Morphological and Molecular Changes in the Cortex and Cerebellum of Immunocompetent Mice Infected with Zika Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Generation of the Viral Stock
2.3. Obtaining Mice Infected with ZIKV for Histological Studies and Differential Expression Assays
2.4. Detection of ZIKV by qRT-PCR and Quantification of the Viral Load
2.5. Histological Analysis and Immunohistochemical Detection of ZIKV
2.6. Differential Expression Assays
2.7. Network Analysis
2.8. Statistical Analysis
3. Results
3.1. Observation of Signs of Illness
3.2. Histological Findings
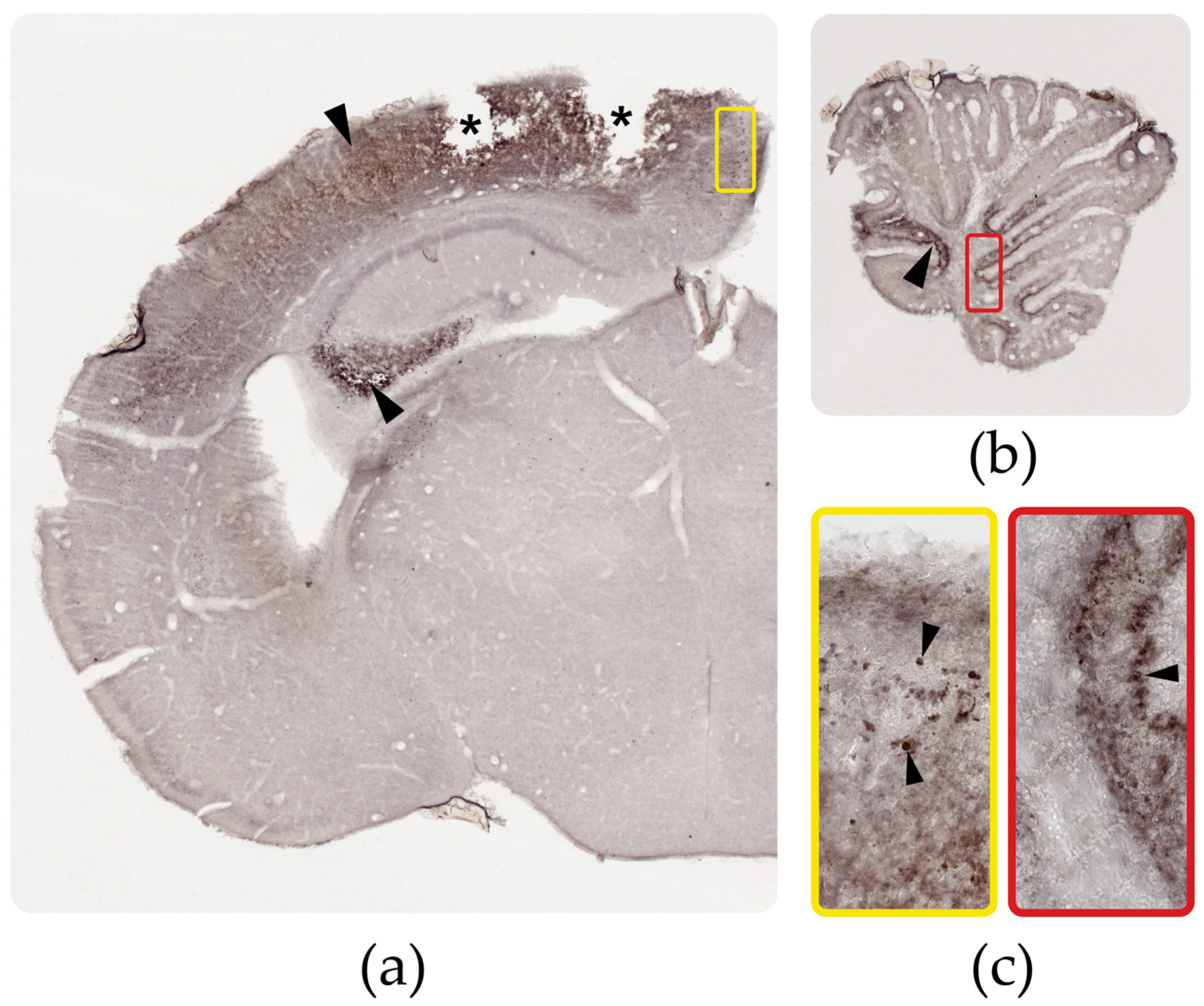
3.2.1. Identification of Calcifications
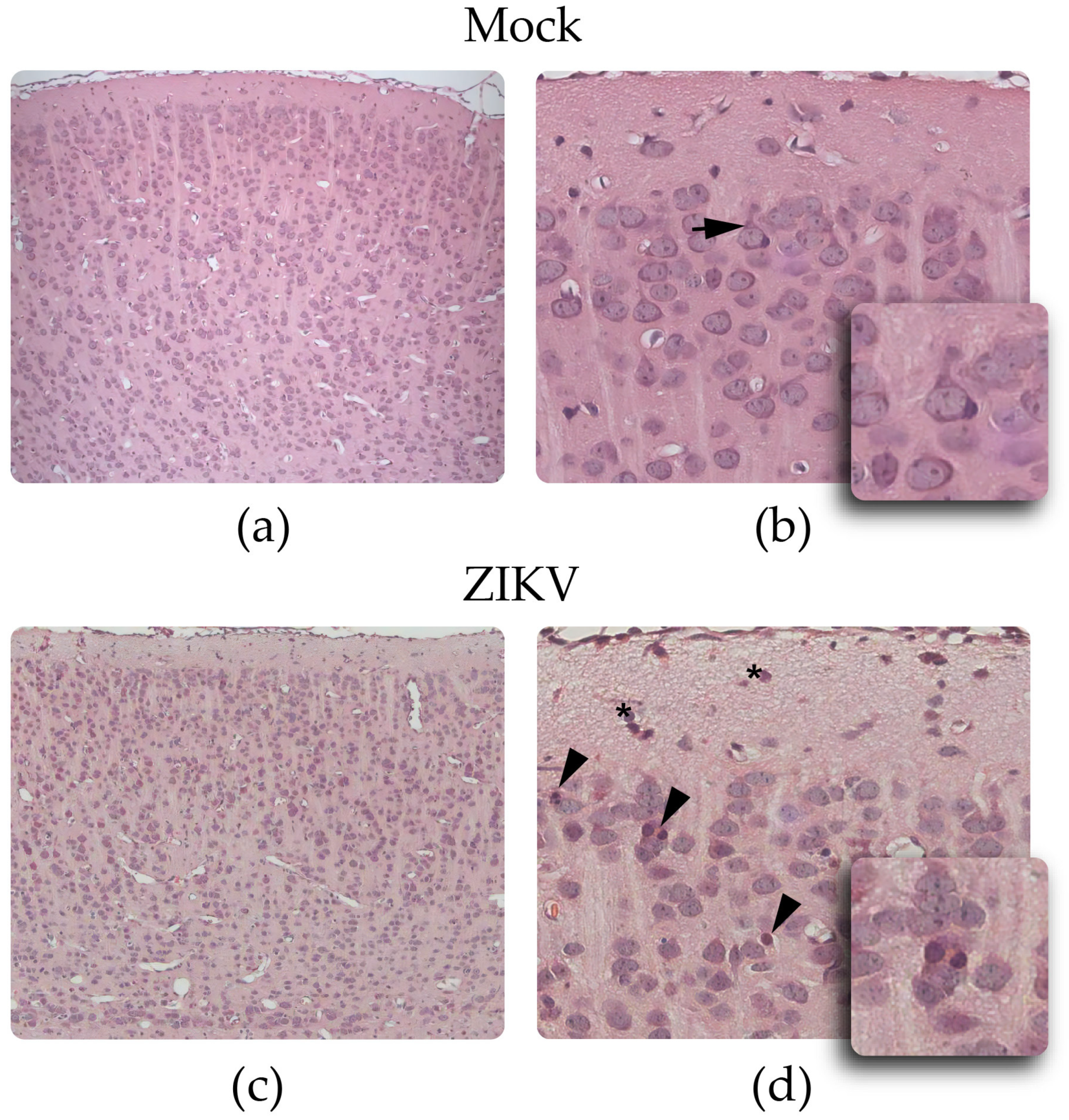
3.2.2. H&E Staining
3.3. Distribution of the ZIKV Antigen, Genome, and Viral Load
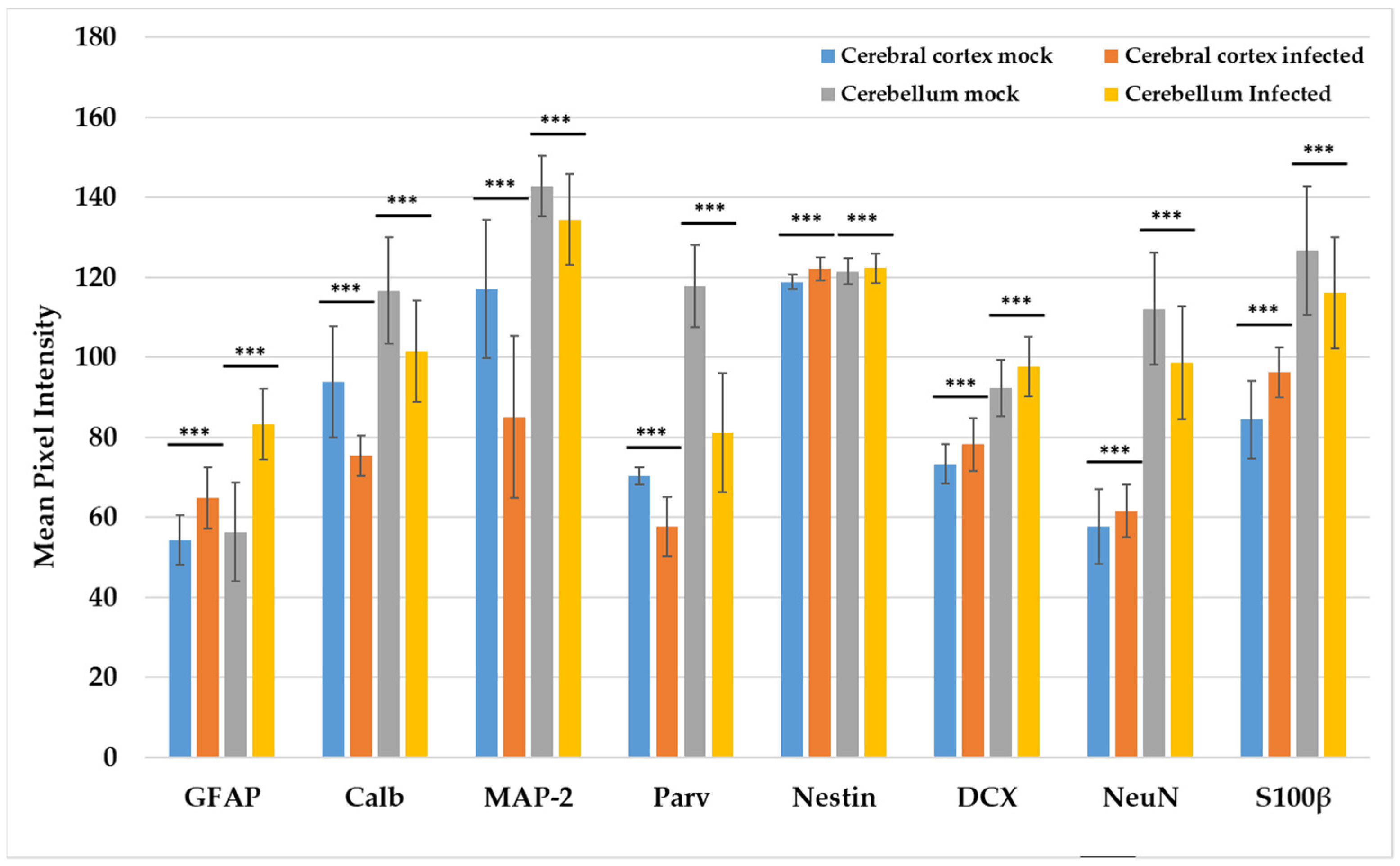
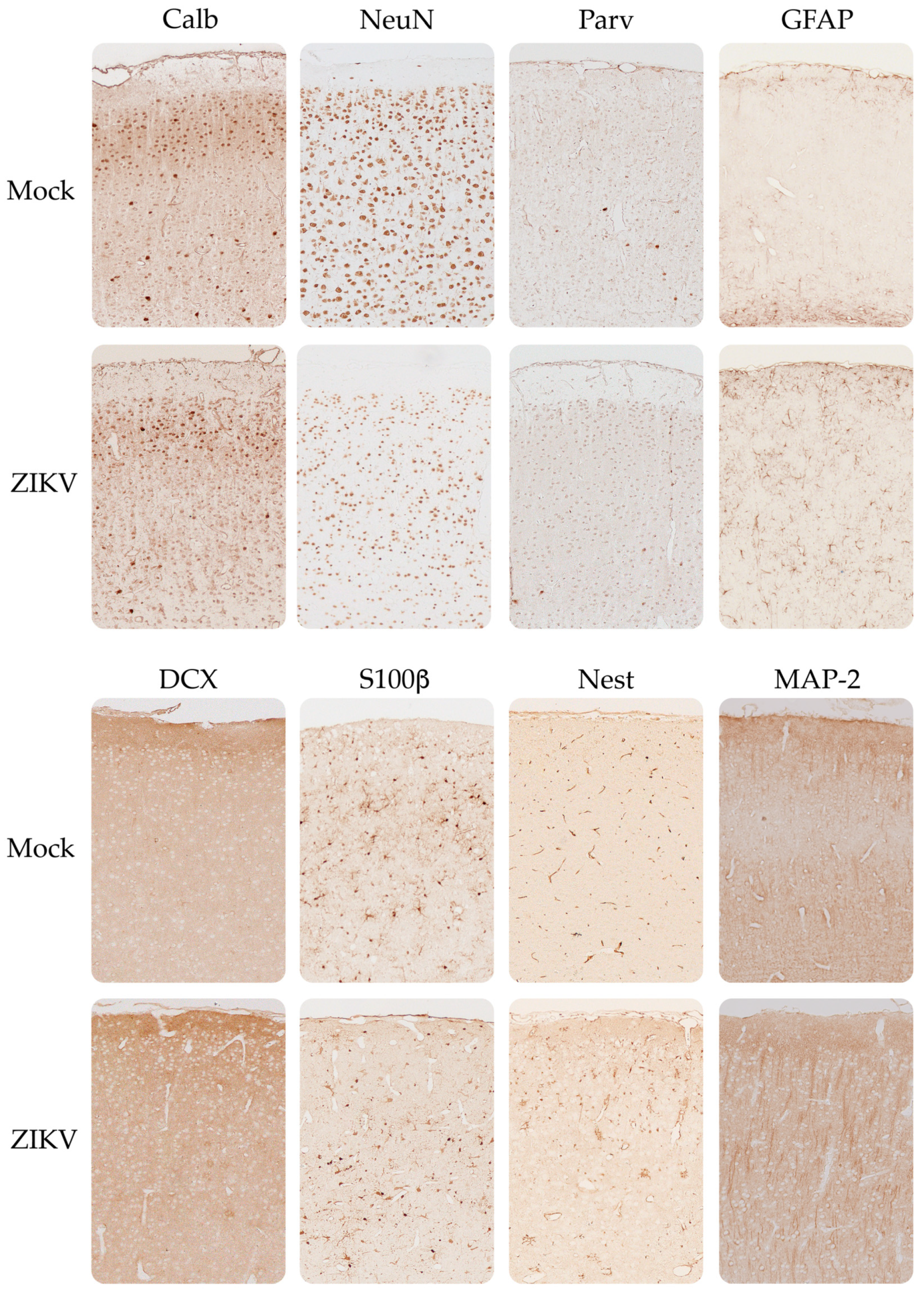
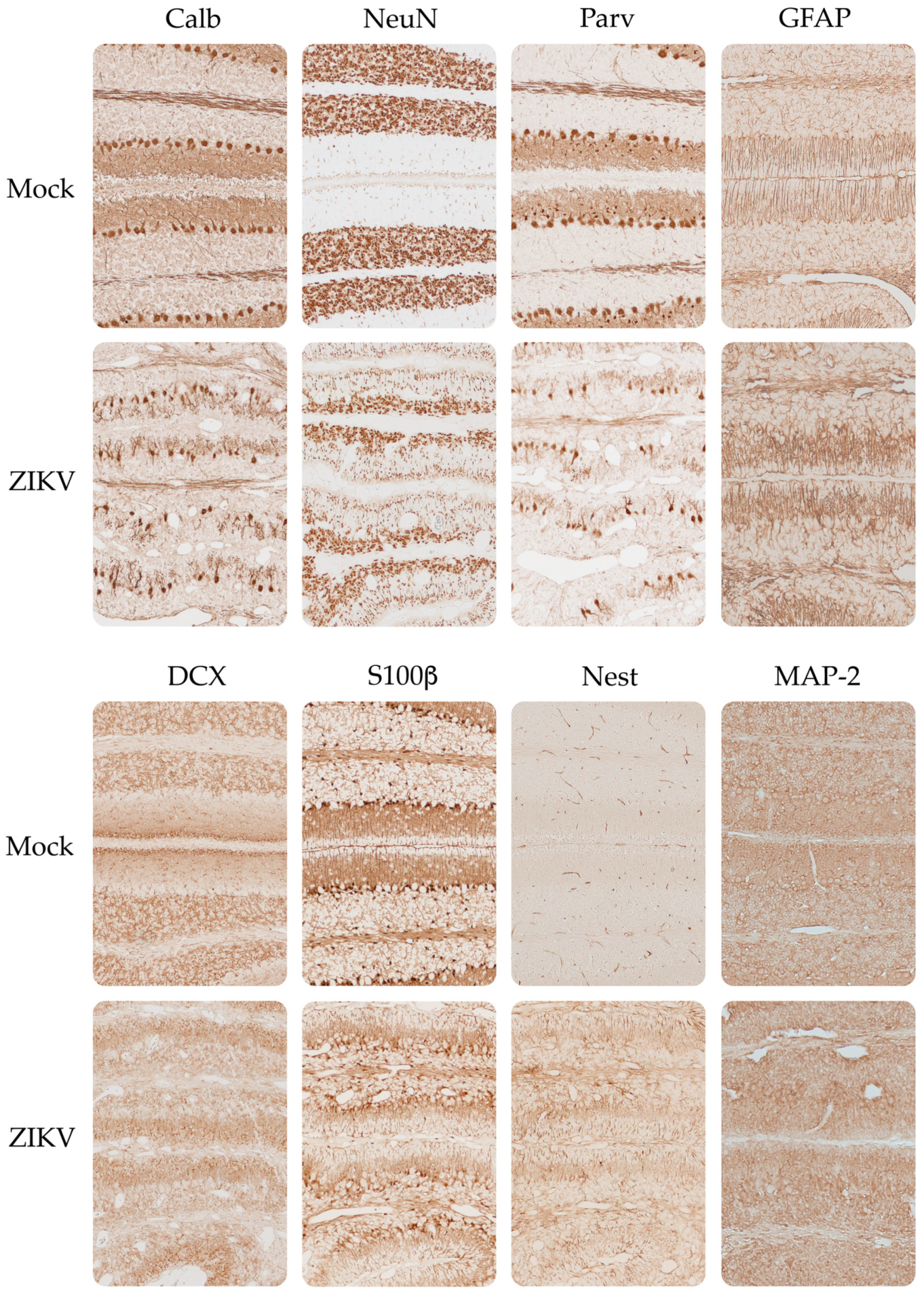
3.4. Expression of Neurodevelopmental Markers in the Cortex and Cerebellum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cadu, R.; Harish, T. Zika virus: A new global threat for 2016. Health 2015, 386, 243–244. [Google Scholar]
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.C.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Vroon, P.; Roosblad, J.; Poese, F.; Wilschut, J.; Codrington, J.; Vreden, S.; Zonneveld, R. Severity of acute Zika virus infection: A prospective emergency room surveillance study during the 2015–2016 outbreak in Suriname. IDCases 2017, 10, 117–121. [Google Scholar] [CrossRef]
- Oliveira Melo, A.; Malinger, G.; Ximenes, R.; Szejnfeld, P.; Alves Sampaio, S.; Bispo de Filippis, A. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Acuña, G.; Perret, C. ¿En qué consiste la infección humana por Virus Zika? Rev. Médica Chile 2016, 144, 1322–1325. [Google Scholar] [CrossRef]
- Chimelli, L.; Melo, A.S.O.; Avvad-Portari, E.; Wiley, C.A.; Camacho, A.H.S.; Lopes, V.S.; Machado, H.N.; Andrade, C.V.; Dock, D.C.A.; Moreira, M.E.; et al. The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol. 2017, 133, 983–999. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Parnell, L.A.; Diamond, M.S.; Mysorekar, I.U. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med. 2017, 214, 2303–2313. [Google Scholar] [CrossRef]
