The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers
Abstract
:1. Introduction
2. Materials and Methods
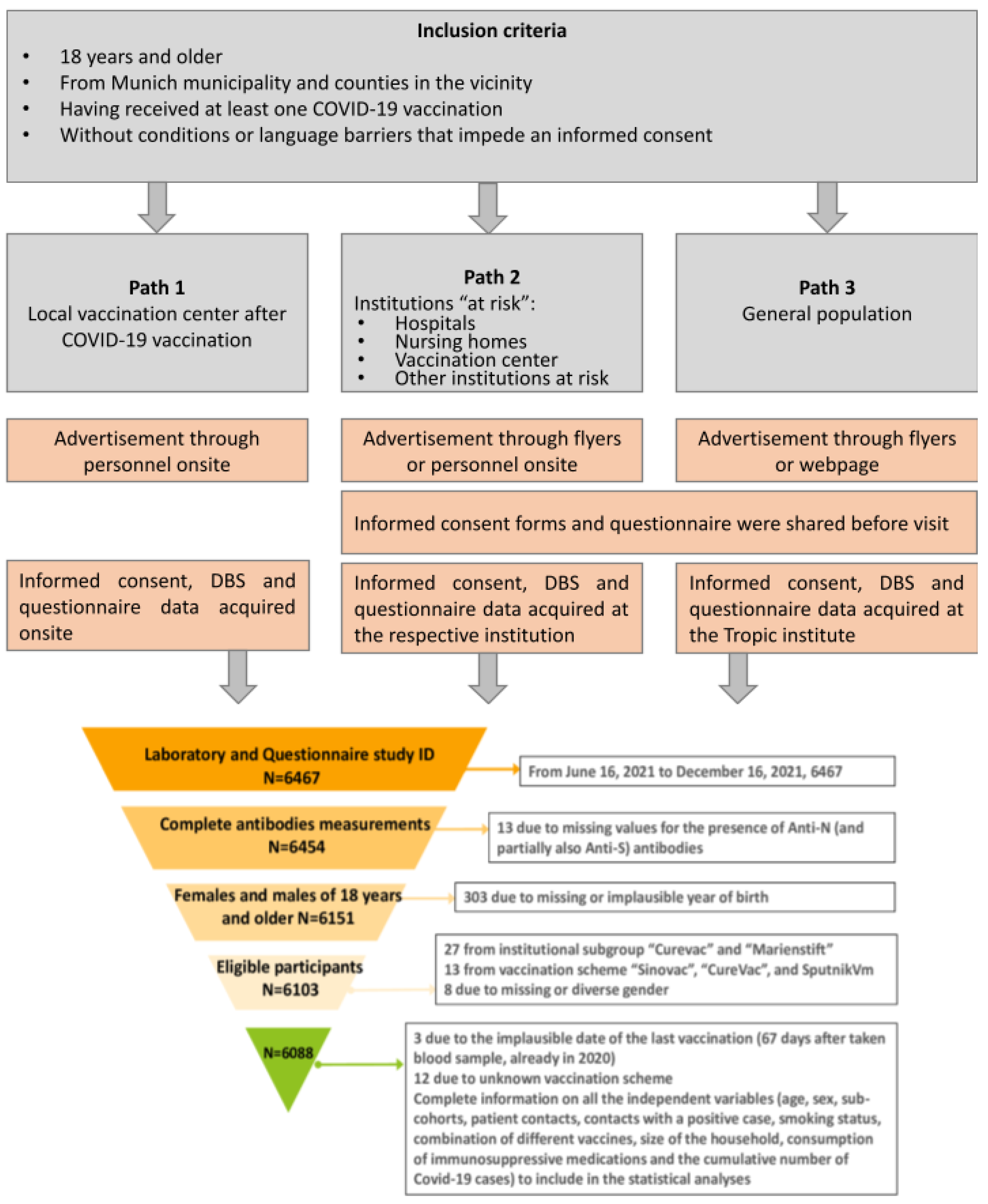
2.1. The KoCo-Impf Cohort: Cohort Design, Inclusion Criteria, and Setting
2.2. Specimen Collection and Laboratory Analyses
2.3. Questionnaire Data
- recruitment (institutional subgroup; recruitment date);
- demographic (date/year of birth; sex; level of education; household size);
- health-related behavior (smoking status; pre-existing medical conditions; medication scheme (intake of immunosuppressive drugs; others));
- employment-related behavior (occupational status; working conditions);
- COVID-19-related health status (vaccination status such as the date and type of first, second, and third vaccination if applicable; infection status, only Polymerase chain reaction (PCR)-confirmed COVID-19-diagnosis; diagnosis period; diagnosis date, month, and year; diagnosis in relation to vaccination and immunization status; diagnosis date after first vaccination; diagnosis date after full immunization (Two doses of Comirnaty, Spikevax or Vaxzevria or one dose of Jcovden at the time of data collection); severity of SARS-CoV-2-infection; previous contact with SARS-CoV-2 infected person; testing frequency; symptoms suggestive for COVID-19).
2.4. Variables Definition
2.5. Statistical Analyses
3. Results
3.1. Cohort Description
- missing or incomplete antibody measurements (n = 13);
- missing or implausible self-reported year of birth (n = 303);
- participation in clinical vaccination trials or recruitment after 16 December 2021 n = 27);
- vaccination with brands not authorized in Germany (n = 13);
- missing or diverse information on sex (n = 8);
- implausible vaccination dates (n = 3)
- unknown vaccination scheme (n = 12).
