Quadruplex Real-Time TaqMan® RT-qPCR Assay for Differentiation of Equine Group A and B Rotaviruses and Identification of Group A G3 and G14 Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Viruses, and Viral RNA
2.2. Bacterial DNA
2.3. Fecal Samples
2.4. Transmission Electron Microscopy (TEM)
2.5. Nucleic Acid Isolation
2.6. RT-PCR Amplification of ERVA VP7 (Segment 9) and ERVB VP6 Genes (Segment 6) and Sanger Sequencing for G-Typing
2.7. Accession Numbers
2.8. Primer and Probe Design
2.9. Synthesis of ERVA and ERVB In Vitro Transcribed RNA for Analytical Performance Evaluation
2.10. ERVA and ERVB-Specific Multiplex TaqMan® Real-Time RT-PCR Assays Targeting G3 VP7, G14 VP7 and NSP3 Genes of ERVA, and VP6 or NSP5 Genes of ERVB
2.11. Statistical Analysis
3. Results
3.1. Analysis of Fecal Samples Included in this Study by Standard RT-PCR and TEM
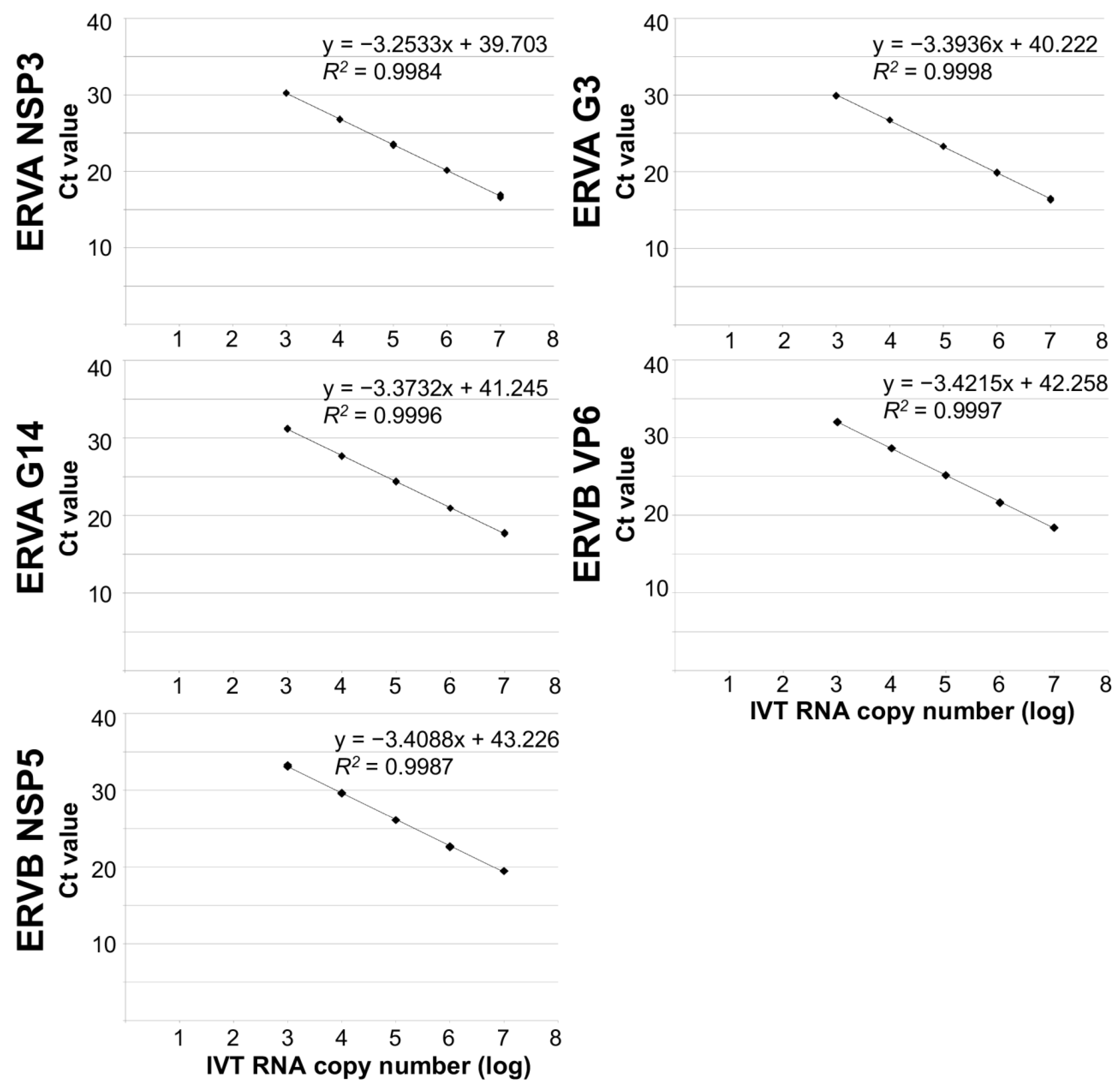
3.2. Analytical Performance of ERVA and ERVB-Specific Multiplex TaqMan® RT-qPCR Assays Targeting ERVA NSP3, G3 VP7, G14 VP7 and ERVB VP6 or NSP5
3.2.1. Analytical Sensitivity and Specificity of ERVA/ERVB-VP6-Specific Multiplex RT-qPCR Assay
3.2.2. Analytical Sensitivity and Specificity of ERVA/ERVB-NSP5-Specific Multiplex RT-qPCR Assay
3.2.3. Precision Assessment of ERVA/ERVB VP6 and ERVA/ERVB NSP5-Specific Multiplex RT-qPCR Assays
3.3. Clinical Performance of the ERVA/ERVB VP6-Specific Multiplex RT-qPCR Assay Targeting ERVA NSP3, G3 VP7, G14 VP7 and ERVB VP6 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, K.E.; Gilkerson, J.R.; Browning, G.F. Equine rotaviruses--current understanding and continuing challenges. Vet. Microbiol. 2013, 167, 135–144. [Google Scholar] [CrossRef]
- Conner, M.E.; Darlington, R.W. Rotavirus infection in foals. Am. J. Vet. Res. 1980, 41, 1699–1703. [Google Scholar] [PubMed]
- Dickson, J.; Smith, V.W.; Coackley, W.; McKean, P.; Adams, P.S. Rotavirus infection of foals. Aust. Vet. J. 1979, 55, 207–208. [Google Scholar]
- Garaicoechea, L.; Mino, S.; Ciarlet, M.; Fernandez, F.; Barrandeguy, M.; Parreno, V. Molecular characterization of equine rotaviruses circulating in Argentinean foals during a 17-year surveillance period (1992–2008). Vet. Microbiol. 2011, 148, 150–160. [Google Scholar] [CrossRef]
- Imagawa, H.; Sekiguchi, K.; Anzai, T.; Fukunaga, Y.; Kanemaru, T.; Ohishi, H.; Higuchi, T.; Kamada, M. Epidemiology of equine rotavirus infection among foals in the breeding region. J. Vet. Med. Sci. 1991, 53, 1079–1080. [Google Scholar] [CrossRef] [Green Version]
- Magdesian, K.G.; Dwyer, R.M.; Gonzalez Arguedas, M. Viral Diarrhea. In Equine Infectious Diseases; Sellon, D.C., Long, M.T., Eds.; Saunders: St. Louis, MO, USA, 2014; pp. 198–203. [Google Scholar]
- Slovis, N.M.; Elam, J.; Estrada, M.; Leutenegger, C.M. Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: A comprehensive molecular study. Equine Vet. J. 2014, 46, 311–316. [Google Scholar] [CrossRef]
- Kopper, J.J. Equine Rotaviral Diarrhea. Vet. Clin. N. Am. Equine Pract. 2023, 39, 47–54. [Google Scholar] [CrossRef]