- de Oliveira, W.K.; Cortez-Escalante, J.; De Oliveira, W.T.G.H.; Carmo, G.M.I.d.; Henriques, C.M.P.; Coelho, G.E.; de França, G.V.A. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- de Fatima Vasco Aragao, M.; Van Der Linden, V.; Brainer-Lima, A.M.; Coeli, R.R.; Rocha, M.A.; Da Silva, P.S.; De Carvalho, M.D.C.G.; van der Linden, A.; De Holanda, A.C.; Valenca, M.M. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: Retrospective case series study. BMJ 2016, 353, i1901. [Google Scholar] [CrossRef] [PubMed]
- de França, T.L.B.; Medeiros, W.R.; de Souza, N.L.; Longo, E.; Pereira, S.A.; França, T.B.d.O.; Sousa, K.G. Growth and Development of Children with Microcephaly Associated with Congenital Zika Virus Syndrome in Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1990. [Google Scholar] [CrossRef]
- Hazin, A.N.; Poretti, A.; Di Cavalcanti Souza Cruz, D.; Tenorio, M.; Van Der Linden, A.; Pena, L.J.; Brito, C.; Gil, L.H.V.; de Barros Miranda-Filho, D.; de Azevedo Marques, E.T.; et al. Computed Tomographic Findings in Microcephaly Associated with Zika Virus. N. Engl. J. Med. 2016, 374, 2193–2195. [Google Scholar] [CrossRef]
- Freitas, B.d.P.; Dias, J.R.d.O.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef]
- Shao, Q.; Herrlinger, S.; Yang, S.-L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.-F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef]
- Yi, L.; Pimentel, H.; Pachter, L. Zika infection of neural progenitor cells perturbs transcription in neurodevelopmental pathways. PLoS ONE 2017, 12, e0175744. [Google Scholar] [CrossRef]
- Garcez, P.P.; Nascimento, J.M.; de Vasconcelos, J.M.; da Costa, R.M.; Delvecchio, R.; Trindade, P.; Loiola, E.C.; Higa, L.M.; Cassoli, J.S.; Vitória, G.; et al. Zika virus disrupts molecular fingerprinting of human neurospheres. Sci. Rep. 2017, 7, srep40780. [Google Scholar] [CrossRef] [PubMed]
- Saade, M.; Ferrero, D.S.; Blanco-Ameijeiras, J.; Gonzalez-Gobartt, E.; Flores-Mendez, M.; Ruiz-Arroyo, V.M.; Martínez-Sáez, E.; Cajal, S.R.Y.; Akizu, N.; Verdaguer, N.; et al. Multimerization of Zika Virus-NS5 Causes Ciliopathy and Forces Premature Neurogenesis. Cell Stem Cell 2020, 27, 920–936.e8. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, X.; Li, S.-H.; Zhou, Y.-P.; Rayner, S.; Xia, H.-M.; Gao, G.F.; Yuan, H.; Tang, Y.-P.; Luo, M.-H. Proteomic Analysis of Zika Virus Infected Primary Human Fetal Neural Progenitors Suggests a Role for Doublecortin in the Pathological Consequences of Infection in the Cortex. Front. Microbiol. 2018, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Deuel, T.A.; Liu, J.S.; Corbo, J.C.; Yoo, S.-Y.; Rorke-Adams, L.B.; Walsh, C.A. Genetic Interactions between Doublecortin and Doublecortin-like Kinase in Neuronal Migration and Axon Outgrowth. Neuron 2006, 49, 41–53. [Google Scholar] [CrossRef]