3.2. Risk Factor Analysis for Anti-N Seropositivity
3.3. Determinants of Antibody Response after SARS-CoV-2 Infection
3.4. Determinants of Antibody Response after SARS-CoV-2 Vaccination and/or Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020, 55, 105924. [Google Scholar] [CrossRef]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 13 April 2023).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 13 April 2023).
- RKI. Table Showing Current COVID-19 Infections per Day as Time Series. Available online: https://npgeo-corona-npge-de.hub.arcgis.com/datasets/dd4580c810204019a7b8eb3e0b329dd6_0/explore (accessed on 16 October 2022).
- RKI. Tabelle mit den Gemeldeten Impfungen nach Bundesländern und Impfquoten nach Altersgruppen. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquotenmonitoring (accessed on 16 September 2021).
- World Health Organization. Guidance on Developing a National Deployment and Vaccination Plan for COVID-19 Vaccines: Interim Guidance, 16 November 2020. Available online: https://apps.who.int/iris/handle/10665/336603?search-result=true&query=Guidance+on+developing+a+national+deployment+and+vaccination+plan+for+COVID-19+vaccines%3A+interim+guidance&scope=&rpp=10&sort_by=score&order=desc (accessed on 11 July 2023).
- World Health Organization. Guidance on Developing a National Deployment and Vaccination Plan for COVID-19 Vaccines: Interim Guidance, 1 June 2021. Available online: https://apps.who.int/iris/handle/10665/341564?search-result=true&query=Guidance+on+developing+a+national+deployment+and+vaccination+plan+for+COVID-19+vaccines%3A+interim+guidance&scope=&rpp=10&sort_by=score&order=desc (accessed on 11 July 2023).
- RKI. Positionspapier der STIKO, Leopoldina und des Deutschen Ethikrats zur Verteilung eines COVID-19-Impfstoffes. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/COVID-19/Positionspapier.html (accessed on 12 January 2023).
- Ledda, C.; Costantino, C.; Motta, G.; Cunsolo, R.; Stracquadanio, P.; Liberti, G.; Maltezou, H.C.; Rapisarda, V. SARS-CoV-2 mRNA Vaccine Breakthrough Infections in Fully Vaccinated Healthcare Personnel: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 9. [Google Scholar] [CrossRef]
- Petráš, M.; Máčalík, R.; Janovská, D.; Čelko, A.M.; Dáňová, J.; Selinger, E.; Doleček, J.; Neradová, S.; Franklová, M.; Dlouhý, P.; et al. Risk factors affecting COVID-19 vaccine effectiveness identified from 290 cross-country observational studies until February 2022: A meta-analysis and meta-regression. BMC Med. 2022, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Lotan, R.; Kalkstein, N.; Peretz, A.; Perez, G.; Ben-Tov, A.; Chodick, G.; Gazit, S.; Patalon, T. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat. Commun. 2021, 12, 6379. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Saathoff, E.; Pritsch, M.; Guggenbühl Noller, J.M.; Kroidl, I.; Olbrich, L.; Thiel, V.; Diefenbach, M.; Riess, F.; Forster, F.; et al. Protocol of a population-based prospective COVID-19 cohort study Munich, Germany (KoCo19). BMC Public Health 2020, 20, 1036. [Google Scholar] [CrossRef]
- Warszawski, J.; Beaumont, A.L.; Seng, R.; de Lamballerie, X.; Rahib, D.; Lydié, N.; Slama, R.; Durrleman, S.; Raynaud, P.; Sillard, P.; et al. Prevalence of SARS-Cov-2 antibodies and living conditions: The French national random population-based EPICOV cohort. BMC Infect. Dis. 2022, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Vivaldi, G.; Jolliffe, D.A.; Holt, H.; Tydeman, F.; Talaei, M.; Davies, G.A.; Lyons, R.A.; Griffiths, C.J.; Kee, F.; Sheikh, A.; et al. Risk factors for SARS-CoV-2 infection after primary vaccination with ChAdOx1 nCoV-19 or BNT162b2 and after booster vaccination with BNT162b2 or mRNA-1273: A population-based cohort study (COVIDENCE UK). Lancet Reg. Health Eur. 2022, 22, 100501. [Google Scholar] [CrossRef]
- Einhauser, S.; Peterhoff, D.; Beileke, S.; Günther, F.; Niller, H.H.; Steininger, P.; Knöll, A.; Korn, K.; Berr, M.; Schütz, A.; et al. Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County-Results from a Population-Based Longitudinal Study in Germany. Viruses 2022, 14, 1168. [Google Scholar] [CrossRef]
