Oral Probenecid for Nonhospitalized Adults with Symptomatic Mild-to-Moderate COVID-19
Abstract
:1. Introduction
2. Methods
2.1. Trial Design and Oversight
2.2. Patient Selection
2.3. Study Procedures
2.4. Statistical Analysis
2.5. Role of the Funding Source
3. Results
3.1. Patient Demographics and Clinical Characteristics
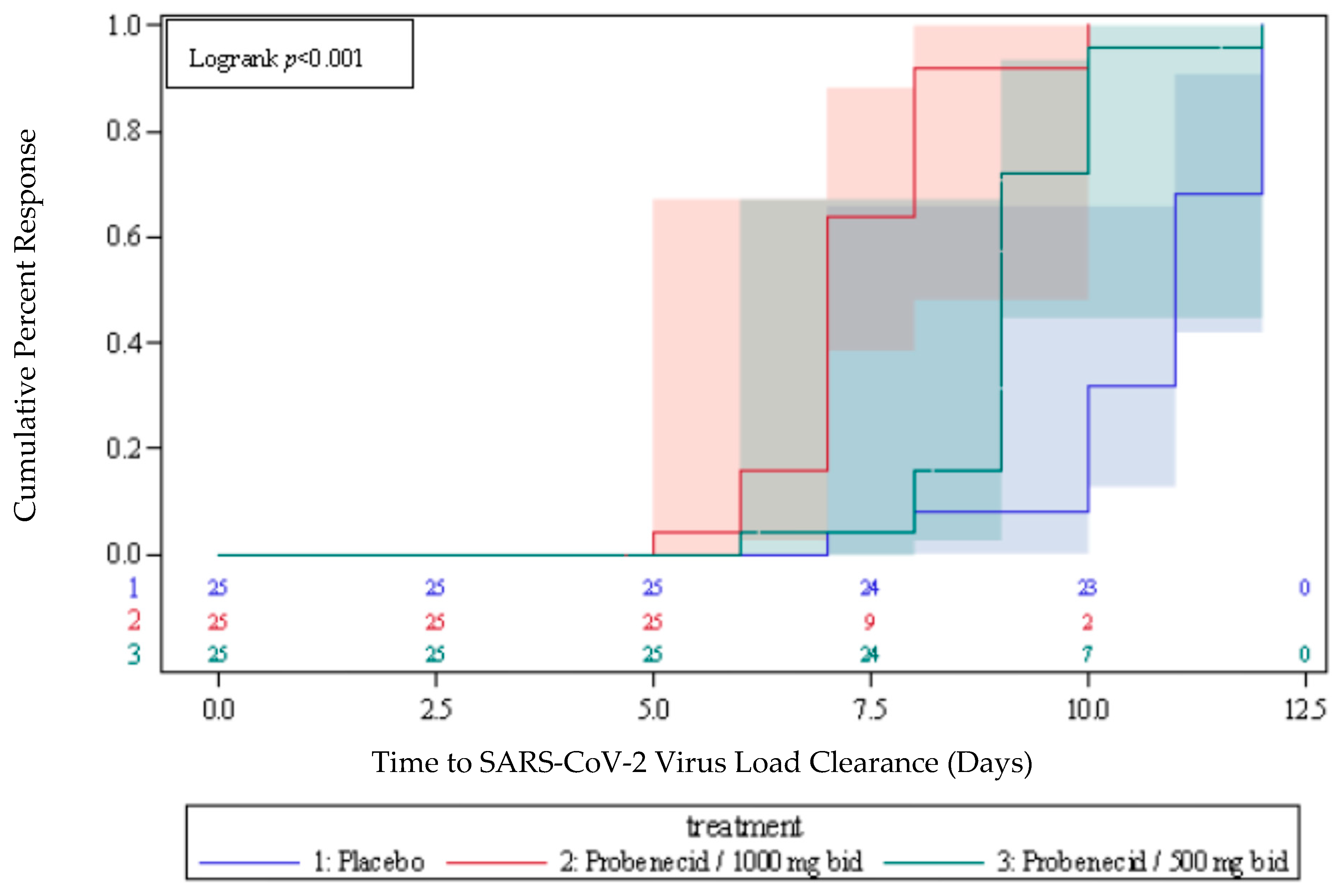
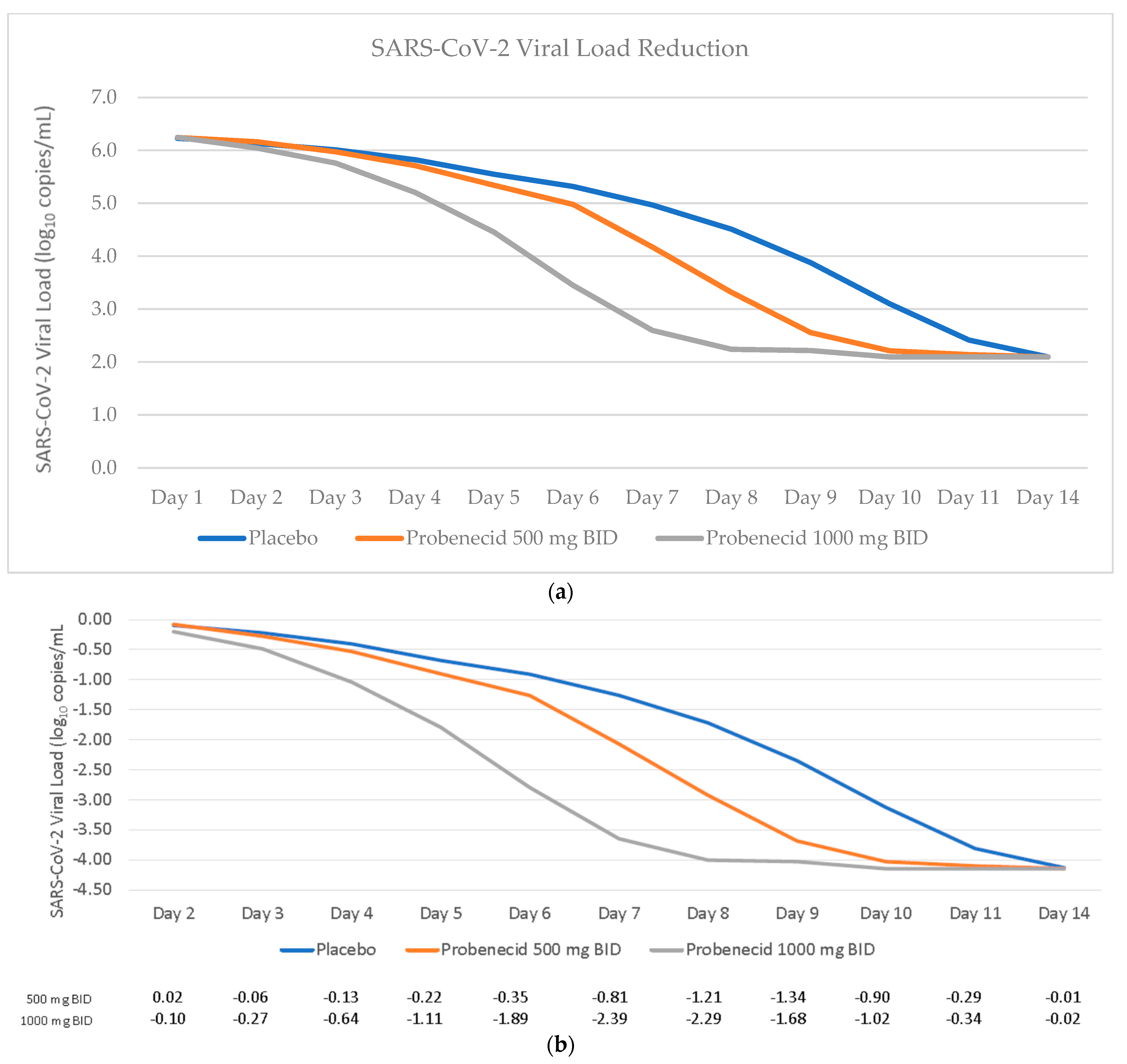
3.2. Time to Viral Clearance and Viral Dynamics
3.3. Time to Symptom Resolution
3.4. Disease Progression
3.5. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- WHO. Coronavirus (COVID-19) Dashboard/WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/?mapFilter=deaths (accessed on 26 January 2023).
- Ponnampalli, S.; Venkata Suryanarayana Birudukota, N.; Kamal, A. COVID-19: Vaccines and therapeutics. Bioorg. Med. Chem. Lett. 2022, 75, 128987. [Google Scholar] [CrossRef] [PubMed]
- FDA. Announces Bebtelovimab Is Not Currently Authorized in Any US Region. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region (accessed on 26 January 2023).