- Koizumi, H.; Tanaka, T.; Gleeson, J.G. doublecortin-like kinase Functions with doublecortin to Mediate Fiber Tract Decussation and Neuronal Migration. Neuron 2006, 49, 55–66. [Google Scholar] [CrossRef]
- Ci, Y.; Liu, Z.-Y.; Zhang, N.-N.; Niu, Y.; Yang, Y.; Xu, C.; Yang, W.; Qin, C.-F.; Shi, L. Zika NS1–induced ER remodeling is essential for viral replicationZika NS1 remodels ER for viral replication. J. Cell Biol. 2020, 219, e201903062. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.-M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef]
- Wells, M.F.; Salick, M.R.; Wiskow, O.; Ho, D.J.; Worringer, K.A.; Ihry, R.J.; Kommineni, S.; Bilican, B.; Klim, J.R.; Hill, E.J.; et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell 2016, 19, 703–708. [Google Scholar] [CrossRef]
- Pena, L.J.; Guarines, K.M.; Silva, A.J.D.; Leal, L.R.S.; Félix, D.M.; Silva, A.; De Oliveira, S.A.; Ayres, C.F.J.; Júnior, A.S.; De Freitas, A.C. In vitro and in vivo models for studying Zika virus biology. J. Gen. Virol. 2018, 99, 1529–1550. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Bradley, J.R.; Pober, J.S. Human Organ Culture: Updating the Approach to Bridge the Gap from In Vitro to In Vivo in Inflammation, Cancer, and Stem Cell Biology. Front. Med. 2017, 4, 148. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Qin, C.-F.; Xu, Z. Update on the Animal Models and Underlying Mechanisms for ZIKV-Induced Microcephaly. Annu. Rev. Virol. 2019, 6, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Caine, E.A.; Jagger, B.W.; Diamond, M.S. Animal Models of Zika Virus Infection during Pregnancy. Viruses 2018, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.N.v.D.; Mao, G.; Yang, Y.; Ornaghi, S.; Davis, J.N. Zika Virus Targeting in the Developing Brain. J. Neurosci. 2017, 37, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.K.; Swiney, B.S.; Williams, S.L.; Huffman, J.N.; Lucas, K.; Wang, S.H.; Kapral, K.M.; Li, A.; Dikranian, K.T. Zika Virus Infection in the Developing Mouse Produces Dramatically Different Neuropathology Dependent on Viral Strain. J. Neurosci. 