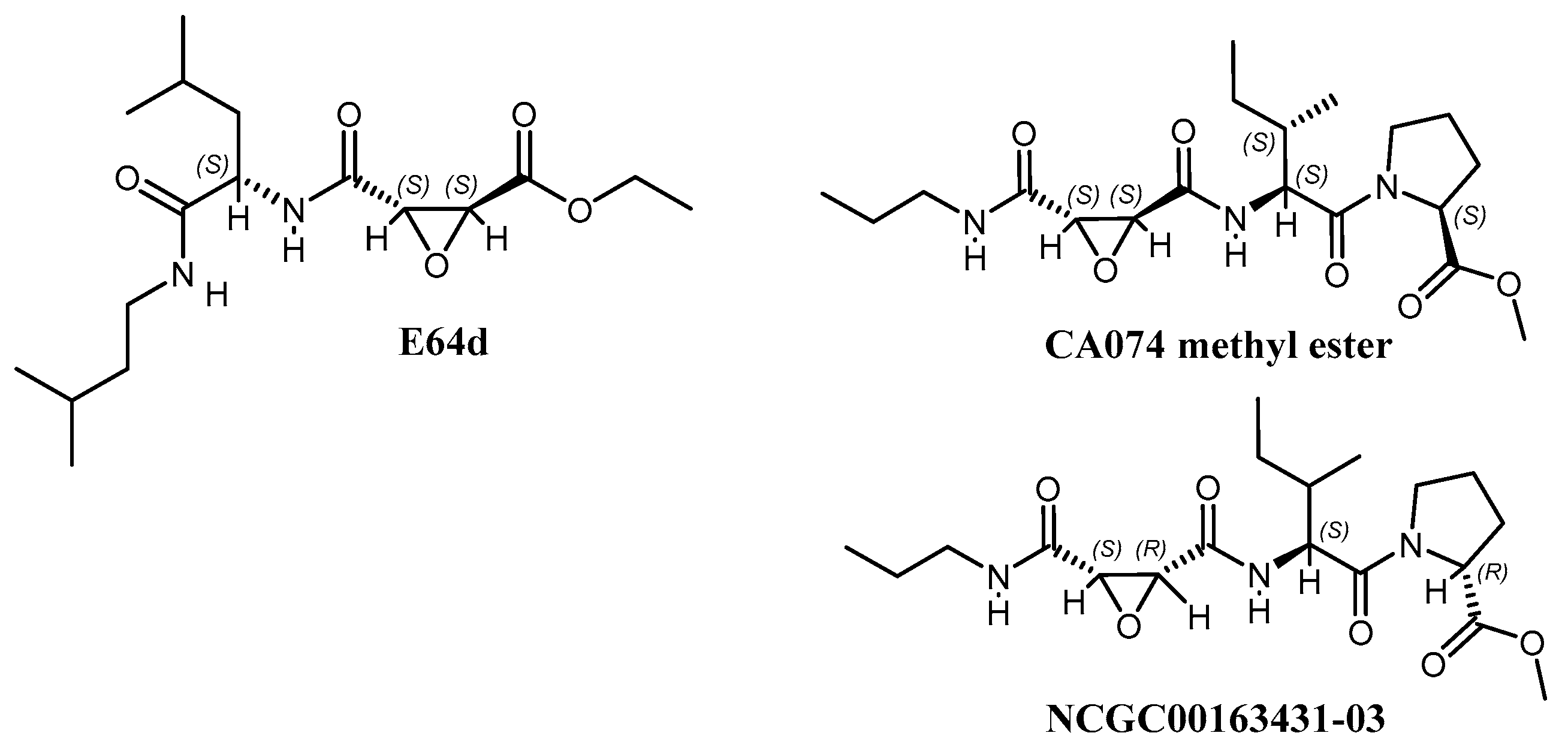
3.6. Synthesized Derivatives of Hybrid CA074/E64d
3.7. Other Compounds Found in the NCATS Library with Anti-Alphaviral Activity in Primary Neurons
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Registration ID | Well | Parent MW (g/mol) | IC50 (µM) | EC50 (µM) | CC50 (µM) |
| MLS001179882-01 | A02 | 292.7 | >200 | >50 | >50 |
| MLS001197204-01 | A03 | 349.3 | 30 ± 20 | >50 | >50 |
| MLS001215963-01 | A04 | 321.3 | 16 ± 3 | NT | |
| MLS001215939-01 | A05 | 339.3 | |||
| MLS001215927-01 | A06 | 325.3 | |||
| MLS001220062-01 | B02 | 556.6 | |||
| MLS001198393-01 | B03 | 442.7 | >200 | >16.67 | >50 |
| MLS001222475-01 | B04 | 431.5 | |||
| MLS001177992-01 | B05 | 303.7 | NT | >50 | >50 |
| MLS001178292-01 | B06 | 364.8 | >200 | >50 | >50 |
| MLS001172952-01 | C02 | 383.8 | >200 | >50 | >50 |
| MLS001178294-01 | C03 | 400.8 | |||
| MLS001097193-01 | C04 | 343.8 | |||
| MLS001222477-01 | C05 | 445.6 | |||
| MLS001173297-01 | C06 | 318.8 | |||
| MLS000577775-01 | D02 | 369.4 | |||
| MLS000553324-01 | D03 | 475.6 | |||
| MLS000760808-01 | D04 | 401.4 | |||
| MLS001030320-01 | D05 | 281.2 | >100 | >50 | >50 |
| MLS001002335-01 | D06 | 361.4 | |||
| MLS001224091-01 | E02 | 387.8 | |||
| MLS000712897-01 | E03 | 316.2 | |||
| MLS001097173-01 | E04 | 323.3 | |||
| MLS000760597-01 | E05 | 338.4 | |||
| MLS001215951-01 | E06 | 331.4 | 18 ± 7 | >50 | >50 |
| MLS000061991-01 | F02 | 254.3 | |||
| MLS000712758-01 | F03 | 270.2 | |||
| MLS001215987-01 | F04 | 382.3 | 14 ± 3 | >50 | >50 |
| MLS000711802-01 | F05 | 271.7 | |||
| MLS000772304-01 | F06 | 263.3 | |||
| MLS001097176-01 | G02 | 393.2 | |||
| MLS001083053-01 | G03 | 284.3 | |||
| MLS000877947-01 | G04 | 292.7 | >200 | >50 | >50 |
| MLS000544331-01 | G05 | 356.4 | |||
| MLS000773385-01 | H02 | 355.4 | 15 ± 2 | >50 | >50 |
| MLS001033759-01 | H03 | 333.4 | |||
| MLS000717223-01 | H04 | 515.6 | |||
| MLS001212815-01 | H05 | 392.9 | >200 | >50 | >50 |
| NCGC00182390-04 | G06 | 404.5 | |||
| NCGC00247785-05 | H06 | 474.6 |

| NCGC ID | Rat_SD/Microsomes t1/2 (m) | Permeability (PAMPA_Pion_Lipid × 10−6 cm/s) | Solubility (μg/mL) | |
|---|---|---|---|---|
| NCGC00389066-01 | ND | >2165 | 10.4 | |
| NCGC00484087-01 | 1.5 | 1089.1 | 4.1 | |
| NCGC00389063-01 | 2.3 | 744.4 | 34.5 | |
| NCGC00165840-02 | >30 | 294.2 | 16.7 | |
| NCGC00420692-01 | 5 | 222.3 | 46.7 | |
| NCGC00478985-01 | 4.7 | 204.3 | 30.8 | |
| NCGC00478984-01 | 11.8 | 175.5 | 31.3 | |
| NCGC00475747-01 | 5.2 | 166.7 | 27.4 | |
| NCGC00475748-01 | >30 | 138.8 | >51 | |
| NCGC00167328-04 | E64d | 1.5 | 89 | 24 |
| NCGC00484012-01 | CA074 | >30 | <1 | >77 |
| NCGC00420691-01 | 6 | 75.9 | 50.1 | |
| NCGC00420693-01 | 4.1 | 67.3 | 55 | |