- Ying, B.; Whitener, B.; VanBlargan, L.A.; Hassan, A.O.; Shrihari, S.; Liang, C.Y.; Karl, C.E.; Mackin, S.; Chen, R.E.; Kafai, N.M.; et al. Protective activity of mRNA vaccines against ancestral and variant SARS-CoV-2 strains. Sci. Transl. Med. 2022, 14, eabm3302. [Google Scholar] [CrossRef]
- Iketani, S.; Mohri, H.; Culbertson, B.; Hong, S.J.; Duan, Y.; Luck, M.I.; Annavajhala, M.K.; Guo, Y.; Sheng, Z.; Uhlemann, A.-C.; et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 2023, 613, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory Syncytial Virus: Virology, Reverse Genetics, and Pathogenesis of Disease. In Challenges and Opportunities for Respiratory Syncytial Virus Vaccines. Current Topics in Microbiology and Immunology; Anderson, L., Graham, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume V372, pp. 3–38. [Google Scholar]
- Perwitasari, O.; Yan, X.; Johnson, S.; White, C.; Brooks, P.; Tompkins, S.M.; Tripp, R.A. Targeting organic anion transporter 3 with probenecid as a novel anti-influenza a virus strategy. Antimicrob. Agents Chemother. 2013, 57, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.; Hogan, R.J.; Martin, D.E.; Blahunka, K.; Sancilio, F.D.; Balyan, R.; Lovern, M.; Still, R.; Tripp, R.A. Probenecid potently inhibits SARS-CoV-2 replication in vivo and in vitro. Sci. Rep. 2021, 11, 8085. [Google Scholar] [CrossRef]
- Rosli, S.; Kirby, F.J.; Lawlor, K.E.; Rainczuk, K.; Drummond, G.R.; Mansell, A.; Tate, M.D. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br. J. Pharmacol. 2019, 176, 3834–3844. [Google Scholar] [CrossRef] [Green Version]
- Silverman, W.; Locovei, S.; Dahl, G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 2008, 295, C761–C767. [Google Scholar] [CrossRef] [Green Version]
- Weaver, A.K.; Head, J.R.; Gould, C.F.; Carlton, E.J.; Remais, J.V. Environmental Factors Influencing COVID-19 Incidence and Severity. Annu. Rev. Public Health 2022, 43, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Chiu, Y.; Stremska, M.; Lucas, C.; Poon, I.; Tung, K.; Elliott, M.; Desai, B.; Lorenz, U.; Bayliss, D.; et al. Pannexin 1 channels facilitate communication between T cells to restrict the severity of airway inflammation. Immunity 2021, 54, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.H.; Wiebelhaus, V.D.; Russe, H.F.; Peck, H.M.; McKinney, S.E. Benemid: An anticatabolite; Its pharmacological properties. Fed. Proc. 1950, 9, 258. [Google Scholar]
- Boger, W.P.; Strickland, S.C. Probenecid (Benemid): Its uses and side effects in 2502 Patients. Am. Med. Assoc. Arch. Intern. Med. 1955, 95, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Koch, S.E.; Tranter, M.; Rubinstein, J. The history and future of probenecid. Cardiovasc. Toxicol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- WHO. COVID-19 Case Definitions. World Health Organization, 2020. Available online: https://apps.who.int/iris/rest/bitstreams/1322790/retrieve (accessed on 26 January 2023).
- PROBENECID-Probenecid Tablet, Film Coated Package Insert. Lannett Company, Inc. Revision 1/2021. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=ab497fd8-00c3-4364-b003-b39d21fbdf38&type=display (accessed on 26 January 2023).
