Plasma Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) as a Possible Biomarker for Severe COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. PCSK9 ELISA
2.3. Statistical Analysis
3. Results
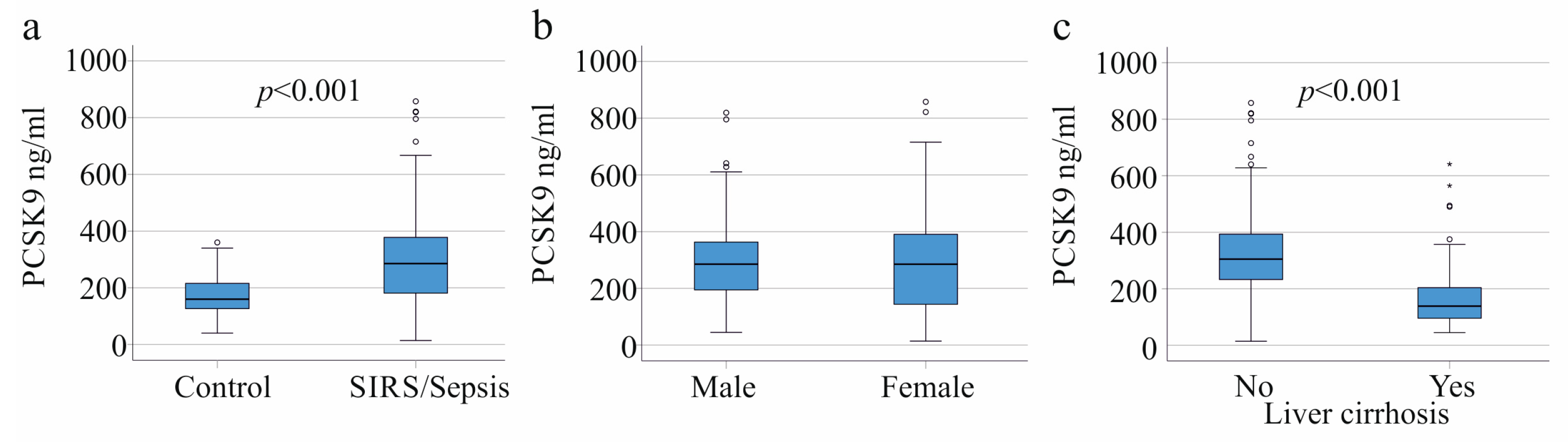
3.1. PCSK9 in Controls, SIRS/Sepsis Patients, and SIRS/Sepsis Patients with Liver Cirrhosis
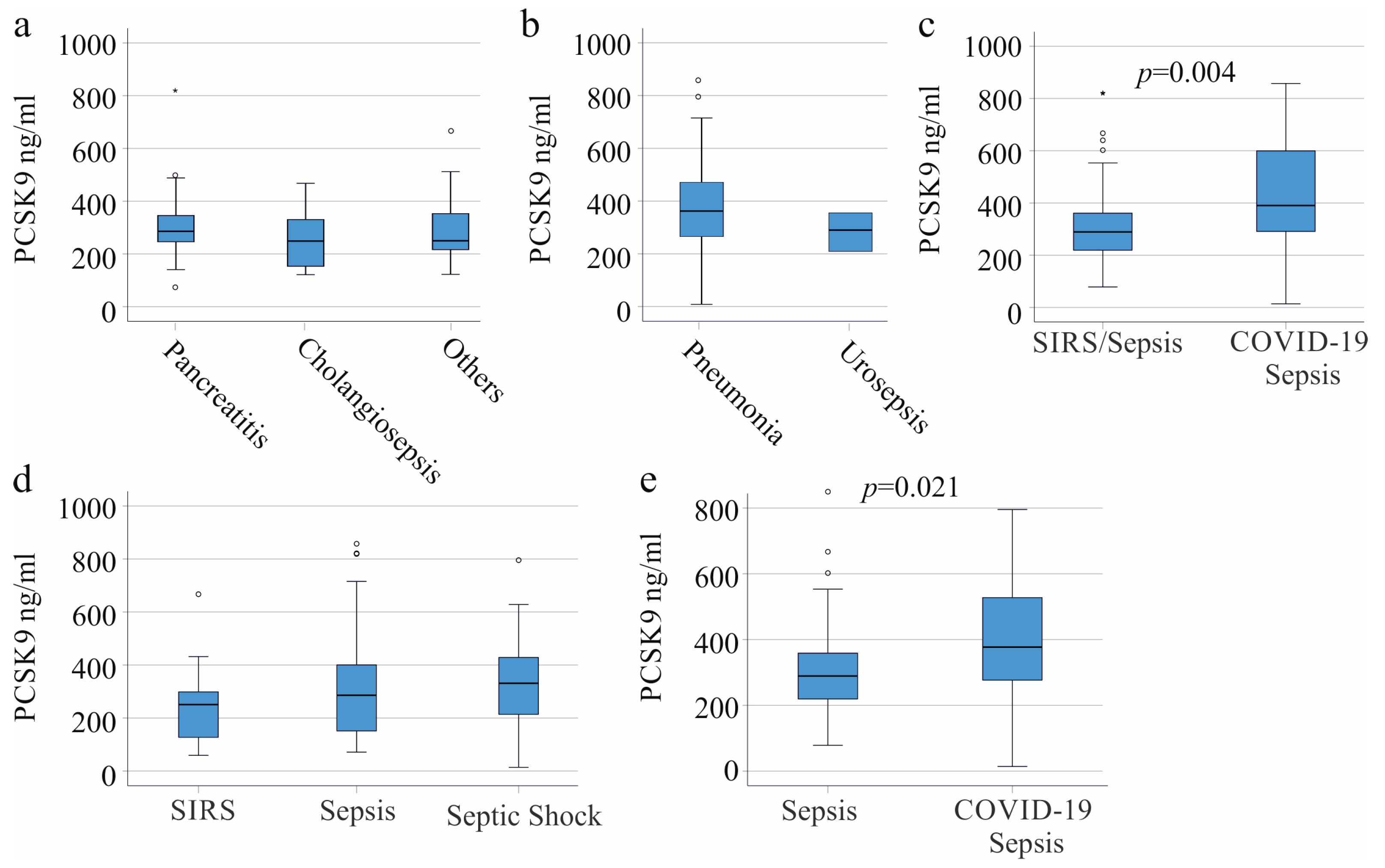
3.2. PCSK9 in SIRS/Sepsis Patients without Liver Cirrhosis Stratified for Underlying Diseases and Infectious Diseases
3.3. Plasma PCSK9 in Relation to Vasopressor Therapy and Interventions
3.4. Plasma PCSK9 in Relation to Inflammation Markers
3.5. Plasma PCSK9 in Gram-Negative and Gram-Positive Infection
3.6. Plasma PCSK9 and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biron, B.M.; Ayala, A.; Lomas-Neira, J.L. Biomarkers for Sepsis: What Is and What Might Be? Biomark. Insights 2015, 10, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Giamarellos-Bourboulis, E.J.; Kyprianou, M.; Fleischmann-Struzek, C.; Pletz, M.W.; Netea, M.G.; Reinhart, K.; Kyriazopoulou, E. Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2021, 49, 2042–2057. [Google Scholar] [CrossRef] [PubMed]
- Barlage, S.; Gnewuch, C.; Liebisch, G.; Wolf, Z.; Audebert, F.X.; Gluck, T.; Frohlich, D.; Kramer, B.K.; Rothe, G.; Schmitz, G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009, 35, 1877–1885. [Google Scholar] [CrossRef]
- Grion, C.M.; Cardoso, L.T.; Perazolo, T.F.; Garcia, A.S.; Barbosa, D.S.; Morimoto, H.K.; Matsuo, T.; Carrilho, A.J. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur. J. Clin. Investig. 2010, 40, 330–338. [Google Scholar] [CrossRef]
- Hofmaenner, D.A.; Arina, P.; Kleyman, A.; Page Black, L.; Salomao, R.; Tanaka, S.; Guirgis, F.W.; Arulkumaran, N.; Singer, M. Association Between Hypocholesterolemia and Mortality in Critically Ill Patients With Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Explor. 2023, 5, e0860. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. HDL in infectious diseases and sepsis. Handb. Exp. Pharm. 2015, 224, 483–508. [Google Scholar] [CrossRef] [Green Version]
- Hofmaenner, D.A.; Kleyman, A.; Press, A.; Bauer, M.; Singer, M. The Many Roles of Cholesterol in Sepsis: A Review. Am. J. Respir. Crit. Care Med. 2022, 205, 388–396. [Google Scholar] [CrossRef]
- Feder, S.; Wiest, R.; Weiss, T.S.; Aslanidis, C.; Schacherer, D.; Krautbauer, S.; Liebisch, G.; Buechler, C. Proprotein convertase subtilisin/kexin type 9 (PCSK9) levels are not associated with severity of liver disease and are inversely related to cholesterol in a cohort of thirty eight patients with liver cirrhosis. Lipids Health Dis. 2021, 20, 6. [Google Scholar] [CrossRef]