2019, 40, 1145–1161. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Craig, A.; Luo, N.L.; Beardsley, D.J.; Wingate-Pearse, N.; Walker, D.W.; Hohimer, A.; Back, S.A. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp. Neurol. 2003, 181, 231–240. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Miner, J.J.; Sene, A.; Richner, J.M.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Rückert, C.; Govero, J.; et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef]
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.e4. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Regla-Nava, J.A.; Chai, G.; Sheets, N.; Tang, W.; Terskikh, A.V.; Shresta, S.; Gleeson, J.G. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell 2016, 19, 593–598. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Álvarez-Díaz, D.A.; Rengifo, A.C.; Torres-Fernández, O.; Usme-Ciro, J.A.; Rivera, J.A.; Santamaría, G.; Naizaque, J.; Monroy-Gómez, J.; Sarmiento, L.; et al. Complete Genome Sequence of a Colombian Zika Virus Strain Obtained from BALB/c Mouse Brain after Intraperitoneal Inoculation. Microbiol. Resour. Announc. 2019, 8, e01719-18. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.P.; Barros-Aragão, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; de Sousa, V.L.; Souza, A.S.; et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef]
- Costa, V.V.; Del Sarto, J.L.; Rocha, R.F.; Silva, F.R.; Doria, J.G.; Olmo, I.G.; Marques, R.E.; Queiroz-Junior, C.M.; Foureaux, G.; Araújo, J.M.S.; et al. N-Methyl-d-Aspartate (NMDA) Receptor Blockade Prevents Neuronal Death Induced by Zika Virus Infection. mBio 2017, 8, e00350-17. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Velandia-Romero, M.L.; Acosta-Losada, O.; Castellanos, J.E. In vivo infection by a neuroinvasive neurovirulent dengue virus. J. NeuroVirol. 2012, 18, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.M.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef]
- Cui, L.; Zou, P.; Chen, E.; Yao, H.; Zheng, H.; Wang, Q.; Zhu, J.-N.; Jiang, S.; Lu, L.; Zhang, J. Visual and Motor Deficits in Grown-up Mice with Congenital Zika Virus Infection. EBioMedicine 2017, 20, 193–201. [Google Scholar] [CrossRef]
- Ybot-Gonzalez, P.; Gaston-Massuet, C.; Girdler, G.; Klingensmith, J.; Arkell, R.; Greene, N.D.E.; Copp, A.J. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development 2007, 134, 3203–3211. [Google Scholar] [CrossRef]