- Wagner, R.; Peterhoff, D.; Beileke, S.; Günther, F.; Berr, M.; Einhauser, S.; Schütz, A.; Niller, H.H.; Steininger, P.; Knöll, A.; et al. Estimates and Determinants of SARS-CoV-2 Seroprevalence and Infection Fatality Ratio Using Latent Class Analysis: The Population-Based Tirschenreuth Study in the Hardest-Hit German County in Spring 2020. Viruses 2021, 13, 1118. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Visci, G.; Violante, F.S.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Larese Fillon, F.; Negro, C.; Janke, C.; Castelletti, N.; et al. Determinants of anti-S immune response at 6 months after COVID-19 vaccination in a multicentric European cohort of healthcare workers—ORCHESTRA project. Front. Immunol. 2022, 13, 986085. [Google Scholar] [CrossRef]
- Porru, S.; Monaco, M.G.L.; Spiteri, G.; Carta, A.; Pezzani, M.D.; Lippi, G.; Gibellini, D.; Tacconelli, E.; Dalla Vecchia, I.; Sala, E.; et al. SARS-CoV-2 Breakthrough Infections: Incidence and Risk Factors in a Large European Multicentric Cohort of Health Workers. Vaccines 2022, 10, 1193. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Ceci, S.; Patano, A.; Corriero, A.; Vimercati, L.; Azzollini, D.; Marinelli, G.; Coloccia, G.; Piras, F.; et al. Effectiveness of SARS-CoV-2 Vaccines for Short- and Long-Term Immunity: A General Overview for the Pandemic Contrast. Int. J. Mol. Sci. 2022, 23, 8485. [Google Scholar] [CrossRef] [PubMed]
- Moncunill, G.; Aguilar, R.; Ribes, M.; Ortega, N.; Rubio, R.; Salmerón, G.; Molina, M.J.; Vidal, M.; Barrios, D.; Mitchell, R.A.; et al. Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naïve healthcare workers. EBioMedicine 2022, 75, 103805. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; Santos de Oliveira, M.H.; Catahay, J.A.; Khan, M.S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef]
- Notarte, K.I.; Ver, A.T.; Velasco, J.V.; Pastrana, A.; Catahay, J.A.; Salvagno, G.L.; Yap, E.P.H.; Martinez-Sobrido, L.B.; Torrelles, J.; Lippi, G.; et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: A systematic review. Crit. Rev. Clin. Lab. Sci. 2022, 59, 373–390. [Google Scholar] [CrossRef]
- Yang, S.L.; Mat Ripen, A.; Leong, C.T.; Lee, J.V.; Yen, C.H.; Chand, A.K.; Koh, K.; Abdul Rahim, N.A.B.; Gokilavanan, V.; Mohamed, N.; et al. COVID-19 breakthrough infections and humoral immune response among BNT162b2 vaccinated healthcare workers in Malaysia. Emerg. Microbes Infect. 2022, 11, 1262–1271. [Google Scholar] [CrossRef]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef]
- Deng, J.; Ma, Y.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Comparison of the Effectiveness and Safety of Heterologous Booster Doses with Homologous Booster Doses for SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10752. [Google Scholar] [CrossRef]
- Lv, J.; Wu, H.; Xu, J.; Liu, J. Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: A systematic review. Infect. Dis. Poverty 2022, 11, 53. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, Z.; Si, S.; Alifu, X.; Zhou, H.; Chi, P.; Zhuang, Y.; Mo, M.; Yu, Y. Immunogenicity and Safety of Homologous and Heterologous Prime-Boost Immunization with COVID-19 Vaccine: Systematic Review and Meta-Analysis. Vaccines 2022, 10, 798. [Google Scholar] [CrossRef]
- Pritsch, M.; Radon, K.; Bakuli, A.; Le Gleut, R.; Olbrich, L.; Guggenbüehl Noller, J.M.; Saathoff, E.; Castelletti, N.; Garí, M.; Pütz, P.; et al. Prevalence and Risk Factors of Infection in the Representative COVID-19 Cohort Munich. Int. J. Environ. Res. Public Health 2021, 18, 3572. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Bakuli, A.; Pütz, P.; Le Gleut, R.; Guggenbuehl Noller, J.M.; Olbrich, L.; Saathoff, E.; Garí, M.; Schälte, Y.; Frahnow, T.; et al. From first to second wave: Follow-up of the prospective COVID-19 cohort (KoCo19) in Munich (Germany). BMC Infect. Dis. 2021, 21, 925. [Google Scholar] [CrossRef] [PubMed]
- Le Gleut, R.; Pütz, P.; Radon, K.; Reinkemeyer, C.; Castelletti, N. The Representative COVID-19 Cohort Munich (KoCo19): From the Beginning of the Pandemic to the Delta Virus Variant. BMC Infect. Dis. 2023, 23, 466. [Google Scholar] [CrossRef] [PubMed]
- Orchestra. Available online: https://orchestra-cohort.eu/ (accessed on 19 January 2023).
- Paul-Ehrlich-Institut. Nicht-interventionelle Studie (Anwendungsbeobachtung) NIS-Nr.: 619. Available online: https://www.pei.de/SharedDocs/awb/nis-0601-0700/0619.html?nn=173154] (accessed on 5 June 2023).