| NCGC00478986-01 | 6.2 | 62.3 | 29.8 | |
| NCGC00488908-01 | >30 | 7.6 | >60 | |
| NCGC00481260-01 | 54.5 | 11.8 | >49 | |
| NCGC00408977-01 | >30 | 8.4 | >51 | |
| NCGC00384435-05 | 31.2 | 7.8 | >49 | |
| NCGC00481259-01 | >30 | 1.2 | >49 | |
| NCGC00384435-04 | 31.6 | 9 | >49 | |
| NCGC00384435-02 | 22.5 | 16.5 | >49 | |
| NCGC00476231-01 | >30 | 12.3 | >47 | |
| NCGC00390171-01 | >30 | 5.6 | >45 | |
| NCGC00389061-01 | 2.5 | 28.2 | >45 | |
| NCGC00476230-01 | 23.6 | 12.9 | >44 | |
| NCGC00420690-01 | 6.3 | 31.7 | 40 | |
| NCGC00484094-01 | CA074me | 11.1 | 33 | 22 |
| NCGC00023595-05 | E64c | >30 | <1 | 33 |
| NCGC00488906-01 | >30 | ND | 15.9 | |
| NCGC00487434-01 | >30 | ND | 22 | |
| NCGC00389074-01 | 1.5 | ND | 20.7 | |
| NCGC00488907-01 | 6.4 | <4.3 |
References
- Reichert, E.; Clase, A.; Bacetty, A.; Larsen, J. Alphavirus antiviral drug development: Scientific gap analysis and prospective research areas. Biosecur. Bioterror. 2009, 7, 413–427. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Rom, A.M.; Holland, L.E. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch. Virol. 2010, 155, 2035–2039. [Google Scholar] [CrossRef]
- Hu, X.; Compton, J.R.; Leary, D.H.; Olson, M.A.; Lee, M.S.; Cheung, J.; Ye, W.; Ferrer, M.; Southall, N.; Jadhav, A.; et al. Kinetic, Mutational, and Structural Studies of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease. Biochemistry 2016, 55, 3007–3019. [Google Scholar] [CrossRef] [Green Version]
- Division of Vector-Borne Diseases. National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). 2021. Available online: https://www.cdc.gov/easternequineencephalitis/statistics-maps/historic-data.html (accessed on 28 May 2023).
- Gardner, C.L.; Burke, C.W.; Tesfay, M.Z.; Glass, P.J.; Klimstra, W.B.; Ryman, K.D. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: Impact of altered cell tropism on pathogenesis. J. Virol. 2008, 82, 10634–10646. [Google Scholar] [CrossRef] [Green Version]
- Maclachlan; James, N.; Dubovi, E.J. Fenner’s Veterinary Virology, 5th ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–581. [Google Scholar]
- Pittman, P.R.; Makuch, R.S.; Mangiafico, J.A.; Cannon, T.L.; Gibbs, P.H.; Peters, C.J. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine 1996, 14, 337–343. [Google Scholar] [CrossRef]
- Pratt, W.D.; Gibbs, P.; Pitt, M.L.; Schmaljohn, A.L. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine 1998, 16, 1056–1064. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Stephenson, E.H. Protective efficacies of live attenuated and formaldehyde-inactivated Venezuelan equine encephalitis virus vaccines against aerosol challenge in hamsters. J. Clin. Microbiol. 1984, 19, 429–431. [Google Scholar] [CrossRef] [Green Version]
