Different Patterns of Codon Usage and Amino Acid Composition across Primate Lentiviruses
Abstract
:1. Introduction
2. Materials and Methods
3. Results
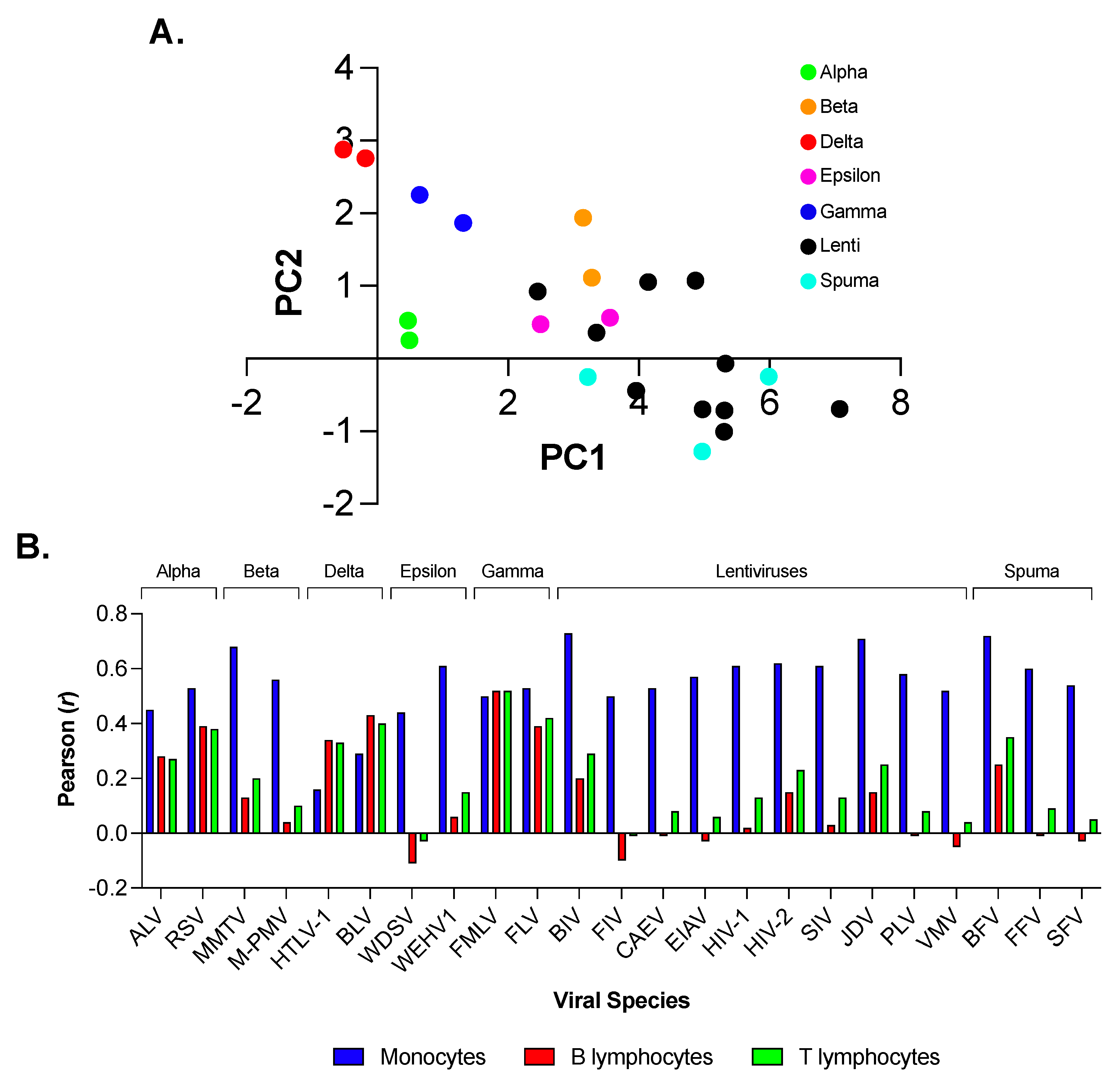
3.1. Principal Component Analysis (PCA) of Codon Usage in the Family of Retroviridae Revealed a Considerable Degree of Variation
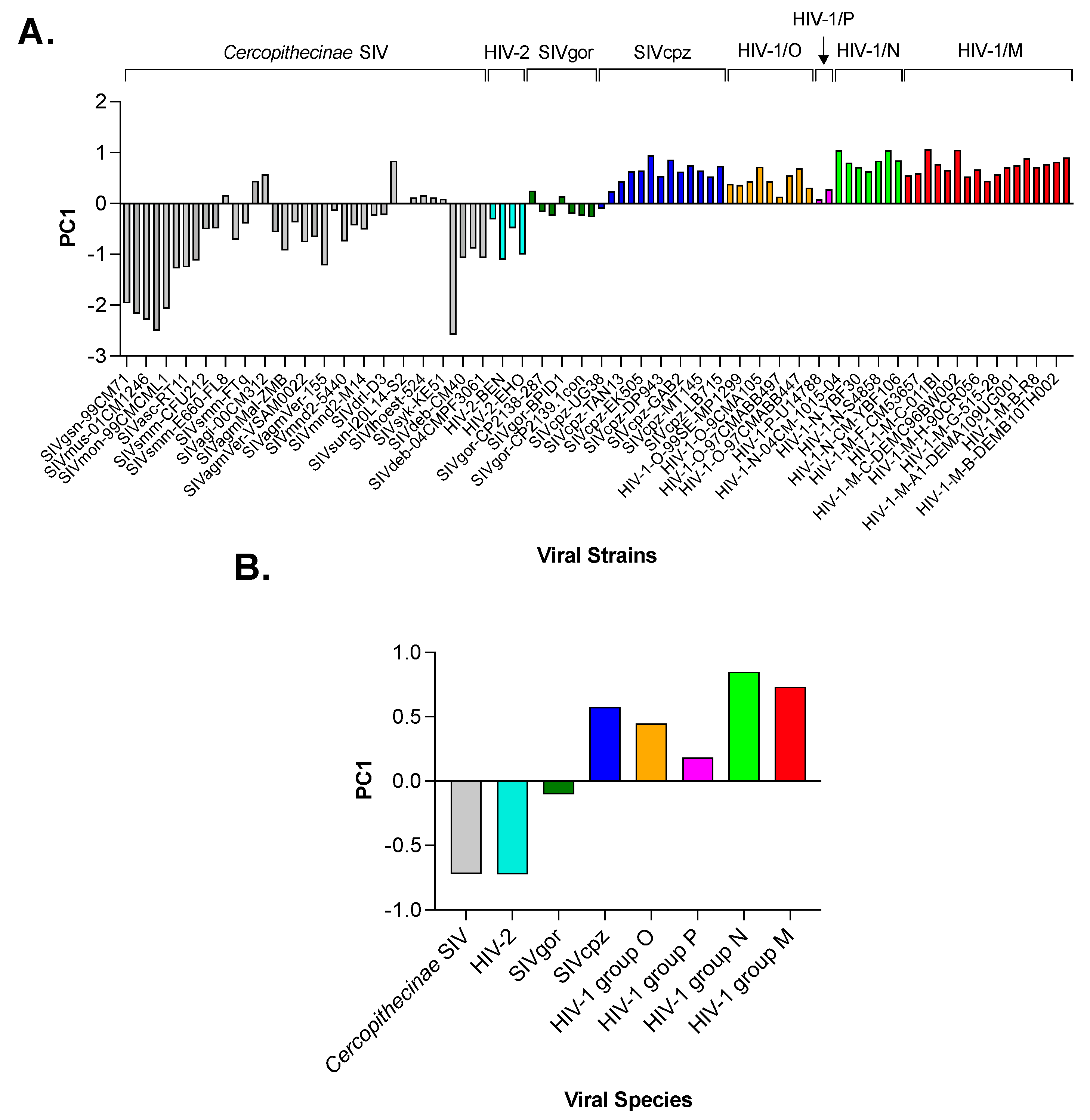
3.2. Principal Component Analysis (PCA) in Primate Lentiviruses Revealed a Trend in the Use of Synonymous and Nonsynonymous Codons
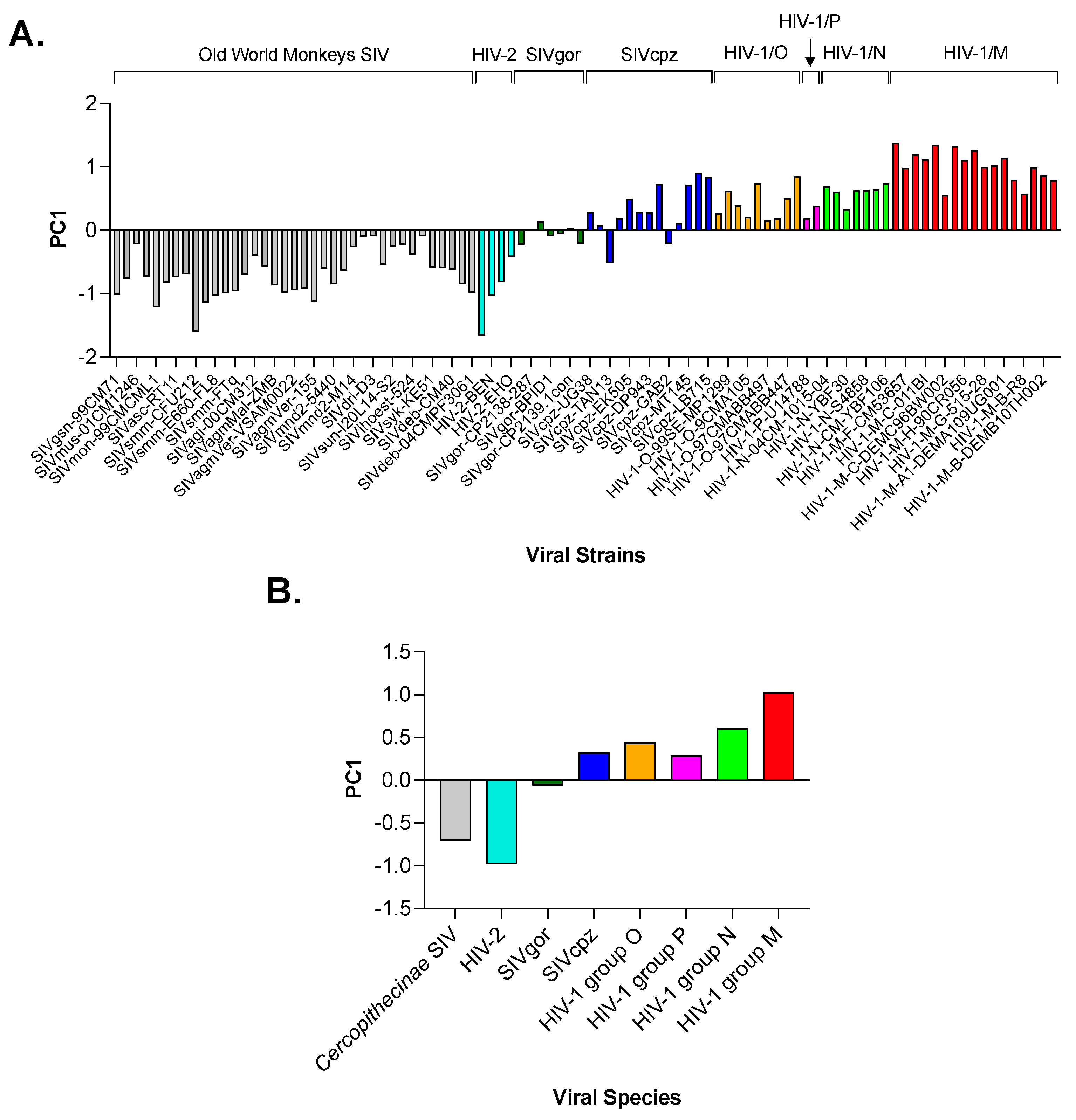
3.3. Principal Component Analysis (PCA) in Primate Lentiviruses Revealed a Trend in the Amino Acid Composition
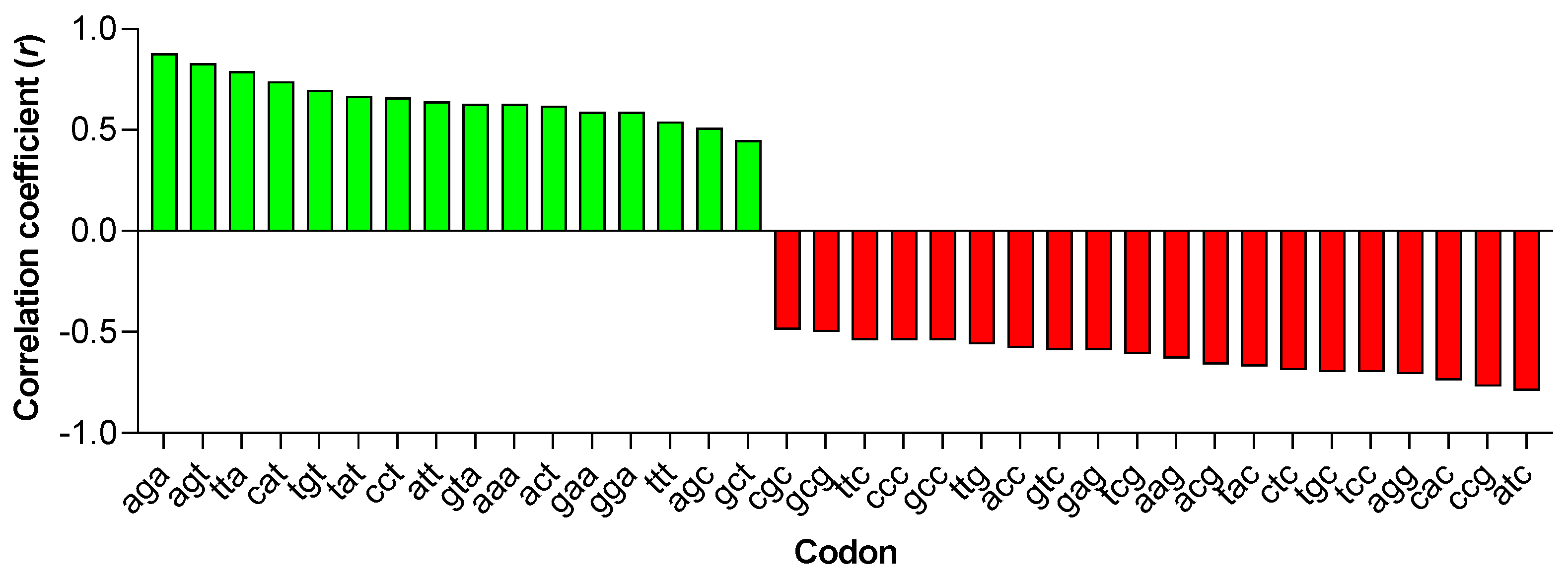
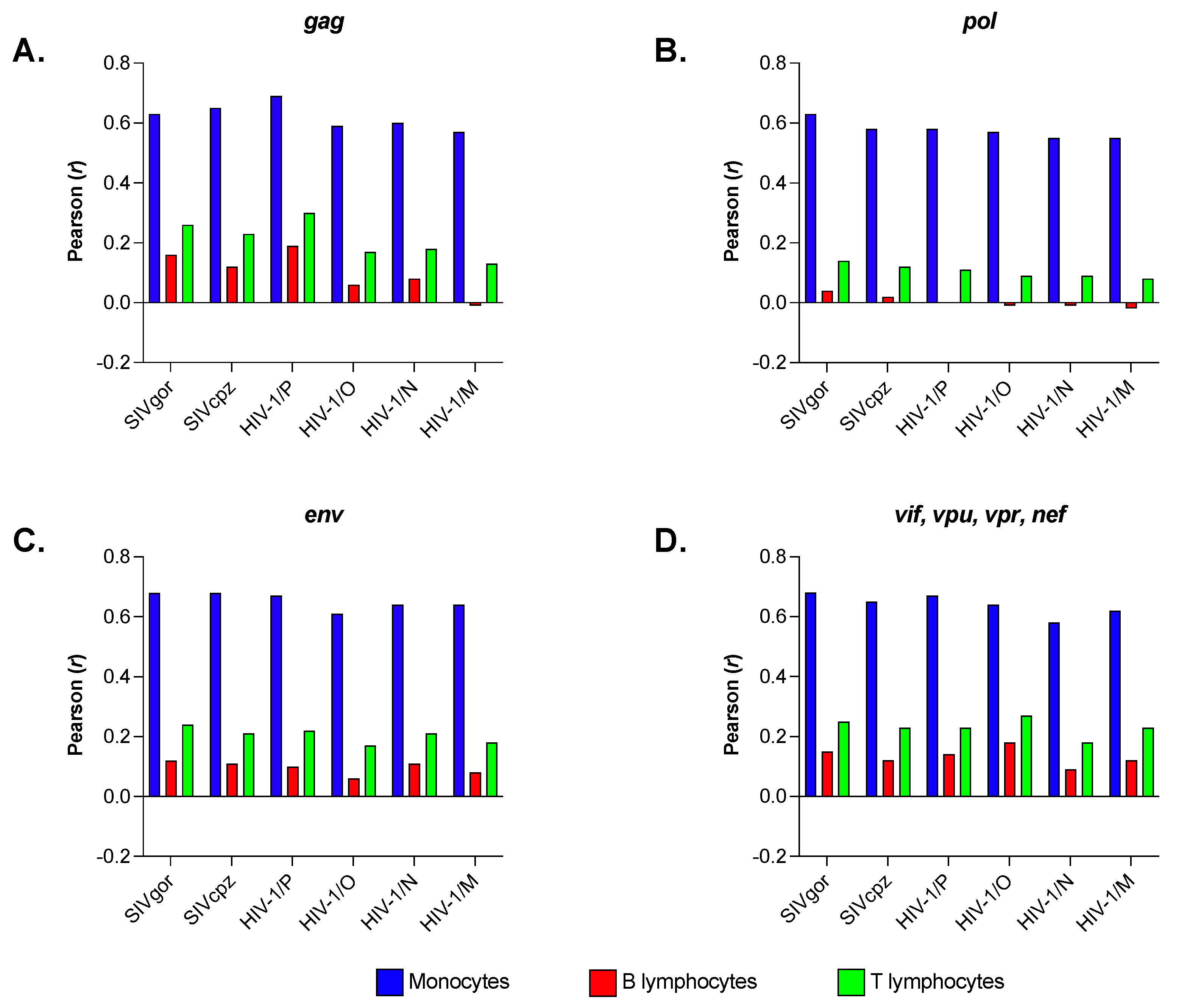
3.4. Primate Lentiviruses Have a Codon Usage Significantly Correlated to That of Human Monocytes (Innate Immunity) but Not to That of Human B and T Lymphocytes (Adaptive Immunity)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Hemert, F.J.; Berkhout, B. The tendency of lentiviral open reading frames to become A-rich: Constraints imposed by viral genome organization and cellular tRNA availability. J. Mol. Evol. 1995, 41, 132–140. [Google Scholar] [CrossRef] [PubMed]
- van der Kuyl, A.C.; Berkhout, B. The biased nucleotide composition of the HIV genome: A constant factor in a highly variable virus. Retrovirology 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hemert, F.; van der Kuyl, A.C.; Berkhout, B. On the nucleotide composition and structure of retroviral