- Beyerl, J.; Rubio-Acero, R.; Castelletti, N.; Paunovic, I.; Kroidl, I.; Khan, Z.N.; Bakuli, A.; Tautz, A.; Oft, J.; Hoelscher, M.; et al. A dried blood spot protocol for high throughput analysis of SARS-CoV-2 serology based on the Roche Elecsys anti-N assay. EBioMedicine 2021, 70, 103502. [Google Scholar] [CrossRef]
- Rubio-Acero, R.; Castelletti, N.; Fingerle, V.; Olbrich, L.; Bakuli, A.; Wölfel, R.; Girl, P.; Müller, K.; Jochum, S.; Strobl, M.; et al. In Search of the SARS-CoV-2 Protection Correlate: Head-to-Head Comparison of Two Quantitative S1 Assays in Pre-characterized Oligo-/Asymptomatic Patients. Infect. Dis. Ther. 2021, 10, 1505–1518. [Google Scholar] [CrossRef]
- Olbrich, L.; Castelletti, N.; Schälte, Y.; Garí, M.; Pütz, P.; Bakuli, A.; Pritsch, M.; Kroidl, I.; Saathoff, E.; Guggenbuehl Noller, J.M.; et al. Head-to-head evaluation of seven different seroassays including direct viral neutralisation in a representative cohort for SARS-CoV-2. J. Gen Virol. 2021, 102, 001653. [Google Scholar] [CrossRef]
- Brochot, E.; Demey, B.; Touzé, A.; Belouzard, S.; Dubuisson, J.; Schmit, J.L.; Duverlie, G.; Francois, C.; Castelain, S.; Helle, F. Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals. Front. Microbiol. 2020, 11, 584251. [Google Scholar] [CrossRef]
- Borremans, B.; Gamble, A.; Prager, K.C.; Helman, S.K.; McClain, A.M.; Cox, C.; Savage, V.; Lloyd-Smith, J.O. Quantifying antibody kinetics and RNA detection during early-phase SARS-CoV-2 infection by time since symptom onset. Elife 2020, 7, 9. [Google Scholar] [CrossRef]
- Moons, K.G.; Donders, R.A.; Stijnen, T.; Harrell, F.E., Jr. Using the outcome for imputation of missing predictor values was preferred. J. Clin. Epidemiol. 2006, 59, 1092–1101. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: New York, NJ, USA, 2017. [Google Scholar]
- Bauer, A.; Weigert, M.; Jalal, H. APCtools: Descriptive and Model-based Age-Period-Cohort Analysis. J. Open Source Softw. 2022, 7, 4056. [Google Scholar] [CrossRef]
- Bayerisches Staatsministerium für Gesundheit und Pflege. Verordnung zur Änderung der Elften Bayerischen Infektionsschutzmaßnahmenverordnung; Bayerisches Staatsministerium für Gesundheit und Pflege: Munich, Germany, 2021; p. 34. [Google Scholar]
- Bayerisches Staatsministerium für Gesundheit und Pflege. Verordnung zur Änderung der Dreizehnten Bayerischen Infektionsschutzmaßnahmenverordnung; Bayerisches Staatsministerium für Gesundheit und Pflege: Munich, Germany, 2021; p. 584. [Google Scholar]
- Dzinamarira, T.; Nkambule, S.J.; Hlongwa, M.; Mhango, M.; Iradukunda, P.G.; Chitungo, I.; Dzobo, M.; Mapingure, M.P.; Chingombe, I.; Mashora, M.; et al. Risk Factors for COVID-19 Infection Among Healthcare Workers. A First Report from a Living Systematic Review and meta-Analysis. Saf. Health Work 2022, 13, 263–268. [Google Scholar] [CrossRef] [PubMed]
- RKI. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19). Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-01-06.pdf?__blob=publicationFile (accessed on 6 January 2022).
- Nah, E.H.; Cho, S.; Park, H.; Kim, S.; Noh, D.; Kwon, E.; Cho, H.I. Antibody response after two doses of homologous or heterologous SARS-CoV-2 vaccines in healthcare workers at health promotion centers: A prospective observational study. J. Med. Virol. 2022, 94, 4719–4726. [Google Scholar] [CrossRef] [PubMed]
- STIKO. Mitteilung der STIKO zur COVID-19-Impfung: Impfabstand und heterologes Impfschema nach Erstimpfung mit Vaxzevria; STIKO: Berlin, Germany, 2021. [Google Scholar]
- STIKO. Pressemitteilung der STIKO zur COVID-19-Impfung mit mRNA-Impfstoff bei Personen unter 30 Jahren; STIKO: Berlin, Germany, 2021. [Google Scholar]
- Paul, G.; Strnad, P.; Wienand, O.; Krause, U.; Plecko, T.; Effenberger-Klein, A.; Giel, K.E.; Junne, F.; Galante-Gottschalk, A.; Ehehalt, S.; et al. The humoral immune response more than one year after SARS-CoV-2 infection: Low detection rate of anti-nucleocapsid antibodies via Euroimmun ELISA. Infection 2023, 51, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Vande Weygaerde, Y.; André, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021, 136, 104765. [Google Scholar] [CrossRef]