- Tripp, R.A.; Martin, D.E. Repurposing Probenecid to Inhibit SARS-CoV-2, Influenza Virus, and Respiratory Syncytial Virus (RSV) Replication. Viruses 2022, 14, 612. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Li, H.M.; Bogoyevitch, M.A.; Jans, D.A. c-Jun N-terminal kinase activity is required for efficient respiratory syncytial virus production. Biochem. Biophys. Res. Commun. 2017, 483, 64–68. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, L.; Xie, Z. Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus. Front. Cell Infect. Microbiol. 2022, 12, 858629. [Google Scholar] [CrossRef]
- Cheng, M.H.; Kim, S.J. Inhibitory Effect of Probenecid on Osteoclast Formation via JNK.; ROS and COX-2. Biomol. Ther. 2020, 28, 104–109. [Google Scholar] [CrossRef]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Khandelwal, N.; Thachamvally, R.; Tripathi, B.N.; Barua, S.; Kashyap, S.K.; Maherchandani, S.; Kumar, N. Role of MAPK/MNK1 signaling in virus replication. Virus Res. 2018, 253, 48–61. [Google Scholar] [CrossRef]
- Sehgal, V.; Ram, P.T. Network Motifs in JNK Signaling. Genes Cancer 2013, 4, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martovetsky, G.; Tee, J.B.; Nigam, S.K. Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol. Pharmacol. 2013, 84, 808–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karyakarte, R.P.; Das, R.; Taji, N.; Yanamandra, S.; Shende, S.; Joshi, S.; Karekar, B.; Bawale, R.; Tiwari, R.; Jadhav, M.; et al. An Early and Preliminary Assessment of the Clinical Severity of the Emerging SARS-CoV-2 Omicron Variants in Maharashtra, India. Cureus 2022, 14, e31352. [Google Scholar] [CrossRef] [PubMed]

| Probenecid 1000 mg | Probenecid 500 mg | Placebo | |
|---|---|---|---|
| N = 25 | N = 25 | N = 25 | |
| Age, median (range) years | 39.1 (23–65) | 40.5 (24–64) | 44.0 (22–64) |
| Sex | |||
| Male | 15 | 19 | 18 |
| Female | 10 | 6 | 7 |
| Race | |||
| Asian | 25 | 25 | 24 |
| Black or African American | 1 | ||
| BMI, median (range) kg/m2 | 24.8 (20.0–30.5) | 25.3 (19.3–33.2) | 25.3 (20.2–30.0) |
| Co-Morbidities | |||
| Diabetes Mellitus (%) | 2 (8%) | 2 (8%) | 5 (20%) |
| Hypertension | 4 (16%) | 6 (24%) | 6 (24%) |
| Baseline SARS-CoV-2 RNA, median (range) log10 copies/mL | 6.3 (6.00–6.48) | 6.3 (6.01–6.54) | 6.2 (5.95–6.55) |
| Vaccine Status | |||
| Y | 17 (68%) | 17 (68%) | 16 (64%) |
| N | 8 (32%) | 8 (32%) | 9 (36%) |
| # of Symptoms/Patient at Baseline (median, range) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Frequency of Symptoms at Baseline | |||
| Fever | 25 (100%) | 25 (100%) | 24 (96%) |
| Cough | 21 (84%) | 23 (92%) | 20 (80%) |
| Sore Throat | 18 (72%) | 19 (76%) | 17 (68%) |
| Body Aches and Pains | 9 (36%) | 7 (28%) | 7 (28%) |
| Loss of Taste/Smell | 6 (24%) | 5 (20%) | 9 (36%) |
| Headache | 5 (20%) | 4 (16%) | 5 (20%) |
| Other | 11 (44%) | 10 (40%) | 14 (56%) |
| Tiredness | 1 (4%) | 1 (4%) | 0 |
| Shortness of Breath/Difficulty Breathing | 0 | 0 | 1 (4%) |
| Visit | Statistics | Placebo | Probenecid 500 mg Bid | Probenecid 1000 mg Bid |
|---|---|---|---|---|
| Baseline | n | 25 | 25 | 25 |
| Baseline | Mean | 2 | 2 | 2 |
| Baseline | SD | 0 | 0 | 0 |
| Day 1 | n | 25 | 25 | 25 |
| Day 1 | Mean | 2 | 2 | 2 |
| Day 1 | SD | 0 | 0 | 0 |
| Day 3 ± 1 | n | 25 | 25 | 25 |
| Day 3 ± 1 | Mean | 2 | 2 | 2 |
| Day 3 ± 1 | SD | 0 | 0 | 0.2 |
| Change from baseline to Day 3 ± 1 | LSmean | 0.04 | 0 | 0.04 |