RNA genomes. Virus Res. 2014, 193, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kustin, T.; Stern, A. Biased Mutation and Selection in RNA Viruses. Mol. Biol. Evol. 2021, 38, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, B.; van Hemert, F.J. The unusual nucleotide content of the HIV RNA genome results in a biased amino acid composition of HIV proteins. Nucleic Acids Res. 1994, 22, 1705–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkhout, B.; Grigoriev, A.; Bakker, M.; Lukashov, V.V. Codon and amino acid usage in retroviral genomes is consistent with virus-specific nucleotide pressure. AIDS Res. Hum. Retroviruses 2002, 18, 133–141. [Google Scholar] [CrossRef]
- Cho, M.; Min, X.; Son, H.S. Analysis of evolutionary and genetic patterns in structural genes of primate lentiviruses. Genes Genom. 2022, 44, 773–791. [Google Scholar] [CrossRef]
- Karlin, S.; Doerfler, W.; Cardon, L.R. Why is CpG suppressed in the genomes of virtually all small eukaryotic viruses but not in those of large eukaryotic viruses? J. Virol. 1994, 68, 2889–2897. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Virk, N.; Chen, W.; Ji, S.; Ji, S.; Sun, Y.; Wu, X. CpG usage in RNA viruses: Data and hypotheses. PLoS ONE 2013, 8, e74109. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, K.; Payne, B.; Feyertag, F.; Alvarez-Ponce, D. The Codon Statistics Database: A Database of Codon Usage Bias. Mol. Biol. Evol. 2022, 39. [Google Scholar] [CrossRef]