- O’Rahilly, R.; Müller, F. Neurulation in the Normal Human Embryo. In Ciba Foundation Symposium 181-Neural Tube Defects: Neural Tube Defects: Ciba Foundation Symposium 181; Wiley Online Library: Hoboken, NJ, USA, 2007; pp. 70–89. [Google Scholar]
- Fernandes, N.C.; Nogueira, J.S.; Réssio, R.A.; Cirqueira, C.S.; Kimura, L.M.; Fernandes, K.R.; Cunha, M.S.; Souza, R.P.; Guerra, J.M. Experimental Zika virus infection induces spinal cord injury and encephalitis in newborn Swiss mice. Exp. Toxicol. Pathol. 2017, 69, 63–71. [Google Scholar] [CrossRef]
- Morrison, T.E.; Diamond, M.S. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91, e00009-17. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugières, P.; Fourati, S.; de Langavant, L.C.; De Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.R.F.; Frontera, J.A.; de Filippis, A.M.B.; do Nascimento, O.J.M. Neurologic complications associated with the Zika virus in Brazilian adults. JAMA Neurol. 2017, 74, 1190–1198. [Google Scholar] [CrossRef]
- Dick, G.W.A. Zika virus (II). Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 521–534. [Google Scholar] [CrossRef]
- Bell, T.M.; Field, E.J.; Narang, H.K. Zika virus infection of the central nervous system of mice. Arch. Virol. 1971, 35, 183–193. [Google Scholar] [CrossRef]
- Rivera, J.; Rengifo, A.C.; Santamaría, G.; Corchuelo, S.; Álvarez-Díaz, D.; Parra, E.A.; Naizaque, J.; Monroy-Gómez, J.; Torres-Fernández, O. Immunoreactivity of Zika virus infection in mouse retina. Biomedica 2019, 39, 8–10. [Google Scholar] [CrossRef]
- Ireland, D.D.C.; Manangeeswaran, M.; Lewkowicz, A.P.; Engel, K.; Clark, S.M.; Laniyan, A.; Sykes, J.; Lee, H.-N.; McWilliams, I.L.; Kelley-Baker, L.; et al. Long-term persistence of infectious Zika virus: Inflammation and behavioral sequela in mice. PLoS Pathog. 2020, 16, e1008689. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Korobko, I. EU Strategy for the Protection and Welfare of Animals for 2012–2015. Ukr. J. Int’l L 2013, 97. Available online: https://heinonline.org/HOL/LandingPage?handle=hein.journals/ukrjil2013&div=101&id=&page= (accessed on 30 May 2023).
- Chosewood, L.C.; Wilson, D.E. Biosafety in Microbiological and Biomedical Laboratories; US Department of Health and Human Services, Public Health Service Centers: Washington, DC, USA, 2009.