- Grewal, T.; Buechler, C. Emerging Insights on the Diverse Roles of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Chronic Liver Diseases: Cholesterol Metabolism and Beyond. Int. J. Mol. Sci. 2022, 23, 1070. [Google Scholar] [CrossRef]
- Grimm, J.; Peschel, G.; Muller, M.; Schacherer, D.; Wiest, R.; Weigand, K.; Buechler, C. Rapid Decline of Serum Proprotein Convertase Subtilisin/Kexin 9 (PCSK9) in Non-Cirrhotic Patients with Chronic Hepatitis C Infection Receiving Direct-Acting Antiviral Therapy. J. Clin. Med. 2021, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.G.; Mannino, D.M.; Moss, M. Cirrhosis as a risk factor for sepsis and death: Analysis of the National Hospital Discharge Survey. Chest 2003, 124, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Rannikko, J.; Jacome Sanz, D.; Ortutay, Z.; Seiskari, T.; Aittoniemi, J.; Huttunen, R.; Syrjanen, J.; Pesu, M. Reduced plasma PCSK9 response in patients with bacteraemia is associated with mortality. J. Intern. Med. 2019, 286, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, W.; Sun, S.; Zhang, Y.; Chen, Z. PCSK9: A Potential Therapeutic Target for Sepsis. J. Immunol. Res. 2020, 2020, 2687692. [Google Scholar] [CrossRef]
- Grin, P.M.; Dwivedi, D.J.; Chathely, K.M.; Trigatti, B.L.; Prat, A.; Seidah, N.G.; Liaw, P.C.; Fox-Robichaud, A.E. Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor. Sci. Rep. 2018, 8, 10496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walley, K.R.; Thain, K.R.; Russell, J.A.; Reilly, M.P.; Meyer, N.J.; Ferguson, J.F.; Christie, J.D.; Nakada, T.A.; Fjell, C.D.; Thair, S.A.; et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014, 6, 258ra143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamialahmadi, T.; Panahi, Y.; Safarpour, M.A.; Ganjali, S.; Chahabi, M.; Reiner, Z.; Solgi, S.; Vahedian-Azimi, A.; Kianpour, P.; Banach, M.; et al. Association of Serum PCSK9 Levels with Antibiotic Resistance and Severity of Disease in Patients with Bacterial Infections Admitted to Intensive Care Units. J. Clin. Med. 2019, 8, 1742. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Wei, W.Q.; Chaugai, S.; Carranza Leon, B.G.; Kawai, V.; Carranza Leon, D.A.; Jiang, L.; Zhong, X.; Liu, G.; Ihegword, A.; et al. A Genetic Approach to the Association Between PCSK9 and Sepsis. JAMA Netw. Open. 2019, 2, e1911130. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zhang, W.; Burgner, D.; Tonkin, A.; Zhu, C.; Sun, C.; Magnussen, C.G.; Ernst, M.E.; Breslin, M.; Nicholls, S.J.; et al. The association between PCSK9 inhibitor use and sepsis–A systematic review and meta-analysis of 20 double-blind, randomized, placebo-controlled trials. Am. J. Med. 2023, 136, 558–567. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur. Heart J. Cardiovasc. Pharm. 2019, 5, 237–245. [Google Scholar] [CrossRef]
- Navarese, E.P.; Podhajski, P.; Gurbel, P.A.; Grzelakowska, K.; Ruscio, E.; Tantry, U.; Magielski, P.; Kubica, A.; Niezgoda, P.; Adamski, P.; et al. PCSK9 Inhibition During the Inflammatory Stage of SARS-CoV-2 Infection. J. Am. Coll. Cardiol. 2023, 81, 224–234. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Iodice, S.; Tersalvi, G.; Rota, I.; Ghidini, S.; Terranova, L.; Valenti, L.; Amati, F.; Aliberti, S.; et al. Prognostic parameters of in-hospital mortality in COVID-19 patients-An Italian experience. Eur. J. Clin. Investig. 2021, 51, e13629. [Google Scholar] [CrossRef] [PubMed]
- Metkus, T.S.; Kim, B.S.; Jones, S.R.; Martin, S.S.; Schulman, S.P.; Leucker, T.M. Plasma Proprotein Convertase Subtilisin/kexin Type 9 (PCSK9) in the Acute Respiratory Distress Syndrome. Front. Med. 2022, 9, 876046. [Google Scholar] [CrossRef] [PubMed]
- Rannikko, J.; Syrjanen, J.; Seiskari, T.; Aittoniemi, J.; Huttunen, R. Sepsis-related mortality in 497 cases with blood culture-positive sepsis in an emergency department. Int. J. Infect. Dis. 2017, 58, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis: Clinical utility and limitations. Crit. Care Med. 2008, 36, 941–952. [Google Scholar] [CrossRef]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023; in press. [Google Scholar] [CrossRef]
- Kim, J.H. Clinical Utility of Procalcitonin on Antibiotic Stewardship: A Narrative Review. Infect. Chemother. 2022, 54, 610–620. [Google Scholar] [CrossRef]
- Pieri, G.; Agarwal, B.; Burroughs, A.K. C-reactive protein and bacterial infection in cirrhosis. Ann. Gastroenterol. 2014, 27, 113–120. [Google Scholar]
- Dong, R.; Wan, B.; Lin, S.; Wang, M.; Huang, J.; Wu, Y.; Wu, Y.; Zhang, N.; Zhu, Y. Procalcitonin and Liver Disease: A Literature Review. J. Clin. Transl. Hepatol. 2019, 7, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Jorda, A.; Gabler, C.; Blaschke, A.; Wolfl-Duchek, M.; Gelbenegger, G.; Nussbaumer-Proll, A.; Radtke, C.; Zeitlinger, M.; Bergmann, F. Community-acquired and hospital-acquired bacterial co-infections in patients hospitalized with Covid-19 or influenza: A retrospective cohort study. Infection, 2023; in press. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bone, R.C. Sepsis, sepsis syndrome, and the systemic inflammatory response syndrome (SIRS). Gulliver in Laputa. JAMA 1995, 273, 155–156. [Google Scholar] [CrossRef]