- Pedersen, A.K.; Wiuf, C.; Christiansen, F.B. A codon-based model designed to describe lentiviral evolution. Mol. Biol. Evol. 1998, 15, 1069–1081. [Google Scholar] [CrossRef] [Green Version]
- Vidyavijayan, K.K.; Hassan, S.; Precilla, L.K.; Ashokkumar, M.; Chandrasekeran, P.; Swaminathan, S.; Hanna, L.E. Biased Nucleotide Composition and Differential Codon Usage Pattern in HIV-1 and HIV-2. AIDS Res. Hum. Retroviruses 2017, 33, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M. What can AIDS virus codon usage tell us? Nature 1986, 324, 114. [Google Scholar] [CrossRef] [PubMed]
- Ruzman, M.A.; Ripen, A.M.; Mirsafian, H.; Ridzwan, N.F.; Mohamad, S.B. Analysis of synonymous codon usage bias in human monocytes, B, and T lymphocytes based on transcriptome data. Gene Rep. 2021, 23, 101034. [Google Scholar] [CrossRef]
- Kumar, A.; Abbas, W.; Herbein, G. HIV-1 latency in monocytes/macrophages. Viruses 2014, 6, 1837–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenhuis, R.T.; Abreu, C.M.; Costa, P.A.G.; Ferreira, E.A.; Ratliff, J.; Pohlenz, L.; Shirk, E.N.; Rubin, L.H.; Blankson, J.N.; Gama, L.; et al. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat. Microbiol. 2023, 8, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Wright, F. The ‘effective number of codons’ used in a gene. Gene 1990, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Li, W.H. The codon Adaptation Index—A measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotelling, H. Relations between two sets of variates. Biometrika 1936, 28, 321–377. [Google Scholar] [CrossRef]