- Kohn, D.F.; Martin, T.E.; Foley, P.L.; Morris, T.H.; Swindle, M.M.; Vogler, G.A.; Wixson, S.K. Guidelines for the assessment and management of pain in rodents and rabbits. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 97–108. [Google Scholar] [PubMed]
- Álvarez-Díaz, D.A.; Valencia-Álvarez, E.; Rivera, J.A.; Rengifo, A.C.; Usme-Ciro, J.A.; Peláez-Carvajal, D.; Lozano-Jiménez, Y.Y.; Torres-Fernández, O. An updated RT-qPCR assay for the simultaneous detection and quantification of chikungunya, dengue and zika viruses. Infect. Genet. Evol. 2021, 93, 104967. [Google Scholar] [CrossRef] [PubMed]
- Biosystems, A. Creating Standard Curves with Genomic DNA or Plasmid DNA Templates for Use in Quantitative PCR; F Hoffmann-La Roche Ltd.: Basel, Switzerland, 2013. [Google Scholar]
- García del Moral, R. Laboratorio de Anatomía Patológica; Interamericana-McGraw-Hill: Bogota, Colombia, 1993; ISBN 8448102290. [Google Scholar]
- Jonkers, B.; Sterk, J.; Wouterlood, F. Transcardial perfusion fixation of the CNS by means of a compressed-air-driven device. J. Neurosci. Methods 1984, 12, 141–149. [Google Scholar] [CrossRef]
- Alshammari, M.A.; Alshammari, T.K.; Laezza, F. Improved Methods for Fluorescence Microscopy Detection of Macromolecules at the Axon Initial Segment. Front. Cell. Neurosci. 2016, 10, 5. [Google Scholar] [CrossRef]
- Paxinos, G.; Puelles, L.; Watson, C. The Mouse Nervous System; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Corchuelo, S.; Gómez, C.Y.; Rosales, A.A.; Santamaria, G.; Rivera, J.A.; Saad, E.P.; Torres-Fernández, O.; Rengifo, A.C. CISH and IHC for the Simultaneous Detection of ZIKV RNA and Antigens in Formalin-Fixed Paraffin-Embedded Cell Blocks and Tissues. Curr. Protoc. 2021, 1, e319. [Google Scholar] [CrossRef]
- Monroy-Gómez, J.; Santamaría, G.; Torres-Fernández, O. Overexpression of MAP2 and NF-H Associated with Dendritic Pathology in the Spinal Cord of Mice Infected with Rabies Virus. Viruses 2018, 10, 112. [Google Scholar] [CrossRef]
- Martines, R.B.; Bhatnagar, J.; de Oliveira Ramos, A.M.; Davi, H.P.F.; Iglezias, S.D.; Kanamura, C.T.; Keating, M.K.; Hale, G.; Silva-Flannery, L.; Muehlenbachs, A.; et al. Pathology of congenital Zika syndrome in Brazil: A case series. Lancet 2016, 388, 898–904. [Google Scholar] [CrossRef]
- Moore, R.; Champeval, D.; Denat, L.; Tan, S.-S.; Faure, F.; Julien-Grille, S.; Larue, L. Involvement of cadherins 7 and 20 in mouse embryogenesis and melanocyte transformation. Oncogene 2004, 23, 6726–6735. [Google Scholar] [CrossRef]
- Altshuler, A.; Verbuk, M.; Bhattacharya, S.; Abramovich, I.; Haklai, R.; Hanna, J.H.; Kloog, Y.; Gottlieb, E.; Shalom-Feuerstein, R. RAS Regulates the Transition from Naive to Primed Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 1088–1101. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, Z.; Wang, M.; Zhang, W.; Yang, R.; An, H.; Yang, R.; van Abel, D.; van Dijk, M.; Yang, X.; et al. Stox1 as a novel transcriptional suppressor of Math1 during cerebellar granule neurogenesis and medulloblastoma formation. Cell Death Differ. 2016, 23, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C T difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Álvarez-Díaz, D.A.; Usme-Ciro, J.A.; Corchuelo, S.; Naizaque, J.R.; Rivera, J.A.; Castiblanco-Martínez, H.D.; Torres-Fernández, O.; Rengifo, A.C. 5′/3′ RACE method for sequencing the 5′and 3′ untranslated regions of Zika virus. Arch. Virol. 2023, 168, 204. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Tseng, C.-K.; Lin, C.-K.; Wei, C.-K.; Lee, J.-C.; Young, K.-C. ICR suckling mouse model of Zika virus infection for disease modeling and drug validation. PLoS Negl. Trop. Dis. 2018, 12, e0006848. [Google Scholar] [CrossRef]
- Anfasa, F.; Siegers, J.Y.; van der Kroeg, M.; Mumtaz, N.; Raj, V.S.; de Vrij, F.M.S.; Widagdo, W.; Gabriel, G.; Salinas, S.; Simonin, Y.; et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere 2017, 2, e00292-17. [Google Scholar] [CrossRef]
- Lossia, O.V.; Conway, M.J.; Tree, M.O.; Williams, R.J.; Goldthorpe, S.C.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. Zika virus induces astrocyte differentiation in neural stem cells. J. NeuroVirol. 2017, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jorgačevski, J.; Korva, M.; Potokar, M.; Lisjak, M.; Avšič-Županc, T.; Zorec, R. ZIKV Strains Differentially Affect Survival of Human Fetal Astrocytes versus Neurons and Traffic of ZIKV-Laden Endocytotic Compartments. Sci. Rep. 2019, 9, 8069. [Google Scholar] [CrossRef]
- Stefanik, M.; Formanová, P.; Bily, T.; Vancová, M.; Eyer, L.; Palus, M.; Salát, J.; Braconi, C.T.; Zanotto, P.M.D.A.; Gould, E.A.; et al. Characterisation of Zika virus infection in primary human astrocytes. BMC Neurosci. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Huttner, W.B.; Kosodo, Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 2005, 17, 648–657. [Google Scholar] [CrossRef]
- Kalay, E.; Yigit, G.; Aslan, Y.; Brown, K.E.; Pohl, E.; Bicknell, L.S.; Kayserili, H.; Li, Y.; Tüysüz, B.; Nürnberg, G.; et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat. Genet. 2010, 43, 23–26. [Google Scholar] [CrossRef]
- Sir, J.-H.; Barr, A.R.; Nicholas, A.K.; Carvalho, O.P.; Khurshid, M.; Sossick, A.; Reichelt, S.; D’Santos, C.; Woods, C.G.; Gergely, F. A primary microcephaly protein complex forms a ring around parental centrioles. Nat. Genet. 2011, 43, 1147–1153. [Google Scholar] [CrossRef]
- Kosodo, Y.; Röper, K.; Haubensak, W.; Marzesco, A.M.; Corbeil, D.; Huttner, W.B. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004, 23, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Ramani, A.; Karow, U.; Gottardo, M.; Natarajan, K.; Gooi, L.M.; Goranci-Buzhala, G.; Krut, O.; Peters, F.; Nikolic, M. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 2017, 20, 397–406.e5. [Google Scholar] [CrossRef]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell. Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Namiki, J.; Suzuki, S.; Masuda, T.; Ishihama, Y.; Okano, H. Nestin Protein Is Phosphorylated in Adult Neural Stem/Progenitor Cells and Not Endothelial Progenitor Cells. Stem Cells Int. 2012, 2012, 430138. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Weng, Y.-C.; Thammisetty, S.S.; Phaneuf, D.; Lalancette-Hebert, M.; Kriz, J. Molecular imaging of nestin in neuroinflammatory conditions reveals marked signal induction in activated microglia. J. Neuroinflamm. 2017, 14, 45. [Google Scholar] [CrossRef]