- Nemoto, M.; Matsumura, T. Equine rotavirus infection. J. Equine Sci. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Uprety, T.; Sreenivasan, C.C.; Hause, B.M.; Li, G.; Odemuyiwa, S.O.; Locke, S.; Morgan, J.; Zeng, L.; Gilsenan, W.F.; Slovis, N.; et al. Identification of a Ruminant Origin Group B Rotavirus Associated with Diarrhea Outbreaks in Foals. Viruses 2021, 13, 1330. [Google Scholar] [CrossRef]
- Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009). Arch. Virol. 2010, 155, 133–146. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Banyai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef] [Green Version]
- Matthijnssens, J.; Attoui, H.; Banyai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Newman, J.F.; Brown, F.; Bridger, J.C.; Woode, G.N. Characterisation of a rotavirus. Nature 1975, 258, 631–633. [Google Scholar] [CrossRef]
- Eren, E.; Zamuda, K.; Patton, J.T. Modeling of the rotavirus group C capsid predicts a surface topology distinct from other rotavirus species. Virology 2016, 487, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Z.; Settembre, E.C.; Aoki, S.T.; Zhang, X.; Bellamy, A.R.; Dormitzer, P.R.; Harrison, S.C.; Grigorieff, N. Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM. Proc. Natl. Acad. Sci. USA 2009, 106, 10644–10648. [Google Scholar] [CrossRef]
- Settembre, E.C.; Chen, J.Z.; Dormitzer, P.R.; Grigorieff, N.; Harrison, S.C. Atomic model of an infectious rotavirus particle. EMBO J. 2011, 30, 408–416. [Google Scholar] [CrossRef]
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef] [Green Version]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef]
- Johne, R.; Schilling-Loeffler, K.; Ulrich, R.G.; Tausch, S.H. Whole Genome Sequence Analysis of a Prototype Strain of the Novel Putative Rotavirus Species L. Viruses 2022, 14, 462. [Google Scholar] [CrossRef]
- Johne, R.; Tausch, S.H.; Ulrich, R.G.; Schilling-Loeffler, K. Genome analysis of the novel putative rotavirus species K. Virus Res. 2023, 334, 199171. [Google Scholar] [CrossRef]
- Aoki, S.T.; Settembre, E.C.; Trask, S.D.; Greenberg, H.B.; Harrison, S.C.; Dormitzer, P.R. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science 2009, 324, 1444–1447. [Google Scholar] [CrossRef] [Green Version]
- Estes, M.K.; Graham, D.Y.; Mason, B.B. Proteolytic enhancement of rotavirus infectivity: Molecular mechanisms. J. Virol. 1981, 39, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Zeller, M.; Patton, J.T.; Heylen, E.; De Coster, S.; Ciarlet, M.; Van Ranst, M.; Matthijnssens, J. Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in Rotarix and RotaTeq. J. Clin. Microbiol. 2012, 50, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gomara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef] [Green Version]