- Reusch, J.; Wagenhäuser, I.; Gabel, A.; Eggestein, A.; Höhn, A.; Lâm, T.T.; Frey, A.; Schubert-Unkmeir, A.; Dölken, L.; Frantz, S.; et al. Influencing factors of anti-SARS-CoV-2-spike-IgG antibody titers in healthcare workers: A cross-section study. J. Med. Virol. 2023, 95, e28300. [Google Scholar] [CrossRef]
- Sarrigeorgiou, I.; Moschandreou, D.; Dimitriadis, A.; Tsinti, G.; Sotiropoulou, E.; Ntoukaki, E.; Eliadis, P.; Backovic, M.; Labropoulou, S.; Escriou, N.; et al. Combined monitoring of IgG and IgA anti-Spike and anti-Receptor binding domain long term responses following BNT162b2 mRNA vaccination in Greek healthcare workers. PLoS ONE 2022, 17, e0277827. [Google Scholar] [CrossRef]
- Günther, F.; Einhauser, S.; Peterhoff, D.; Wiegrebe, S.; Niller, H.H.; Beileke, S.; Steininger, P.; Burkhardt, R.; Küchenhoff, H.; Gefeller, O.; et al. Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves-Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study. Int. J. Environ. Res. Public Health 2022, 19, 16996. [Google Scholar] [CrossRef]
- Haddad, C.; Bou Malhab, S.; Sacre, H.; Salameh, P. Smoking and COVID-19: A scoping review. Tob. Use Insights 2021, 14, 1179173X21994612. [Google Scholar] [CrossRef]
- Sopori, M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- Usman, M.S.; Siddiqi, T.J.; Khan, M.S.; Patel, U.K.; Shahid, I.; Ahmed, J.; Kalra, A.; Michos, E.D. Is there a smoker’s paradox in COVID-19? BMJ Evid.-Based Med. 2021, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Kroidl, I.; Rubio-Acero, R.; Olbrich, L.; Castelletti, N.; Wieser, A. Studying Temporal Titre Evolution of Commercial SARS-CoV-2 Assays Reveals Significant Shortcomings of Using BAU Standardization for Comparison; LMU Munich: Munich, Germany, 2023. [Google Scholar]
- Whitaker, H.J.; Gower, C.; Otter, A.D.; Simmons, R.; Kirsebom, F.; Letley, L.; Quinot, C.; Ireland, G.; Linley, E.; Ribeiro, S. Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: Impact of variant, vaccination, and choice of assay cut-off. MedRxiv 2021. [Google Scholar] [CrossRef]
- Plumb, I.D.; Fette, L.M.; Tjaden, A.H.; Feldstein, L.; Saydah, S.; Ahmed, A.; Link-Gelles, R.; Wierzba, T.F.; Berry, A.A.; Friedman-Klabanoff, D. Estimated COVID-19 vaccine effectiveness against seroconversion from SARS-CoV-2 Infection, March–October, 2021. Vaccine 2023, 41, 2596–2604. [Google Scholar] [CrossRef]
- Yu, H.; Guan, F.; Miller, H.; Lei, J.; Liu, C. The role of SARS-CoV-2 nucleocapsid protein in antiviral immunity and vaccine development. Emerg. Microbes Infect. 2023, 12, 2164219. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Agnello, L.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine. Sci. Rep. 2022, 12, 8679. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Heo, J.Y.; Seo, Y.B.; Yoon, Y.K.; Sohn, J.W.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Choi, J.Y.; Kim, H.J.; et al. Six-month longitudinal immune kinetics after mRNA-1273 vaccination: Correlation of peak antibody response with long-term, cross-reactive immunity. Front. Immunol. 2022, 13, 1035441. [Google Scholar] [CrossRef] [PubMed]



| Variable Name | Definition (Type of Variable) |
|---|---|
| Quantitative anti-N/S | The detected amount of Ro-N-Ig/Ro-RBD-Ig from DBS (continuous) |
| Qualitative anti-N/S | A positive anti-N/S result is defined when the amount of Ro-N-Ig/Ro-RBD-Ig is ≥0.105/0.115 (positive/negative) |
| Age **** | Age of participants in years (continuous) |
| Cumulative cases | Cumulative number of COVID-19 cases from the beginning of the pandemic till the recruitment date (continuous) |
| Intake of immunosuppressive drugs **** | Current intake of medications that may suppress the immune system (yes, no) |