- McGrath, E.L.; Rossi, S.L.; Gao, J.; Widen, S.G.; Grant, A.C.; Dunn, T.J.; Azar, S.R.; Roundy, C.M.; Xiong, Y.; Prusak, D.J.; et al. Differential Responses of Human Fetal Brain Neural Stem Cells to Zika Virus Infection. Stem Cell Rep. 2017, 8, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Lalancette-Hébert, M.; Phaneuf, D.; Soucy, G.; Weng, Y.C.; Kriz, J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain 2009, 132, 940–954. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Johnston, H.M.; Morris, B.J. NMDA and Nitric Oxide Increase Microtubule-Associated Protein 2 Gene Expression in Hippocampal Granule Cells. J. Neurochem. 2002, 63, 379–382. [Google Scholar] [CrossRef]
- Hurtado, A.P.; Rengifo, A.C.; Torres-Fernández, O. Immunohistochemical Overexpression of MAP-2 in the Cerebral Cortex of Rabies-Infected Mice. Int. J. Morphol. 2015, 33, 465–470. [Google Scholar] [CrossRef]
- Li, X.-Q.; Sarmento, L.; Fu, Z.F. Degeneration of Neuronal Processes after Infection with Pathogenic, but Not Attenuated, Rabies Viruses. J. Virol. 2005, 79, 10063–10068. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.S.; Andres, S.; Makowski, C.; Meitinger, T.; Hoefele, J. MAP2—A Candidate Gene for Epilepsy, Developmental Delay and Behavioral Abnormalities in a Patient With Microdeletion 2q34. Front. Genet. 2018, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; MacDonald, S.M.; Altamura, C.R. Dendritic Cytoskeletal Protein Expression in Mental Retardation: An Immunohistochemical Study of the Neocortex in Rett Syndrome. Cereb. Cortex 2000, 10, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Williams, S.K.; Diem, R. Calcium-Binding Proteins as Determinants of Central Nervous System Neuronal Vulnerability to Disease. Int. J. Mol. Sci. 2019, 20, 2146. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.-Y.; Jeong, H.; Kang, M.J.; Park, R.; Shin, H.J.; Han, S.-H.; Son, S.M.; Song, H.; Baik, S.H.; Moon, M.; et al. Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death Differ. 2014, 21, 1575–1587. [Google Scholar] [CrossRef]
- Büttner, C.; Heer, M.; Traichel, J.; Schwemmle, M.; Heimrich, B. Zika Virus-Mediated Death of Hippocampal Neurons Is Independent From Maturation State. Front. Cell. Neurosci. 2019, 13, 389. [Google Scholar] [CrossRef]
- Ascoli, G.A.; Alonso-Nanclares, L.; Anderson, S.A.; Barrionuevo, G.; Benavides-Piccione, R.; Burkhalter, A.; Buzsáki, G.; Cauli, B.; Defelipe, J.; Fairén, A.; et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008, 9, 557–568. [Google Scholar] [CrossRef]
- Monroy-Gomez, J.; Torres-Fernandez, O. Calbindin and parvalbumin distribution in spinal cord of normal and rabies-infected mice. Biomedica 2013, 33, 564–573. [Google Scholar]
- Bastianelli, E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum 2003, 2, 242–262. [Google Scholar] [CrossRef]
- Walker, C.L.; Little, M.-T.E.; Roby, J.A.; Armistead, B.; Gale, M.; Rajagopal, L.; Nelson, B.R.; Ehinger, N.; Mason, B.; Nayeri, U.; et al. Zika virus and the nonmicrocephalic fetus: Why we should still worry. Am. J. Obstet. Gynecol. 2018, 220, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hashizume, Y.; Yoshida, M.; Wang, Y.; Goto, Y.; Mitsuma, N.; Ishikawa, K.; Mizusawa, H. Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathol. 2000, 100, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Wang, Z.; Xu, W.; Ding, Q.; Zhao, Y.; Han, J.; Sun, J. A Rare Novel CLCN2 Variation and Risk of Gilles de la Tourette Syndrome: Whole-Exome Sequencing in a Multiplex Family and a Follow-Up Study in a Chinese Population. Front. Psychiatry 2020, 11, 543911. [Google Scholar] [CrossRef]
- Depienne, C.; Bugiani, M.; Dupuits, C.; Galanaud, D.; Touitou, V.; Postma, N.; van Berkel, C.; Polder, E.; Tollard, E.; Darios, F.; et al. Brain white matter oedema due to ClC-2 chloride channel deficiency: An observational analytical study. Lancet Neurol. 2013, 12, 659–668. [Google Scholar] [CrossRef]
- Smith, R.; Clayton, G.; Wilcox, C.; Escudero, K.; Staley, K. Differential expression of an inwardly rectifying chloride conductance in rat brain neurons: A potential mechanism for cell-specific modulation of postsynaptic inhibition. J. Neurosci. 1995, 15, 4057–4067. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.K.; Reese, A.L.; Kuryshev, Y.; Wen, X.; McKiernan, K.A.; Gray, E.E.; Wu, C.; Huguenard, J.R.; Maduke, M.; Du Bois, J. Development and validation of a potent and specific inhibitor for the CLC-2 chloride channel. Proc. Natl. Acad. Sci. USA 2020, 117, 32711–32721. [Google Scholar] [CrossRef] [PubMed]
- Blanz, J.; Schweizer, M.; Auberson, M.; Maier, H.; Muenscher, A.; Hübner, C.A.; Jentsch, T.J. Leukoencephalopathy upon Disruption of the Chloride Channel ClC-2. J. Neurosci. 2007, 27, 6581–6589. [Google Scholar] [CrossRef]
- Owens, D.F.; Boyce, L.H.; Davis, M.B.E.; Kriegstein, A.R. Excitatory GABA Responses in Embryonic and Neonatal Cortical Slices Demonstrated by Gramicidin Perforated-Patch Recordings and Calcium Imaging. J. Neurosci. 1996, 16, 6414–6423. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Parrish, A.R.; Nahm, S.-S.; Abbott, L.C.; McCool, B.A.; Frye, G.D. Effects of early postnatal ethanol intubation on GABAergic synaptic proteins. Dev. Brain Res. 2002, 138, 177–185. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; Madeiro Da Costa, R.; Higa, L.M.; Trindade, P.; DelVecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Molnár, Z.; Price, D.J. Brain Development. In Kaufman’s Atlas of Mouse Development Supplement; Elsevier: Amsterdam, The Netherlands, 2016; pp. 239–252. [Google Scholar]