- Strauss, E.G.; Strauss, J.H. Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2234–2236. [Google Scholar] [CrossRef]
- Morazzani, E.M.; Compton, J.R.; Leary, D.H.; Berry, A.V.; Hu, X.; Marugan, J.; Glass, P.J.; Legler, P.M. Proteolytic cleavage of host proteins by the Group IV viral proteases of Venezuelan equine encephalitis virus and Zika virus. Antivir. Res. 2019, 164, 106–122. [Google Scholar] [CrossRef]
- Zhou, Z.; Jia, X.; Xue, Q.; Dou, Z.; Ma, Y.; Zhao, Z.; Jiang, Z.; He, B.; Jin, Q.; Wang, J. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-I-like receptor-mediated innate immune response. Proc. Natl. Acad. Sci. USA 2014, 111, E245–E254. [Google Scholar] [CrossRef]
- Doctor, K.Z.; Gilmour, E.; Recarte, M.; Beatty, T.R.; Shifa, I.; Stangel, M.; Schwisow, J.; Leary, D.H.; Legler, P.M. Automated SSHHPS Analysis Predicts a Potential Host Protein Target Common to Several Neuroinvasive (+)ssRNA Viruses. Viruses 2023, 15, 542. [Google Scholar] [CrossRef]
- Falk, M.M.; Grigera, P.R.; Bergmann, I.E.; Zibert, A.; Multhaup, G.; Beck, E. Foot-and-mouth disease virus protease 3C induces specific proteolytic cleavage of host cell histone H3. J. Virol. 1990, 64, 748–756. [Google Scholar]
- Grigera, P.R.; Tisminetzky, S.G. Histone H3 modification in BHK cells infected with foot-and-mouth disease virus. Virology 1984, 136, 10–19. [Google Scholar]
- Reynolds, N.D.; Aceves, N.M.; Liu, J.L.; Compton, J.R.; Leary, D.H.; Freitas, B.T.; Pegan, S.D.; Doctor, K.Z.; Wu, F.Y.; Hu, X.; et al. The SARS-CoV-2 SSHHPS Recognized by the Papain-like Protease. ACS Infect. Dis. 2021, 7, 1483–1502. [Google Scholar] [CrossRef]
- Lim, B.-K.; Peter, A.K.; Xiong, D.; Narezkina, A.; Yung, A.; Dalton, N.D.; Hwang, K.-K.; Yajima, T.; Chen, J.; Knowlton, K.U. Inhibition of Coxsackievirus-associated dystrophin cleavage prevents cardiomyopathy. J. Clin. Investig. 2013, 123, 5146–5151. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Compton, J.R.; Legler, P.M. Analysis of Group IV Viral SSHHPS Using In Vitro and In Silico Methods. J. Vis. Exp. 2019, 154, e60421. [Google Scholar] [CrossRef]
- Smith, E.C.; Sexton, N.R.; Denison, M.R. Thinking Outside the Triangle: Replication Fidelity of the Largest RNA Viruses. Annu. Rev. Virol. 2014, 1, 111–132. [Google Scholar] [CrossRef]
- Dahlin, J.L.; Nissink, J.W.; Strasser, J.M.; Francis, S.; Higgins, L.; Zhou, H.; Zhang, Z.; Walters, M.A. PAINS in the assay: Chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J. Med. Chem. 2015, 58, 2091–2113. [Google Scholar] [CrossRef] [Green Version]