- Morrison, D.F. Multivariate Statistical Methods; McGraw-Hill: New York, NY, USA, 1976. [Google Scholar]
- Ringner, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, A.; Romerio, F. Extending the Coding Potential of Viral Genomes with Overlapping Antisense ORFs: A Case for the De Novo Creation of the Gene Encoding the Antisense Protein ASP of HIV-1. Viruses 2022, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Le, S.Y.; Malim, M.H.; Cullen, B.R.; Maizel, J.V. A highly conserved RNA folding region coincident with the Rev response element of primate immunodeficiency viruses. Nucleic Acids Res. 1990, 18, 1613–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirsafian, H.; Ripen, A.M.; Manaharan, T.; Mohamad, S.B.; Merican, A.F. Toward a Reference Gene Catalog of Human Primary Monocytes. OMICS 2016, 20, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Hotelling, H. The selection of variates for use in prediction with some comments on the problem of nuisance parameters. Ann. Math. Stat. 1940, 11, 271–283. [Google Scholar] [CrossRef]
- Dawson, B.; Trapp, R.G. Basic and Clinical Biostatistics, 3rd ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Iowa State University Press: Ames, IA, USA, 1967; p. 593. [Google Scholar]
- RoyChoudhury, S.; Mukherjee, D. Complex codon usage pattern and compositional features of retroviruses. Comput. Math. Methods Med. 2013, 2013, 848123. [Google Scholar] [CrossRef] [Green Version]