- Matthijnssens, J.; Mino, S.; Papp, H.; Potgieter, C.; Novo, L.; Heylen, E.; Zeller, M.; Garaicoechea, L.; Badaracco, A.; Lengyel, G.; et al. Complete molecular genome analyses of equine rotavirus A strains from different continents reveal several novel genotypes and a largely conserved genotype constellation. J. Gen. Virol. 2012, 93, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Tsunemitsu, H.; Imagawa, H.; Hata, H.; Higuchi, T.; Sato, S.; Orita, Y.; Sugita, S.; Bannai, H.; Tsujimura, K.; et al. Molecular characterization and analysis of equine rotavirus circulating in Japan from 2003 to 2008. Vet. Microbiol. 2011, 152, 67–73. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ons, E.; De Coster, S.; Conceicao-Neto, N.; Gryspeerdt, A.; Van Ranst, M.; Raue, R. Molecular characterization of equine rotaviruses isolated in Europe in 2013: Implications for vaccination. Vet. Microbiol. 2015, 176, 179–185. [Google Scholar] [CrossRef]
- Nemoto, M.; Niwa, H.; Murakami, S.; Miki, R.; Higuchi, T.; Bannai, H.; Tsujimura, K.; Kokado, H. Molecular analyses of G3A/G3B and G14 equine group A rotaviruses detected between 2012 and 2018 in Japan. J. Gen. Virol. 2019, 100, 913–931. [Google Scholar] [CrossRef]
- Carossino, M.; Barrandeguy, M.E.; Li, Y.; Parreno, V.; Janes, J.; Loynachan, A.T.; Balasuriya, U.B.R. Detection, molecular characterization and phylogenetic analysis of G3P[12] and G14P[12] equine rotavirus strains co-circulating in central Kentucky. Virus Res. 2018, 255, 39–54. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; de Figueiredo, M.R.; Fialho, A.M.; Cantelli, C.P.; Miagostovich, M.P.; Fumian, T.M. Nosocomial acute gastroenteritis outbreak caused by an equine-like G3P[8] DS-1-like rotavirus and GII.4 Sydney[P16] norovirus at a pediatric hospital in Rio de Janeiro, Brazil, 2019. Hum. Vaccines Immunother. 2021, 17, 4654–4660. [Google Scholar] [CrossRef]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.C.C.; Boen, L.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J. Gen. Virol. 2019, 100, 7–25. [Google Scholar] [CrossRef]
- Perkins, C.; Mijatovic-Rustempasic, S.; Ward, M.L.; Cortese, M.M.; Bowen, M.D. Genomic Characterization of the First Equine-Like G3P[8] Rotavirus Strain Detected in the United States. Genome Announc. 2017, 5, e01317–e01341. [Google Scholar] [CrossRef] [Green Version]
- Silva Serra, A.C.; Junior, E.C.; Cruz, J.F.; Lobo, P.S.; Junior, E.T.; Bandeira, R.S.; Bezerra, D.A.; Mascarenhas, J.D.; Santos Guerra, S.F.; Soares, L.S. Molecular analysis of G3P[6] rotavirus in the Amazon region of Brazil: Evidence of reassortment with equine-like strains. Future Microbiol. 2021, 16, 847–862. [Google Scholar] [CrossRef]
- Barrandeguy, M.; Parreno, V.; Lagos Marmol, M.; Pont Lezica, F.; Rivas, C.; Valle, C.; Fernandez, F. Prevention of rotavirus diarrhoea in foals by parenteral vaccination of the mares: Field trial. Dev. Biol. Stand. 1998, 92, 253–257. [Google Scholar]
- Powell, D.G.; Dwyer, R.M.; Traub-Dargatz, J.L.; Fulker, R.H.; Whalen, J.W., Jr.; Srinivasappa, J.; Acree, W.M.; Chu, H.J. Field study of the safety, immunogenicity, and efficacy of an inactivated equine rotavirus vaccine. J. Am. Vet. Med. Assoc. 1997, 211, 193–198. [Google Scholar]