| Sex **** | Sex of the participant (male, female) |
| Smoking status **** | Current smoking status (never smoker, current smoker, past smoker) |
| Contact with patients **** | Direct contact with patients (yes, no) |
| Contact with positives **** | Previous contact with COVID-19 affected/SARS-CoV-2 infected person (yes, no, or unwittingly) |
| Household size **** | Number of household members including participant (1, 2, 3, 4, 5, >5) |
| Institutional subgroup | Categorization according to the institution of recruitment (Hospitals *: Medical center of LMU, Tropical Institute **, MK Bogenhausen, MK Harlaching, MK Neuperlach, MK Schwabing, MK Thalkirchner Straße, Barmherzige Brüder, Seefeld, Institutions of long-term care: Eichenau, MS Heilig Geist, MS Rümannstraße, Obersendling Others: Vaccination center Riem, Friedenheimer Brücke, General population ***) |
| Breakthrough Infection (BTI) **** | An infection happened at least 2 weeks after the second dose (yes, no, not applicable) |
| Time since infection **** | Time between the sampling date and the positive PCR (infected in less than 3 months, infected between 3 and 6 months, infected between 6 and 12 months, infected after 12 months, no infection) |
| Combination of vaccination scheme and former infection (immunity) | A composite variable containing information on the previous infection (based on anti-N result) and the undergone vaccination scheme (infection yes, not vaccinated, infection yes + one vaccination, infection yes + two vaccinations, infection yes + three vaccinations, infection no + one vaccination, infection no + two vaccinations, infection no + three vaccinations) |
| Time since second vaccination **** | Time between the second vaccination and the sampling date (continuous) |
| Vaccination scheme **** | A combination of types of vaccination and number of vaccinations, including BioNTech/Pfizer, Moderna, AstraZeneca, Johnson & Johnson/Janssen (no vaccination, one vaccination, two vaccinations, three vaccinations) |
| Covariate | Category | Number of Participants N (%) | Qualitative Anti-N N (%) | Qualitative Anti-S N (%) | Quantitative Anti-N Mean Value (SD) | Quantitative Anti-S Mean Value (SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||
| Overall cohort | 6088 (100.0) | 424 (6.9) | 5664 (93.1) | 5767 (94.8) | 321 (5.2) | 0.94 (1.52) | 0.06 (0.01) | 83.54 (200.35) | 0.03 (0.02) | |
| Sex | Female | 4379 (72.0) | 296 (6.7) | 4083 (93.3) | 4199 (95.9) | 180 (4.1) | 0.88 (1.33) | 0.06 (0.01) | 82.39 (199.08) | 0.03 (0.02) |
| Male | 1709 (28.0) | 128 (7.4) | 1581 (92.6) | 1568 (91.8) | 141 (8.2) | 1.10 (1.86) | 0.06 (0.01) | 86.68 (204.17) | 0.03 (0.02) | |
| Institutional subgroup | Barmherzige Brüder | 188 (3.0) | 40 (21.2) | 148 (78.8) | 187 (99.5) | 1 (0.5) | 0.98 (1.04) | 0.07 (0.008) | 55.02 (106.23) | 0.06 (NA) |
| Eichenau | 34 (0.5) | 5 (14.7) | 29 (85.3) | 34 (100.0) | 0 (0.0) | 1.59 (2.00) | 0.07 (0.004) | 447.20 (427.47) | - * | |
| Friedenheimer Brücke | 34 (0.5) | 1 (2.9) | 33 (97.1) | 34 (100.0) | 0 (0.0) | 0.88 (NA) | 0.08 (0.006) | 82.45 (122.71) | - | |
| General population | 671 (11.0) | 50 (7.5) | 621 (92.5) | 366 (54.6) | 306 (45.4) | 1.33 (2.25) | 0.07 (0.02) | 43.84 (121.03) | 0.03 (0.02) | |
| Medical Center of LMU | 3689 (60.6) | 213 (5.7) | 3476 (94.3) | 3680 (99.8) | 9 (0.2) | 0.86 (1.53) | 0.06 (0.01) | 85.62 (205.49) | 0.04 (0.04) | |
| MK, Bogenhausen | 238 (3.9) | 23 (9.6) | 215 (90.4) | 238 (100.0) | 0 (0.0) | 1.42 (1.78) | 0.07 (0.01) | 62.67 (172.21) | - | |
| MK, Harlaching | 154 (2.5) | 14 (9.1) | 140 (90.9) | 154 (100.0) | 0 (0.0) | 0.87 (1.19) | 0.07 (0.006) | 43.20 (60.97) | - | |
| MK, Neuperlach | 112 (1.8) | 5 (4.4) | 107 (95.6) | 112 (100.0) | 0 (0.0) | 0.45 (0.38) | 0.07 (0.005) | 33.44 (32.95) | - | |
| MK, Schwabing | 281 (4.6) | 13 (4.7) | 268 (95.3) | 281 (100.0) | 0 (0.0) | 0.36 (0.35) | 0.07 (0.009) | 48.08 (128.11) | - | |