- Pandit, A.; Sinha, S. Differential trends in the codon usage patterns in HIV-1 genes. PLoS ONE 2011, 6, e28889. [Google Scholar] [CrossRef]
- Koning, F.A.; Newman, E.N.; Kim, E.Y.; Kunstman, K.J.; Wolinsky, S.M.; Malim, M.H. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J. Virol. 2009, 83, 9474–9485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doehle, B.P.; Schafer, A.; Cullen, B.R. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology 2005, 339, 281–288. [Google Scholar] [CrossRef] [Green Version]
- OhAinle, M.; Kerns, J.A.; Li, M.M.; Malik, H.S.; Emerman, M. Antiretroelement activity of APOBEC3H was lost twice in recent human evolution. Cell Host Microbe 2008, 4, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkhout, B.; van Hemert, F.J. Silent codon positions in the A-rich HIV RNA genome that do not easily become A: Restrictions imposed by the RNA sequence and structure. Virus Evol. 2022, 8, veac072. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Hahn, B.H. The evolution of HIV-1 and the origin of AIDS. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Marthas, M. Simian Immunodeficiency Virus: Animal Models of Disease. In Encyclopedia of Virology; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 594–603. [Google Scholar]
- Mayrose, I.; Stern, A.; Burdelova, E.O.; Sabo, Y.; Laham-Karam, N.; Zamostiano, R.; Bacharach, E.; Pupko, T. Synonymous site conservation in the HIV-1 genome. BMC Evol. Biol. 2013, 13, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazykin, G.A.; Dushoff, J.; Levin, S.A.; Kondrashov, A.S. Bursts of nonsynonymous substitutions in HIV-1 evolution reveal instances of positive selection at conservative protein sites. Proc. Natl. Acad. Sci. USA 2006, 103, 19396–19401. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, J.; Fellay, J.; Bartha, I.; Douek, D.C.; Telenti, A. Mapping of positive selection sites in the HIV-1 genome in the context of RNA and protein structural constraints. Retrovirology 2011, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, Z.K.; Williams, K.C. Monocytes in HIV and SIV Infection and Aging: Implications for Inflamm-Aging and Accelerated Aging. Viruses 2022, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J. Clin. Investig. 2023, 133, e167417. [Google Scholar] [CrossRef]
- van Weringh, A.; Ragonnet-Cronin, M.; Pranckeviciene, E.; Pavon-Eternod, M.; Kleiman, L.; Xia, X. HIV-1 modulates the tRNA pool to improve translation efficiency. Mol. Biol. Evol. 2011, 28, 1827–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Real, F.; Zhu, A.; Huang, B.; Belmellat, A.; Sennepin, A.; Vogl, T.; Ransy, C.; Revol, M.; Arrigucci, R.; Lombes, A.; et al. S100A8-mediated metabolic adaptation controls HIV-1 persistence in macrophages in vivo. Nat. Commun. 2022, 13, 5956. [Google Scholar] [CrossRef] [PubMed]