- Sheoran, A.S.; Karzenski, S.S.; Whalen, J.W.; Crisman, M.V.; Powell, D.G.; Timoney, J.F. Prepartum equine rotavirus vaccination inducing strong specific IgG in mammary secretions. Vet. Rec. 2000, 146, 672–673. [Google Scholar]
- Nemoto, M.; Inagaki, M.; Tamura, N.; Bannai, H.; Tsujimura, K.; Yamanaka, T.; Kokado, H. Evaluation of inactivated vaccines against equine group A rotaviruses by use of a suckling mouse model. Vaccine 2018, 36, 5551–5555. [Google Scholar] [CrossRef] [PubMed]
- Papp, H.; Matthijnssens, J.; Martella, V.; Ciarlet, M.; Banyai, K. Global distribution of group A rotavirus strains in horses: A systematic review. Vaccine 2013, 31, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- Browning, G.F.; Chalmers, R.M.; Fitzgerald, T.A.; Snodgrass, D.R. Serological and genomic characterization of L338, a novel equine group A rotavirus G serotype. J. Gen. Virol. 1991, 72 Pt 5, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Browning, G.F.; Fitzgerald, T.A.; Chalmers, R.M.; Snodgrass, D.R. A novel group A rotavirus G serotype: Serological and genomic characterization of equine isolate FI23. J. Clin. Microbiol. 1991, 29, 2043–2046. [Google Scholar]
- Hardy, M.E.; Woode, G.N.; Xu, Z.C.; Williams, J.D.; Conner, M.E.; Dwyer, R.M.; Powell, D.G. Analysis of serotypes and electropherotypes of equine rotaviruses isolated in the United States. J. Clin. Microbiol. 1991, 29, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemoto, M.; Tsunemitsu, H.; Murase, H.; Nambo, Y.; Sato, S.; Orita, Y.; Imagawa, H.; Bannai, H.; Tsujimura, K.; Yamanaka, T.; et al. Antibody response in vaccinated pregnant mares to recent G3BP[12] and G14P[12] equine rotaviruses. Acta Vet. Scand. 2012, 54, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miño, S.; Adúriz, M.; Barrandeguy, M.; Parreño, V. Molecular Characterization of Equine Rotavirus Group A Detected in Argentinean Foals During 2009–2014. J. Equine Vet. Sci. 2017, 59, 64–70. [Google Scholar] [CrossRef]
- Brown, D.W.; Beards, G.M.; Chen, G.M.; Flewett, T.H. Prevalence of antibody to group B (atypical) rotavirus in humans and animals. J. Clin. Microbiol. 1987, 25, 316–319. [Google Scholar] [CrossRef] [Green Version]
- Marthaler, D.; Rossow, K.; Gramer, M.; Collins, J.; Goyal, S.; Tsunemitsu, H.; Kuga, K.; Suzuki, T.; Ciarlet, M.; Matthijnssens, J. Detection of substantial porcine group B rotavirus genetic diversity in the United States, resulting in a modified classification proposal for G genotypes. Virology 2012, 433, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyabe, F.M.; Dall Agnol, A.M.; Leme, R.A.; Oliveira, T.E.S.; Headley, S.A.; Fernandes, T.; de Oliveira, A.G.; Alfieri, A.F.; Alfieri, A.A. Porcine rotavirus B as primary causative agent of diarrhea outbreaks in newborn piglets. Sci. Rep. 2020, 10, 22002. [Google Scholar] [CrossRef]