| MK, Thalkirchner Straße | 67 (1.1) | 4 (5.9) | 63 (94.1) | 67 (100.0) | 0 (0.0) | 2.15 (2.27) | 0.07 (0.006) | 40.60 (46.19) | - | |
| MS, Heilig Geist | 60 (0.9) | 14 (23.3) | 46 (76.7) | 60 (100.0) | 0 (0.0) | 0.61 (0.69) | 0.06 (0.02) | 140.81 (380.16) | - | |
| MS, Rümannstraße | 36 (0.5) | 2 (5.5) | 34 (94.5) | 36 (100.0) | 0 (0.0) | 0.58 (0.67) | 0.06 (0.005) | 531.93 (574.09) | - | |
| Obersendling | 27 (0.4) | 4 (14.8) | 23 (85.2) | 27 (100.0) | 0 (0.0) | 0.88 (0.66) | 0.08 (0.004) | 54.03 (113.73) | - | |
| Seefeld | 83 (1.3) | 5 (6.1) | 78 (93.9) | 83 (100.0) | 0 (0.0) | 1.26 (0.52) | 0.06 (0.01) | 138.71 (285.03) | - | |
| Tropical Institute | 48 (0.8) | 2 (4.1) | 46 (95.9) | 46 (95.9) | 2 (4.1) | 0.16 (0.05) | 0.07 (0.01) | 78.37 (115.27) | 0.05 (0.02) | |
| Vaccination center Riem | 366 (6.0) | 29 (7.9) | 337 (92.1) | 363 (99.2) | 3 (0.8) | 0.76 (0.85) | 0.07 (0.007) | 101.04 (148.18) | 0.06 (0.04) | |
| Contact with patients | Yes | 3505 (57.5) | 261 (7.4) | 3244 (92.6) | 3493 (99.7) | 12 (0.3) | 0.90 (1.42) | 0.06 (0.01) | 94.39 (227.33) | 0.03 (0.03) |
| No | 1833 (30.2) | 111 (6.1) | 1722 (93.9) | 1647 (89.9) | 186 (10.1) | 0.89 (1.39) | 0.06 (0.02) | 65.44 (140.44) | 0.03 (0.02) | |
| Unknown ** | 750 (12.3) | 52 (6.8) | 698 (93.2) | 627 (83.8) | 123 (16.2) | 1.26 (2.09) | 0.07 (0.02) | 70.64 (167.82) | 0.03 (0.02) | |
| Contact with positives | Yes | 2804 (45.9) | 278 (9.9) | 2526 (90.1) | 2747 (97.9) | 57 (2.1) | 1.00 (1.62) | 0.06 (0.01) | 89.99 (215.54) | 0.03 (0.02) |
| No or unwittingly | 3284 (54.1) | 146 (4.4) | 3138 (95.6) | 3020 (91.9) | 264 (8.1) | 0.84 (1.28) | 0.06 (0.01) | 77.70 (185.37) | 0.03 (0.02) | |
| Smoking status | Never smoker | 4177 (68.5) | 315 (7.5) | 3862 (92.5) | 3967 (94.9) | 210 (5.1) | 0.96 (1.57) | 0.06 (0.02) | 86.29 (205.12) | 0.03 (0.02) |
| Current smoker | 1062 (17.5) | 49 (4.6) | 1013 (95.4) | 1009 (95.1) | 53 (4.9) | 0.52 (0.61) | 0.06 (0.01) | 73.95 (188.65) | 0.03 (0.02) | |
| Past smoker | 798 (13.1) | 56 (7.1) | 742 (92.9) | 740 (92.8) | 58 (7.2) | 1.20 (1.71) | 0.07 (0.01) | 82.21 (190.29) | 0.03 (0.02) | |
| Unknown | 51 (0.9) | 4 (7.8) | 47 (92.2) | 51 (100.0) | 0 (0.0) | 0.91 (0.65) | 0.06 (0.007) | 80.02 (201.80) | - | |
| Vaccination scheme | No vacc. *** | 353 (5.7) | 40 (11.3) | 313 (88.7) | 53 (15.0) | 300 (85.0) | 1.65 (2.64) | 0.07 (0.02) | 13.25 (50.72) | 0.03 (0.02) |
| One vaccination | 380 (6.1) | 123 (32.5) | 257 (67.5) | 367 (96.6) | 13 (3.4) | 1.15 (1.53) | 0.07 (0.01) | 98.05 (226.56) | 0.04 (0.04) | |
| Two vaccinations | 5001 (82.2) | 245 (4.9) | 4756 (95.1) | 4997 (99.9) | 4 (0.1) | 0.75 (1.23) | 0.06 (0.01) | 55.40 (136.23) | 0.06 (0.03) | |
| Three vaccinations | 354 (5.8) | 16 (4.4) | 338 (95.6) | 350 (98.9) | 4 (1.1) | 0.79 (1.07) | 0.06 (0.01) | 480.65 (416.65) | 0.04 (0.04) | |
| Household size | One person | 1586 (25.9) | 117 (7.3) | 1469 (92.7) | 1477 (93.2) | 109 (6.8) | 1.01 (1.57) | 0.06 (0.01) | 80.86 (197.26) | 0.03 (0.02) |
| 2 people | 2219 (36.5) | 140 (6.3) | 2079 (93.7) | 2107 (94.9) | 112 (5.1) | 1.08 (1.65) | 0.06 (0.01) | 84.91 (209.09) | 0.03 (0.02) | |
| 3 people | 969 (15.8) | 68 (7.1) | 901 (92.9) | 924 (95.4) | 45 (4.6) | 0.89 (1.53) | 0.06 (0.01) | 82.79 (172.72) | 0.04 (0.03) | |
| 4 people | 890 (14.8) | 67 (7.6) | 823 (92.4) | 859 (96.6) | 31 (3.4) | 0.70 (1.13) | 0.06 (0.01) | 83.94 (213.37) | 0.02 (0.02) | |
| 5 people or more | 331 (5.4) | 23 (6.9) | 308 (93.1) | 314 (94.9) | 17 (5.1) | 0.50 (0.67) | 0.06 (0.01) | 92.08 (205.55) | 0.04 (0.03) | |
| Unknown | 93 (1.5) | 9 (8.8) | 84 (91.2) | 86 (93.2) | 7 (6.8) | 1.15 (2.29) | 0.07 (0.01) | 68.55 (163.15) | 0.04 (0.03) | |
| Intake of immunosuppressive drugs | Yes | 178 (2.9) | 11 (6.1) | 167 (93.9) | 166 (93.3) | 12 (6.7) | 1.09 (1.21) | 0.06 (0.02) | 103.35 (234.73) | 0.03 (0.02) |
| No | 5855 (96.0) | 406 (6.9) | 5449 (93.1) | 5550 (94.8) | 305 (5.2) | 0.94 (1.53) | 0.06 (0.01) | 82.39 (199.94) | 0.03 (0.02) | |