- Ganor, Y.; Real, F.; Sennepin, A.; Dutertre, C.A.; Prevedel, L.; Xu, L.; Tudor, D.; Charmeteau, B.; Couedel-Courteille, A.; Marion, S.; et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat. Microbiol. 2019, 4, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Desdouits, M.; Garzetti, L.; Podini, P.; Alfano, M.; Rubartelli, A.; Furlan, R.; Benaroch, P.; Poli, G. Extracellular ATP induces the rapid release of HIV-1 from virus containing compartments of human macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, E3265–E3273. [Google Scholar] [CrossRef]
- Rodrigues, V.; Ruffin, N.; San-Roman, M.; Benaroch, P. Myeloid Cell Interaction with HIV: A Complex Relationship. Front. Immunol. 2017, 8, 1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primate Lentiviruses | A | T | G | C | A + T | G + C |
|---|---|---|---|---|---|---|
| PC1 vs. first codon position | 0.83 | −0.75 | 0.27 | −0.76 | 0.60 | −0.60 |
| PC1 vs. second codon position | −0.32 | 0.52 | 0.74 | −0.51 | 0.11 | −0.11 |
| PC1 vs. third codon position | 0.83 | 0.85 | −0.88 | −0.84 | 0.95 | −0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavesi, A.; Romerio, F. Different Patterns of Codon Usage and Amino Acid Composition across Primate Lentiviruses. Viruses 2023, 15, 1580. https://doi.org/10.3390/v15071580
Pavesi A, Romerio F. Different Patterns of Codon Usage and Amino Acid Composition across Primate Lentiviruses. Viruses. 2023; 15(7):1580. https://doi.org/10.3390/v15071580
Chicago/Turabian StylePavesi, Angelo, and Fabio Romerio. 2023. "Different Patterns of Codon Usage and Amino Acid Composition across Primate Lentiviruses" Viruses 15, no. 7: 1580. https://doi.org/10.3390/v15071580