- Ruge, D.R.; Dunning, F.M.; Piazza, T.M.; Molles, B.E.; Adler, M.; Zeytin, F.N.; Tucker, W.C. Detection of six serotypes of botulinum neurotoxin using fluorogenic reporters. Anal. Biochem. 2011, 411, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Kitz, R.; Wilson, I.B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J. Biol. Chem. 1962, 237, 3245–3249. [Google Scholar]
- Murshudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. Sect. D Biol. Crystallogr. 1997, 53, 240–255. [Google Scholar]
- Brunger, A.T.; Adams, P.D.; Clore, G.M.; DeLano, W.L.; Gros, P.; Grosse-Kunstleve, R.W.; Jiang, J.S.; Kuszewski, J.; Nilges, M.; Pannu, N.S.; et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 905–921. [Google Scholar]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Spurgers, K.B.; Hurt, C.R.; Cohen, J.W.; Eccelston, L.T.; Lind, C.M.; Lingappa, V.R.; Glass, P.J. Validation of a cell-based ELISA as a screening tool identifying anti-alphavirus small-molecule inhibitors. J. Virol. Methods 2013, 193, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.T.; White, M.A.; Watowich, S.J. The crystal structure of the Venezuelan equine encephalitis alphavirus nsP2 protease. Structure 2006, 14, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Cao, S.; Su, P.C.; Patel, R.; Shah, D.; Chokshi, H.B.; Szukala, R.; Johnson, M.E.; Hevener, K.E. Hit identification and optimization in virtual screening: Practical recommendations based on a critical literature analysis. J. Med. Chem. 2013, 56, 6560–6572. [Google Scholar] [CrossRef] [Green Version]
- Montaser, M.; Lalmanach, G.; Mach, L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol. Chem. 2002, 383, 1305–1308. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangere, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rebe, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef]
- Deregnaucourt, J.; Andre, E.; Tisne-Versailles, J. Use of Antihistamine Agents for the Preventive or Early Treatment of Inflammatory Syndromes, in Particular Those Triggered by Togaviruses. U.S. Patent 8242110B2, 2012. [Google Scholar]
- Schroeder, C.E.; Yao, T.; Sotsky, J.; Smith, R.A.; Roy, S.; Chu, Y.K.; Guo, H.; Tower, N.A.; Noah, J.W.; McKellip, S.; et al. Development of (E)-2-((1,4-dimethylpiperazin-2-ylidene)amino)-5-nitro-N-phenylbenzamide, ML336: Novel 2-amidinophenylbenzamides as potent inhibitors of venezuelan equine encephalitis virus. J. Med. Chem. 2014, 57, 8608–8621. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Parvathareddy, J.; Yang, D.; Bansal, S.; O’Connell, K.; Golden, J.E.; Jonsson, C.B. Emergence and Magnitude of ML336 Resistance in Venezuelan Equine Encephalitis Virus Depend on the Microenvironment. J. Virol. 2020, 94, e00317-20. [Google Scholar]