| Unknown | 55 (1.1) | 7 (10.9) | 48 (89.1) | 51 (93.8) | 4 (6.2) | 0.81 (0.64) | 0.06 (0.008) | 144.25 (233.67) | 0.01 (0.01) | |
| Time since infection | Less than three months ago | 11 (0.1) | 7 (63.6) | 4 (36.4) | 10 (90.9) | 1 (9.1) | 0.74 (1.53) | 0.03 (0.03) | 835.43 (653.70) | 0.04 (NA) |
| Three to less than six months ago | 10 (0.1) | 3 (30.0) | 7 (70.0) | 10 (100.0) | 0 (0.0) | 0.74 (1.00) | 0.05 (0.03) | 184.22 (387.26) | - | |
| Six to twelve months ago | 81 (1.3) | 57 (70.3) | 24 (29.7) | 81 (100.0) | 0 (0.0) | 1.04 (1.75) | 0.06 (0.03) | 357.00 (500.08) | - | |
| More than twelve months ago | 118 (1.9) | 71 (59.6) | 47 (40.4) | 116 (98.4) | 2 (1.6) | 0.76 (1.10) | 0.06 (0.02) | 221.56 (301.96) | 0.05 (0.05) | |
| No infection | 5582 (91.8) | 0 (0.0) | 5582 (100.0) | 5268 (94.4) | 314 (5.6) | - | 0.06 (0.01) | 67.39 (166.41) | 0.03 (0.02) | |
| Unknown | 286 (4.8) | 286 (100.0) | 0 (0.0) | 282 (98.7) | 4 (1.3) | 0.98 (1.56) | - | 220.78 (323.30) | 0.05 (0.03) | |
| Breakthrough Infection (BTI) | Yes | 63 (1.1) | 28 (46.4) | 35 (53.6) | 62 (98.6) | 1 (1.4) | 0.58 (0.85) | 0.05 (0.03) | 546.24 (532.41) | 0.09 (NA) |
| No | 6018 (98.8) | 396 (6.5) | 5622 (93.5) | 5698 (94.8) | 320 (5.2) | 0.97 (1.55) | 0.06 (0.01) | 78.58 (187.13) | 0.03 (0.02) | |
| Not applicable | 7 (0.1) | 0 (0.0) | 7 (100.0) | 7 (100.0) | 0 (0.0) | - | 0.07 (0.02) | 21.87 (19.57) | - | |
| Vaccination scheme and infection (immunity) | Infection yes, not vaccinated | 40 (0.7) | 40 (100.0) | 0 (0.0) | 36 (90.0) | 4 (10.0) | 1.65 (2.64) | - | 18.30 (60.95) | 0.05 (0.03) |
| Infection yes + one vaccination | 123 (2.0) | 123 (100.0) | 0 (0.0) | 123 (100.0) | 0 (0.0) | 1.15 (1.53) | - | 238.20 (341.43) | - | |
| Infection yes + two vaccinations | 245 (4.0) | 245 (100.0) | 0 (0.0) | 245 (100.0) | 0 (0.0) | 0.75 (1.23) | - | 294.99 (398.29) | - | |
| Infection yes + three vaccinations | 16 (0.3) | 16 (100.0) | 0 (0.0) | 16 (100.0) | 0 (0.0) | 0.79 (1.07) | - | 437.20 (462.30) | - | |
| Infection no, not vaccinated | 313 (5.1) | 0 (0.0) | 313 (100.0) | 17 (5.5) | 296 (94.5) | - | 0.06 (0.02) | 2.56 (7.37) | 0.03 (0.02) | |
| Infection no + one vaccination | 257 (4.1) | 0 (0.0) | 257 (100.0) | 244 (94.9) | 13 (5.1) | - | 0.07 (0.01) | 27.40 (62.10) | 0.04 (0.03) | |
| Infection no + two vaccinations | 4756 (78.3) | 0 (0.0) | 4756 (100.0) | 4752 (99.9) | 4 (0.1) | - | 0.06 (0.01) | 43.06 (90.88) | 0.06 (0.02) | |
| Infection no + three vaccinations | 338 (5.5) | 0 (0.0) | 338 (100.0) | 334 (98.9) | 4 (1.1) | - | 0.06 (0.01) | 482.71 (414.94) | 0.03 (0.04) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinkemeyer, C.; Khazaei, Y.; Weigert, M.; Hannes, M.; Le Gleut, R.; Plank, M.; Winter, S.; Noreña, I.; Meier, T.; Xu, L.; et al. The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers. Viruses 2023, 15, 1574. https://doi.org/10.3390/v15071574
Reinkemeyer C, Khazaei Y, Weigert M, Hannes M, Le Gleut R, Plank M, Winter S, Noreña I, Meier T, Xu L, et al. The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers. Viruses. 2023; 15(7):1574. https://doi.org/10.3390/v15071574
Chicago/Turabian StyleReinkemeyer, Christina, Yeganeh Khazaei, Maximilian Weigert, Marlene Hannes, Ronan Le Gleut, Michael Plank, Simon Winter, Ivan Noreña, Theresa Meier, Lisa Xu, and et al. 2023. "The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers" Viruses 15, no. 7: 1574. https://doi.org/10.3390/v15071574
APA StyleReinkemeyer, C., Khazaei, Y., Weigert, M., Hannes, M., Le Gleut, R., Plank, M., Winter, S., Noreña, I., Meier, T., Xu, L., Rubio-Acero, R., Wiegrebe, S., Le Thi, T. G., Fuchs, C., Radon, K., Paunovic, I., Janke, C., Wieser, A., Küchenhoff, H., ... Castelletti, N., on behalf of the KoCo-Impf/ORCHESTRA working group. (2023). The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers. Viruses, 15(7), 1574. https://doi.org/10.3390/v15071574