- Otto, P.H.; Rosenhain, S.; Elschner, M.C.; Hotzel, H.; Machnowska, P.; Trojnar, E.; Hoffmann, K.; Johne, R. Detection of rotavirus species A, B and C in domestic mammalian animals with diarrhoea and genotyping of bovine species A rotavirus strains. Vet. Microbiol. 2015, 179, 168–176. [Google Scholar] [CrossRef]
- Chen, F.; Knutson, T.P.; Ciarlet, M.; Sturos, M.; Marthaler, D.G. Complete genome characterization of a rotavirus B (RVB) strain identified in Alpine goat kids with enteritis reveals inter-species transmission with RVB bovine strains. J. Gen. Virol. 2018, 99, 457–463. [Google Scholar] [CrossRef]
- Hayashi-Miyamoto, M.; Murakami, T.; Minami-Fukuda, F.; Tsuchiaka, S.; Kishimoto, M.; Sano, K.; Naoi, Y.; Asano, K.; Ichimaru, T.; Haga, K.; et al. Diversity in VP3, NSP3, and NSP4 of rotavirus B detected from Japanese cattle. Infect. Genet. Evol. 2017, 49, 97–103. [Google Scholar] [CrossRef]
- Shepherd, F.K.; Herrera-Ibata, D.M.; Porter, E.; Homwong, N.; Hesse, R.; Bai, J.; Marthaler, D.G. Whole Genome Classification and Phylogenetic Analyses of Rotavirus B strains from the United States. Pathogens 2018, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Kuga, K.; Miyazaki, A.; Tsunemitsu, H. Genetic divergence and classification of non-structural protein 1 among porcine rotaviruses of species B. J. Gen. Virol. 2011, 92, 2922–2929. [Google Scholar] [CrossRef]
- Saiada, F.; Rahman, H.N.A.; Moni, S.; Karim, M.M.; Pourkarim, M.R.; Azim, T.; Rahman, M. Clinical presentation and molecular characterization of group B rotaviruses in diarrhoea patients in Bangladesh. J. Med. Microbiol. 2011, 60, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.Y.; Glass, R.I.; Penaranda, M.; Dong, H.; Monroe, S.S.; Wen, L.; Estes, M.K.; Eiden, J.; Yolken, R.H.; Saif, L.; et al. Purification and characterization of adult diarrhea rotavirus: Identification of viral structural proteins. J. Virol. 1989, 63, 2191–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.Y.; Monroe, S.S.; Dong, H.; Penaranda, M.; Wen, L.; Gouvea, V.; Allen, J.R.; Hung, T.; Glass, R.I. Coding assignments of the genome of adult diarrhea rotavirus. Arch. Virol. 1992, 125, 53–69. [Google Scholar] [CrossRef]
- Diller, J.R.; Parrington, H.M.; Patton, J.T.; Ogden, K.M. Rotavirus Species B Encodes a Functional Fusion-Associated Small Transmembrane Protein. J. Virol. 2019, 93, e00813–e00819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiden, J.J. Expression and sequence analysis of gene 7 of the IDIR agent (group B rotavirus): Similarity with NS53 of group A rotavirus. Virology 1994, 199, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Carossino, M.; Barrandeguy, M.E.; Erol, E.; Li, Y.; Balasuriya, U.B.R. Development and evaluation of a one-step multiplex real-time TaqMan((R)) RT-qPCR assay for the detection and genotyping of equine G3 and G14 rotaviruses in fecal samples. Virol. J. 2019, 16, 49. [Google Scholar] [CrossRef]