| Change from baseline to Day 3 ± 1 | 95% CI for LSmean | (−0.0251, 0.1051) | (−0.0651, 0.0651) | (−0.0251, 0.1051) |
| Change from baseline to Day 3 ± 1 | p-value | 0.2247 | >0.9999 | 0.2247 |
| Day 5 ± 1 | n | 25 | 25 | 25 |
| Day 5 ± 1 | Mean | 1.8 | 2 | 1.7 |
| Day 5 ± 1 | SD | 0.37 | 0.2 | 0.56 |
| Change from baseline to Day 5 ± 1 | LSmean | 0.28 | −0.12 | 0.16 |
| Change from baseline to Day 5 ± 1 | 95% CI for LSmean | (0.0521, 0.5079) | (−0.3479, 0.1079) | (−0.0679, 0.3879) |
| Change from baseline to Day 5 ± 1 | p-value | 0.0167 | 0.2973 | 0.1659 |
| Day 10 ± 1 | n | 25 | 25 | 25 |
| Day 10 ± 1 | Mean | 1 | 0.2 | 0.1 |
| Day 10 ± 1 | SD | 0.89 | 0.55 | 0.4 |
| Change from baseline to Day 10 ± 1 | LSmean | 0.08 | 0.88 | 0.96 |
| Change from baseline to Day 10 ± 1 | 95% CI for LSmean | (−0.2849, 0.4449) | (0.5151, 1.2449) | (0.5951, 1.3249) |
| Change from baseline to Day 10 ± 1 | p-value | 0.6634 | <0.0001 | <0.0001 |
| Day 15 ± 1 | n | 25 | 25 | 25 |
| Day 15 ± 1 | Mean | 0.1 | 0 | 0 |
| Day 15 ± 1 | SD | 0.4 | 0 | 0 |
| Change from baseline to Day 15 ± 1 | LSmean | 0 | 0.08 | 0.08 |
| Change from baseline to Day 15 ± 1 | 95% CI for LSmean | (−0.1302, 0.1302) | (−0.0502, 0.2102) | (−0.0502, 0.2102) |
| Change from baseline to Day 15 ± 1 | p-value | >0.9999 | 0.2247 | 0.2247 |
| Day 28 ± 3 | n | 25 | 25 | 25 |
| Day 28 ± 3 | Mean | 0 | 0 | 0 |
| Day 28 ± 3 | SD | 0 | 0 | 0 |
| Change from baseline to Day 28 ± 3 | LSmean | 0 | 0 | 0 |
| Change from baseline to Day 28 ± 3 | 95% CI for LSmean | (., .) | (., .) | (., .) |
| Change from baseline to Day 28 ± 3 | p-value | <0.0001 | <0.0001 | <0.0001 |
| System Organ Class Preferred Term | Statistics | Placebo (N = 25) | Probenecid/500 mg Bid (N = 25) | Probenecid/1000 mg Bid (N = 25) | Overall (N = 75) |
|---|---|---|---|---|---|
| GASTROINTESTINAL DISORDERS | n (%) E | 2 (8.0) 2 | 2 (8.0) 2 | 3 (12.0) 5 | 7 (9.3) 9 |
| CONSTIPATION | n (%) E | 1 (4.0) 1 | 1 (4.0) 1 | 1 (4.0) 1 | 3 (4.0) 3 |
| HYPERCHLORHYDRIA | n (%) E | 1 (4.0) 1 | 1 (4.0) 1 | 0 (0.0) 0 | 2 (2.7) 2 |
| NAUSEA | n (%) E | 0 (0.0) 0 | 0 (0.0) 0 | 2 (8.0) 2 | 2 (2.7) 2 |
| VOMITING | n (%) E | 0 (0.0) 0 | 0 (0.0) 0 | 2 (8.0) 2 | 2 (2.7) 2 |
| GENERAL DISORDERS & ADMINISTRATION SITE CONDITIONS | n (%) E | 1 (4.0) 1 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.3) 1 |
| ASTHENIA | n (%) E | 1 (4.0) 1 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.3) 1 |
| NERVOUS SYSTEM DISORDERS | n (%) E | 0 (0.0) 0 | 1 (4.0) 1 | 0 (0.0) 0 | 1 (1.3) 1 |
| HEADACHE | n (%) E | 0 (0.0) 0 | 1 (4.0) 1 | 0 (0.0) 0 | 1 (1.3) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, D.E.; Pandey, N.; Chavda, P.; Singh, G.; Sutariya, R.; Sancilio, F.; Tripp, R.A. Oral Probenecid for Nonhospitalized Adults with Symptomatic Mild-to-Moderate COVID-19. Viruses 2023, 15, 1508. https://doi.org/10.3390/v15071508
Martin DE, Pandey N, Chavda P, Singh G, Sutariya R, Sancilio F, Tripp RA. Oral Probenecid for Nonhospitalized Adults with Symptomatic Mild-to-Moderate COVID-19. Viruses. 2023; 15(7):1508. https://doi.org/10.3390/v15071508
Chicago/Turabian StyleMartin, David E., Neelam Pandey, Purvi Chavda, Gurpreet Singh, Rakesh Sutariya, Frederic Sancilio, and Ralph A. Tripp. 2023. "Oral Probenecid for Nonhospitalized Adults with Symptomatic Mild-to-Moderate COVID-19" Viruses 15, no. 7: 1508. https://doi.org/10.3390/v15071508