- Chung, D.; Schroeder, C.E.; Sotsky, J.; Yao, T.; Roy, S.; Smith, R.A.; Tower, N.A.; Noah, J.W.; McKellip, S.; Sosa, M.; et al. ML336: Development of Quinazolinone-Based Inhibitors against Venezuelan Equine Encephalitis Virus (VEEV). In Probe Reports from the NIH Molecular Libraries Program; Bethesda: Rockville, MD, USA, 2010. [Google Scholar]
- Pluimer, B.R.; Colt, M.; Zhao, Z. G Protein-Coupled Receptors in the Mammalian Blood-Brain Barrier. Front. Cell. Neurosci. 2020, 14, 139. [Google Scholar] [CrossRef]
- Chang, J.; Mancuso, M.R.; Maier, C.; Liang, X.; Yuki, K.; Yang, L.; Kwong, J.W.; Wang, J.; Rao, V.; Vallon, M.; et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017, 23, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Paessler, S.; Aguilar, P.; Anishchenko, M.; Wang, H.-Q.; Aronson, J.; Campbell, G.; Cararra, A.-S.; Weaver, S.C. The Hamster as an Animal Model for Eastern Equine Encephalitisb. J. Infect. Dis. 2004, 189, 2072–2076. [Google Scholar]


| AVERAGE TITER | |||||||
|---|---|---|---|---|---|---|---|
| CA074me | E64D | DMSO | |||||
| [I] | 200 µM | 100 µM | 50 µM | 25µM | 100 µM | 50 µM | |
| VEEV | 1.40 × 102 | 5.49 × 102 | 2.50 × 104 | 1.33 × 105 | 1.48 × 107 | 4.17 × 107 | 5.15 × 108 |
| EEEV | 3.12 × 104 | 3.20 × 106 | 3.18 × 107 | 4.57 × 107 | 2.28 × 108 | 2.27 × 108 | 3.13 × 108 |
| WEEV | 9.73 × 102 | 3.17 × 104 | 5.98 × 105 | 1.54 × 106 | 2.13 × 106 | 2.16 × 106 | 3.08 × 106 |
| CHIKV | 1.58 × 102 | 2.35 × 102 | 2.23 × 102 | 3.42 × 102 | 2.70 × 103 | 1.07 × 104 | 2.99 × 104 |
| STDEV | |||||||
| CA074me | E64D | DMSO | |||||
| [I] | 200 µM | 100 µM | 50 µM | 25µM | 100 µM | 50 µM | |
| VEEV | 7.5 × 101 | 2.0 × 102 | 5.3 × 103 | 4.3 × 104 | 5.4 × 106 | 8.9 × 106 | 9.5 × 107 |
| EEEV | 7.3 × 103 | 2.3 × 105 | 1.5 × 106 | 4.5 × 106 | 9.6 × 107 | 5.9 × 107 | 4.3 × 107 |
| WEEV | 1.8 × 102 | 9.1 × 103 | 2.2 × 105 | 7.1 × 105 | 9.9 × 105 | 9.9 × 105 | 1.1 × 106 |
| CHIKV | 2.3 × 101 | 1.3 × 101 | 5.4 × 101 | 6.4 × 101 | 2.6 × 102 | 9.2 × 102 | 5.3 × 103 |
| PDB ID | 8T8N |
| Space group | P2(1)2(1)2(1) |
| Unit Cell Dimensions (Å) | 60.76, 63.58, 86.28 |
| Wavelength (Å) | 1.54 |
| Resolution Range (Å) a | 63.58–2.32 (2.42–2.32) |
| Unique Reflections | 14,251 (1625) |
| Rsym | 0.051 (0.172) |
| I/σI | 18.03 (6.08) |
| Completeness | 94.6 (92.8) |
| Redundancy | 13.35 (12.79) |
| Refinement Statistics: | |
| Resolution (Å) | 51.19–2.32 |
| No. of reflections | 13,506 |
| Rfactor | 0.191 |
| Rfree b | 0.200 (5%) |
| Number of Atoms: | |
| Protein | 2577 |
| Solvent | 136 |
| Other | 26 |
| Average B-factors (Å2) | |
| Protein | 19.9 |
| Solvent | 23.3 |
| R.m.s.d. from ideal geometry: | |
| Bond lengths (Å) | 0.006 |
| Bond angles (degrees) | 1.23 |
| Ramachandran plot | |
| Most favored regions (%) | 90.0% |
| Additional allowed regions (%) | 10.0% |
| Generously allowed regions (%) | 0.0% |