- Zhang, J.; Guy, J.S.; Snijder, E.J.; Denniston, D.A.; Timoney, P.J.; Balasuriya, U.B. Genomic characterization of equine coronavirus. Virology 2007, 369, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Carossino, M.; Lee, P.A.; Nam, B.; Skillman, A.; Shuck, K.M.; Timoney, P.J.; Tsai, Y.; Ma, L.; Chang, H.G.; Wang, H.T.; et al. Development and evaluation of a reverse transcription-insulated isothermal polymerase chain reaction (RT-iiPCR) assay for detection of equine arteritis virus in equine semen and tissue samples using the POCKIT system. J. Virol. Methods 2016, 234, 7–15. [Google Scholar] [CrossRef]
- Mino, S.; Barrandeguy, M.; Parreno, V.; Parra, G.I. Genetic linkage of capsid protein-encoding RNA segments in group A equine rotaviruses. J. Gen. Virol. 2016, 97, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.O.; Parwani, A.V.; Saif, L.J. The characterization of VP7 (G type) and VP4 (P type) genes of bovine group A rotaviruses from field samples using RT-PCR and RFLP analysis. Arch. Virol. 1996, 141, 1727–1739. [Google Scholar] [CrossRef]
- Freeman, M.M.; Kerin, T.; Hull, J.; McCaustland, K.; Gentsch, J. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J. Med. Virol. 2008, 80, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Validation of laboratory-developed molecular assays for infectious diseases. Clin. Microbiol. Rev. 2010, 23, 550–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006, 12, 304–306. [Google Scholar] [CrossRef] [Green Version]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Bialasiewicz, S.; Whiley, D.M.; Nissen, M.D.; Sloots, T.P. Impact of competitive inhibition and sequence variation upon the sensitivity of malaria PCR. J. Clin. Microbiol. 2007, 45, 1621–1623. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, D.; Hassan, F.; Avadhanula, V.; Piedra, P.A.; Boom, J.; Sahni, L.C.; Weinberg, G.A.; Lindstrom, S.; Rha, B.; Harrison, C.J.; et al. Comparative analysis of three multiplex platforms for the detection of respiratory viral pathogens. J. Clin. Virol. 2022, 156, 105274. [Google Scholar] [CrossRef]
- Das, A.; Wang, Y.; Babiuk, S.; Bai, J.; Dodd, K.; Jia, W. Development of multiplex real-time PCR assays for differential detection of capripoxvirus, parapoxvirus and foot-and-mouth disease virus. Transbound. Emerg. Dis. 2022, 69, 1326–1337. [Google Scholar] [CrossRef]
- Das, A.; Xu, L.; Jia, W. Development of conventional and real time PCR assays for rapid species authentication of mammalian cell lines commonly used in veterinary diagnostic laboratories. Res. Vet. Sci. 2019, 126, 170–177. [Google Scholar] [CrossRef]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Furer, F.; Fraefel, C.; Lechmann, J. Multiplex real-time PCR for the detection and differentiation of equid gammaherpesvirus 2 and 5. J. Virol. Methods 2022, 310, 114615. [Google Scholar] [CrossRef] [PubMed]