- Ahirwar, A.K.; Takhelmayum, R.; Sakarde, A.; Rathod, B.D.; Jha, P.K.; Kumawat, R.; Gopal, N. The study of serum hsCRP, ferritin, IL-6 and plasma D-dimer in COVID-19: A retrospective study. Horm. Mol. Biol. Clin. Investig. 2022, 43, 337–344. [Google Scholar] [CrossRef]
- Kaddoura, R.; Orabi, B.; Salam, A.M. PCSK9 Monoclonal Antibodies: An Overview. Heart Views 2020, 21, 97–103. [Google Scholar] [CrossRef]
- Boyd, J.H.; Fjell, C.D.; Russell, J.A.; Sirounis, D.; Cirstea, M.S.; Walley, K.R. Increased Plasma PCSK9 Levels Are Associated with Reduced Endotoxin Clearance and the Development of Acute Organ Failures during Sepsis. J. Innate Immun. 2016, 8, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, V.; Treuner-Kaueroff, T.; Seehofer, D.; Berg, T.; Becker, S.; Ceglarek, U.; Thiery, J.; Kaiser, T. Low PCSK9 levels are correlated with mortality in patients with end-stage liver disease. PLoS ONE 2017, 12, e0181540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.K.K.; Genga, K.R.; Topchiy, E.; Cirstea, M.; Shimada, T.; Fjell, C.; Russell, J.A.; Boyd, J.H.; Walley, K.R. Reduced Proprotein convertase subtilisin/kexin 9 (PCSK9) function increases lipoteichoic acid clearance and improves outcomes in Gram positive septic shock patients. Sci. Rep. 2019, 9, 10588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Jafri, L.; Hoodbhoy, Z.; Siddiqui, I. Prognostic Value of Serum Procalcitonin in COVID-19 Patients: A Systematic Review. Indian J. Crit. Care Med. 2021, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Fei, S.F.; Tong, D.B.; Xue, C.; Li, J.J. Sex difference in circulating PCSK9 and its clinical implications. Front. Pharmacol. 2022, 13, 953845. [Google Scholar] [CrossRef]
- Marinelli, C.; Zingone, F.; Lupo, M.G.; Marin, R.; D’Inca, R.; Gubbiotti, A.; Massimi, D.; Casadei, C.; Barberio, B.; Ferri, N.; et al. Serum Levels of PCSK9 Are Increased in Patients With Active Ulcerative Colitis Representing a Potential Biomarker of Disease Activity: A Cross-sectional Study. J. Clin. Gastroenterol. 2022, 56, 787–793. [Google Scholar] [CrossRef]
- Ghosh, M.; Galman, C.; Ruding, M.; Angelin, B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J. Lipid Res. 2015, 56, 463–469. [Google Scholar] [CrossRef] [Green Version]