| Disallowed regions (%) | 0.0% |
| Metal | Inhibitor | Ki (µM) | k2 (min−1) | k2/Ki (µM−1min−1) |
|---|---|---|---|---|
| No Metal | CA074 | 1200 ± 500 | 0.23 ± 0.09 | 0.0002 ± 0.0001 |
| MnCl2 | CA074 | 410 ± 60 | 0.08 ± 0.03 | 0.0002 ± 0.0001 |
| MgCl2 | CA074 | 210 ± 90 | 0.04 ± 0.01 | 0.0002 ± 0.0001 |
| CaCl2 | CA074 | 2000 ± 1000 | 0.3 ± 0.2 | 0.0002 ± 0.0002 |
| No Metal | E64d | 40 ± 30 | 0.10 ± 0.06 | 0.002 ± 0.002 |
| Compound ID | VEEV-Trinidad EC50 (µM) | VEEV-TC83 EC50 (µM) | VEEV nsP2 PROTEASE k2/Ki (M−1 s−1) | CHIKV EC50 (µM) | WEEV EC50 (µM) | EEEV EC50 (µM) | CC50 (µM) | CC50 (µM) |
|---|---|---|---|---|---|---|---|---|
| Cell Line | A549 | HeLa | Protease | U2OS | HeLa | A549 | HeLa | A549 |
| NCGC00488909-01 | 1.76 | 1.48 | 862 | 9.85 | 5.02 | 43 | >50 | >50 |
| NCGC00484087-01 | 7.90 | 2.17 | 100 | (Not Tested) | 5.74 | 13.23 | >50 | >50 |
| NCGC00488907-01 | 5.97 | 5.22 | 86 | 25.92 | 8.76 | <50 | >50 | >50 |
| NCGC00488904-01 | 4.29 | 4.09 | 61 | 14.83 | 5.15 | 16.19 | >50 | >50 |
| NCGC00488908-01 | 9.45 | 9.23 | 23 | 50.00 | 14.55 | ≥100 | >50 | >50 |
| NCGC00488906-01 | 26.4 | 26.62 | 12 | 18.48 | 20.36 | >100 | >50 | >50 |
| Compound ID | Microsomal Stability t1/2 | PAMPA | Solubility |
|---|---|---|---|
| Min | ×10−6 cm/s | µg/mL | |
| NCGC00484087-01 | 1.5 | 1089.1 | 4.1 |
| NCGC00484012-01 | >30 | <1 | >77 |
| NCGC00484094-01 | 11.1 | 33 | 22 |
| NCGC00488906-01 | >30 | ND | 15.9 |
| NCGC00488907-01 | 6.4 | <4.3 | ND |
| NCGC00488908-01 | >30 | 7.6 | >60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Morazzani, E.; Compton, J.R.; Harmon, M.; Soloveva, V.; Glass, P.J.; Garcia, A.D.; Marugan, J.J.; Legler, P.M. In Silico Screening of Inhibitors of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease. Viruses 2023, 15, 1503. https://doi.org/10.3390/v15071503
Hu X, Morazzani E, Compton JR, Harmon M, Soloveva V, Glass PJ, Garcia AD, Marugan JJ, Legler PM. In Silico Screening of Inhibitors of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease. Viruses. 2023; 15(7):1503. https://doi.org/10.3390/v15071503
Chicago/Turabian StyleHu, Xin, Elaine Morazzani, Jaimee R. Compton, Moeshia Harmon, Veronica Soloveva, Pamela J. Glass, Andres Dulcey Garcia, Juan J. Marugan, and Patricia M. Legler. 2023. "In Silico Screening of Inhibitors of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease" Viruses 15, no. 7: 1503. https://doi.org/10.3390/v15071503
APA StyleHu, X., Morazzani, E., Compton, J. R., Harmon, M., Soloveva, V., Glass, P. J., Garcia, A. D., Marugan, J. J., & Legler, P. M. (2023). In Silico Screening of Inhibitors of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease. Viruses, 15(7), 1503. https://doi.org/10.3390/v15071503