- Gunson, R.N.; Bennett, S.; Maclean, A.; Carman, W.F. Using multiplex real time PCR in order to streamline a routine diagnostic service. J. Clin. Virol. 2008, 43, 372–375. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Wong, A.A.; Ma, R.; Zelyas, N.; Tipples, G.A. Development and validation of a multiplex reverse transcriptase-PCR assay for simultaneous testing of influenza A, influenza B and SARS-CoV-2. J. Virol. Methods 2021, 293, 114151. [Google Scholar] [CrossRef]
- Parker, J.; Fowler, N.; Walmsley, M.L.; Schmidt, T.; Scharrer, J.; Kowaleski, J.; Grimes, T.; Hoyos, S.; Chen, J. Correction: Analytical Sensitivity Comparison between Singleplex Real-Time PCR and a Multiplex PCR Platform for Detecting Respiratory Viruses. PLoS ONE 2018, 13, e0205483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbussche, F.; Vanbinst, T.; Vandemeulebroucke, E.; Goris, N.; Sailleau, C.; Zientara, S.; De Clercq, K. Effect of pooling and multiplexing on the detection of bluetongue virus RNA by real-time RT-PCR. J. Virol. Methods 2008, 152, 13–17. [Google Scholar] [CrossRef] [PubMed]


| Name | Target | Nucleotide Position | Sequence (5′ to 3′) |
|---|---|---|---|
| NVP3-FDeg 1 | RVA NSP3 | 963–982 a | ACCATCTWCACRTRACCCTC |
| NVP3-R1 1 | RVA NSP3 | 1053–1034 a | GGTCACATAACGCCCCTATA |
| NVP3-Probe 1 | RVA NSP3 | 984–1026 a | JUN-ATGAGCACAATAGTTAAAAGCTAACACTGTCAA-QSY |
| RVA-G3-756F | ERVA VP7 (G3) b | 756–777 | GATGTTACCACGACCACTTGTA |
| RVA-G3-872R | ERVA VP7 (G3) b | 872–854 | AGTTGGATCGGCCGTTATG |
| RVA-G3-779P | ERVA VP7 (G3) b | 779–823 | FAM-TGGGACCACGAGAGAATGTAGCTGT-MGB |
| RVA-G14-ARG869F | ERVA VP7 (G14) c | 869–885 | ATCCGACTACGGCTCCA |
| RVA-G14-ARG1011R | ERVA VP7 (G14) c | 1011–990 | TGCAGCAGAATTTAATGATCGC |
| RVA-G14-ARG886P | ERVA VP7 (G14) c | 886–915 | VIC-CAGATTGGACGAATGATGCGTATAAATTGG-MGB |
| ERVB-VP6-F | ERVB VP6 | 132–153 d | CATCCAGAGTGAATGGGAAGAC |
| ERVB-VP6-R | ERVB VP6 | 230–210 d | TTCTAACGGCCAGCGAAATTA |
| ERVB-VP6-P | ERVB VP6 | 187–209 d | LIZ-CCCTTACACGATACACGCACCGA-QSY |
| ERVB-NSP5-F | ERVB NSP5 | 124–146 e | GCCTTCTGATTCTACGTCAACTA |
| ERVB-NSP5-R | ERVB NSP5 | 238–215 e | CTTGTTGTACGCTTCTTCGTATTC |
| ERVB-NSP5-P | ERVB NSP5 | 160–183 e | LIZ-AACATCAAGTCGTAGCGACGCAGT-QSY |
| ERVA Singleplex | ERVB Singleplex | Quadruplex (ERVA/ERVB VP6) | Quadruplex (ERVA/ERVB NSP5) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | G3 | G14 | NSP3 | VP6 | NSP5 | G3 | G14 | NSP3 | VP6 | G3 | G14 | NSP3 | NSP5 |
| Slope | −3.3936 | −3.3732 | −3.2533 | −3.4215 | −3.4088 | −3.1487 | −3.3054 | −3.3159 | −3.496 | −3.3288 | −3.4355 | −3.4354 | −3.3723 |
| Linearity (R2) | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 |
| Efficiency (%) | 97 | 98 | 103 | 96 | 96.5 | 108 | 100 | 100 | 93.22 | 100 | 95 | 95 | 98 |
| LOD95% (copies/μL) | 2.6 | 5.7 | 27 | 20 | 20 | 67 | 67 | 67 | 747 | 67 | 67 | 67 | 747 |
| Detection rate limit (100%, copies/μL) | 10 | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 1000 | 100 | 100 | 100 | 1000 |
| Ct cut-off | 38 | 39 | 34 | 36 | 37 | 34 | 39 | 35 | 34 | 35 | 36 | 34 | 35 |
| (a) Within-run | ERVA/ERVB VP6 Quadruplex Assay | ERVA/ERVB NSP5 Quadruplex Assay | ||||||