| Parameter | All Patients | Patients with Liver Cirrhosis Excluded | Patients with Liver Cirrhosis (COVID-19 Patients Excluded) | COVID-19 Patients (Patients with Liver Cirrhosis Excluded) |
|---|---|---|---|---|
| Males/Females | 109/47 | 86/38 | 22/8 | 15/6 |
| Age (years) | 59 (21–93) | 57 (21–88) | 58 (31–75) | 63 (29–80) |
| SIRS/Sepsis/Septic shock | 37/40/79 | 27/33/64 | 10/7/15 | 00/2/19 |
| C-reactive protein mg/L | 157 (12–697) *** §§§ | 183 (35–597) *** | 61 (12–236) §§§ | 156 (44–472) |
| Procalcitonin ng/mL | 1.15 (0.05–270) * | 1.17 (0.06–270.00) | 1.17 (0.10–65.18) | 0.57 (0.08–65.40) * |
| Leukocytes n × 109/L | 10.31 (0.06–1586.00) | 10.35 (2.16–37.38) | 10.95 (2.51–1586.00) | 9.62 (2.78–18.47) |
| Intervention/Drug | Patients without Liver Cirrhosis and without COVID-19 (103) | Patients with Liver Cirrhosis and without COVID-19 (30) | Patients with COVID-19 without Liver Cirrhosis (21) | |||
|---|---|---|---|---|---|---|
| N | p-Value | N | p-Value | N | p-Value | |
| Dialysis | 29 | 0.538 | 14 | 0.552 | 9 | 0.651 |
| Ventilation | 54 | 0.160 | 18 | 0.124 | 21 | - |
| Vasopressor therapy | 55 | 0.500 | 19 | 0.030 | 19 | 0.467 |
| Biomarker of Inflammation | Patients without Liver Cirrhosis without COVID-19 (103) | Patients with Liver Cirrhosis without COVID-19 (30) | Patients with COVID-19 without Liver Cirrhosis (21) | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Leukocyte count | 0.127 | 0.200 | 0.404 | 0.027 | 0.143 | 0.537 |
| Procalcitonin | −0.016 | 0.871 | 0.405 | 0.027 | −0.182 | 0.430 |
| C-reactive protein | 0.153 | 0.124 | 0.603 | <0.001 | −0.171 | 0.457 |
| IL-6 | 0.271 | 0.234 | ||||
| Ferritin | 0.038 | 0.871 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mester, P.; Amend, P.; Schmid, S.; Müller, M.; Buechler, C.; Pavel, V. Plasma Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) as a Possible Biomarker for Severe COVID-19. Viruses 2023, 15, 1511. https://doi.org/10.3390/v15071511
Mester P, Amend P, Schmid S, Müller M, Buechler C, Pavel V. Plasma Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) as a Possible Biomarker for Severe COVID-19. Viruses. 2023; 15(7):1511. https://doi.org/10.3390/v15071511
Chicago/Turabian StyleMester, Patricia, Pablo Amend, Stephan Schmid, Martina Müller, Christa Buechler, and Vlad Pavel. 2023. "Plasma Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) as a Possible Biomarker for Severe COVID-19" Viruses 15, no. 7: 1511. https://doi.org/10.3390/v15071511