| Concentration of target (IVT RNA copies/μL) | G3 | G14 | NSP3 | VP6 | G3 | G14 | NSP3 | NSP5 |
| 100,000 | 0.55% | 0.43% | 0.66% | 0.33% | 0.86% | 0.91% | 0.77% | 0.51% |
| 10,000 | 1.62% | 1.05% | 1.11% | 0.53% | 1.11% | 0.85% | 1.91% | 0.97% |
| 1000 | 0.89% | 1.68% | 1.87% | 1.11% | 0.51% | 1.3% | 1.99% | 0.99% |
| (b) Between-run | ERVA/ERVB VP6 Quadruplex Assay | ERVA/ERVB NSP5 Quadruplex Assay | ||||||
| Concentration of target (IVT RNA copies/μL) | G3 | G14 | NSP3 | VP6 | G3 | G14 | NSP3 | NSP5 |
| 100,000 | 1.2% | 1.1% | 0.30% | 0.32% | 1.2% | 1.1% | 0.66% | 0.30% |
| 10,000 | 0.95% | 1.23% | 1.35% | 0.45% | 1.05% | 1.14% | 1.77% | 0.80% |
| 1000 | 1.07% | 2.31% | 2.17% | 0.90% | 0.46% | 0.93% | 1.03% | 0.59% |
| (a) | ERVA VP7-Specific RT-PCR | |||
| Positive | Negative | Total | ||
| NSP3-specific RT-qPCR | Positive | 78 | 0 | 78 |
| Negative | 7 | 108 | 115 | |
| Total | 85 | 108 | 193 | |
| (b) | ERVA Genotype G3 1 | |||
| Positive | Negative | Total | ||
| G3-specific RT-qPCR | Positive | 38 | 0 | 38 |
| Negative | 3 | 152 | 155 | |
| Total | 41 | 152 | 193 | |
| (c) | ERVA Genotype G14 1 | |||
| Positive | Negative | Total | ||
| G14-specific RT-qPCR | Positive | 44 | 0 | 44 |
| Negative | 1 | 148 | 149 | |
| Total | 45 | 148 | 193 | |
| (d) | ERVB VP6-Specific RT-PCR | |||
| Positive | Negative | Total | ||
| VP6-specific RT-qPCR | Positive | 14 | 0 | 14 |
| Negative | 1 | 178 | 179 | |
| Total | 15 | 178 | 193 | |
| Target | ERVA Triplex Sensitivity | ERVA/ERVB VP6 Quadruplex Sensitivity | p-Value * |
|---|---|---|---|
| NSP3 | 100% | 91.8% | 0.0083 |
| G3 | 92.7% | 92.7% | 1 |
| G14 | 100% | 97.8% | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carossino, M.; Balasuriya, U.B.R.; Thieulent, C.J.; Barrandeguy, M.E.; Vissani, M.A.; Parreño, V. Quadruplex Real-Time TaqMan® RT-qPCR Assay for Differentiation of Equine Group A and B Rotaviruses and Identification of Group A G3 and G14 Genotypes. Viruses 2023, 15, 1626. https://doi.org/10.3390/v15081626
Carossino M, Balasuriya UBR, Thieulent CJ, Barrandeguy ME, Vissani MA, Parreño V. Quadruplex Real-Time TaqMan® RT-qPCR Assay for Differentiation of Equine Group A and B Rotaviruses and Identification of Group A G3 and G14 Genotypes. Viruses. 2023; 15(8):1626. https://doi.org/10.3390/v15081626
Chicago/Turabian StyleCarossino, Mariano, Udeni B. R. Balasuriya, Côme J. Thieulent, Maria E. Barrandeguy, Maria Aldana Vissani, and Viviana Parreño. 2023. "Quadruplex Real-Time TaqMan® RT-qPCR Assay for Differentiation of Equine Group A and B Rotaviruses and Identification of Group A G3 and G14 Genotypes" Viruses 15, no. 8: 1626. https://doi.org/10.3390/v15081626
APA StyleCarossino, M., Balasuriya, U. B. R., Thieulent, C. J., Barrandeguy, M. E., Vissani, M. A., & Parreño, V. (2023). Quadruplex Real-Time TaqMan® RT-qPCR Assay for Differentiation of Equine Group A and B Rotaviruses and Identification of Group A G3 and G14 Genotypes. Viruses, 15(8), 1626. https://doi.org/10.3390/v15081626