- Sun, T.; Hevner, R.F. Growth and folding of the mammalian cerebral cortex: From molecules to malformations. Nat. Rev. Neurosci. 2014, 15, 217–232. [Google Scholar] [CrossRef]
- Winkler, C.W.; Clancy, C.S.; Rosenke, R.; Peterson, K.E. Zika virus vertical transmission in interferon receptor1-antagonized Rag1−/− mice results in postnatal brain abnormalities and clinical disease. Acta Neuropathol. Commun. 2022, 10, 46. [Google Scholar] [CrossRef]
- Silbereis, J.; Heintz, T.; Taylor, M.M.; Ganat, Y.; Ment, L.R.; Bordey, A.; Vaccarino, F. Astroglial cells in the external granular layer are precursors of cerebellar granule neurons in neonates. Mol. Cell. Neurosci. 2010, 44, 362–373. [Google Scholar] [CrossRef]
- Yamano, T.; Shimada, M.; Abe, Y.; Ohta, S.; Ohno, M. Destruction of external granular layer and subsequent cerebellar abnormalities. Acta Neuropathol. 1983, 59, 41–47. [Google Scholar] [CrossRef]
- Raaf, J.; Kernohan, J.W. A study of the external granular layer in the cerebellum. The disappearance of the external granular layer and the growth of the molecular and internal granular layers in the cerebellum. Am. J. Anat. 1944, 75, 151–172. [Google Scholar] [CrossRef]
- Ábrahám, H.; Tornóczky, T.; Kosztolányi, G.; Seress, L. Cell formation in the cortical layers of the developing human cerebellum. Int. J. Dev. Neurosci. 2001, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, S.A.; Guerini, J.C.; Fonseca, I.B.; Samar, M.E.; Spitale, L.S. Estudio Morfométrico Comparativo de la Corteza Cerebelosa Humana en Dos Grupos Etarios. Int. J. Morphol. 2012, 30, 825–828. [Google Scholar] [CrossRef][Green Version]
- He, D.; Marie, C.; Zhao, C.; Kim, B.; Wang, J.; Deng, Y.; Clavairoly, A.; Frah, M.; Wang, H.; He, X.; et al. Chd7 cooperates with Sox10 and regulates the onset of CNS myelination and remyelination. Nat. Neurosci. 2016, 19, 678–689. [Google Scholar] [CrossRef]
- Armengol, J.A. Manual de Neurociencia; Delgado, J., Ferrús, A., Mora, F., Rubia, F.J., Eds.; Sintesis: Madrid, Spain, 2001; Volume 33, pp. 635–642. [Google Scholar]
- Delgado, J.J.R.N. Estructura y función del cerebelo. Rev. Neurol. 2001, 33, 635–642. [Google Scholar] [CrossRef]
- Doughty, C.T.; Yawetz, S.; Lyons, J. Emerging Causes of Arbovirus Encephalitis in North America: Powassan, Chikungunya, and Zika Viruses. Curr. Neurol. Neurosci. Rep. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- van Essen, M.J.; Nayler, S.; Becker, E.B.E.; Jacob, J. Deconstructing cerebellar development cell by cell. PLoS Genet. 2020, 16, e1008630. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; D’Arcangelo, G. Role of reelin in the control of brain development. Brain Res. Rev. 1998, 26, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Lakatosova, S.; Ostatnikova, D. Reelin and its complex involvement in brain development and function. Int. J. Biochem. Cell Biol. 2012, 44, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.J.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Zimmer, D.B.; Cornwall, E.H.; Landar, A.; Song, W. The S100 protein family: History, function, and expression. Brain Res. Bull. 1995, 37, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; Karl, J.; Leite, M.; Rotta, L.; Salbego, C.; Rocha, E.; Wofchuk, S.; Gonçalves, C.-A. High glutamate decreases S100B secretion stimulated by serum deprivation in astrocytes. Neuroreport 2002, 13, 1533–1535. [Google Scholar] [CrossRef]
- Tramontina, F.; Leite, M.C.; Gonçalves, D.; Tramontina, A.C.; Souza, D.F.; Frizzo, J.K.; Nardin, P.; Gottfried, C.; Wofchuk, S.T.; Gonçalves, C.-A. High Glutamate Decreases S100B Secretion by a Mechanism Dependent on the Glutamate Transporter. Neurochem. Res. 2006, 31, 815–820. [Google Scholar] [CrossRef]





| Antibody | Supplier | Catalog Number | Host | Clonality | Dilution |
|---|---|---|---|---|---|
| Anti-NeuN | Cell Signaling Technology | 24307S | Rabbit | Monoclonal | 1:600 |
| Anti-Calbindin | Sigma-Aldrich | C9848 | Mouse | Monoclonal | 1:400 |
| Anti-Parvalbumin | Sigma-Aldrich | P3088 | Mouse | Monoclonal | 1:2000 |
| Anti-S100 | Abcam | ab868 | Rabbit | Polyclonal | 1:400 |
| Anti-Nestin | Abcam | ab6142 | Mouse | Monoclonal | 1:100 |
| Anti-DCX | Abcam | ab18723 | Rabbit | Polyclonal | 1:10,000 |
| Anti-MAP-2 | Santa Cruz | Sc 20172 | Rabbit | Polyclonal | 1:2500 |
| Anti-GFAP | Dako | Z0334 | Rabbit | Polyclonal | 1:1000 |
| Anti-ZIKV | CDC’s Division of Vector-Borne Diseases | NA | Mouse | Polyclonal | 1:1000 |
| Cell Morphology-INS | NA | Rabbit | Polyclonal |
| Gene Symbol or Genome Region | Primer Sequence (5′-3′) | TM (°C) | Size Amplicon (bp) | GenBank Accession No. | Author |
|---|---|---|---|---|---|
| * GAPDH | F: AGGTCGGTGTGAACGGATTTG | 62.6 | 95 | (NM_017008) | |
| R: GGGGTCGTTGATGGCAACA | 62.6 | ||||
| ** CDH20 | F: GGGACCACAACAGTCAACATC | 55 | 191 | (AF007116) | [72] |
| R: GCACCATCTCCATCCACAAT | 55 | ||||
| ** DCX | F: CATTTTGACGAACGAGACAAAGC | 60.8 | 63 | (NM_010025) | |
| R: TGGAAGTCCATTCATCCGTGA | 60.9 | ||||
| ** RELN | F: TCTCTTCGTGGGTTGTTTCC | 58.4 | 136 | (NM_011261) | |
| R: GCATGGTTCAGCCTAGAGTG | 60.5 | ||||
| ** GFAP | F: CGGAGACGCATCACCTCTG | 62.1 | 120 | (NM_001131020) | |
| R: TGGAGGAGTCATTCGAGACAA | 60.2 | ||||
| ** NES | F: CCCTGAAGTCGAGGAGCTG | 61.4 | 166 | (NM_016701) | [73] |
| R: CTGCTGCACCTCTAAGCGA | 61.7 | ||||
| ** NeuN | F: GGCAAATGTTCGGGCAATTCG | 63 | 160 | (NM_001039167) | [74] |
| R: TCAATTTTCCGTCCCTCTACGAT | 61.1 | ||||
| ** CALB1 | F: GGCTTCATTTCGACGCTGAC | 61.7 | 184 | (NM_009788) | |
| R: ACGTGAGCCAACTCTACAATTC | 60.3 | ||||
| ** MAP2 | F: CCACTGCCGGACCTGAAGAATG | 60.3 | 164 | (NM001039934) | |
| R: CCCCCAGCAGAATGTTTGATGTTA | 59 | ||||
| ** CEP152 | F: CCCTTTGCAGAACGCCACCAC | 61.2 | 197 | (NM001081091) | |
| R: CCGCAACATTCCGCTTTTACCA | 61.9 | ||||
| ** CLCN2 | F: ATGTATGGCCGGTACACTCAG | 61.3 | 163 | (NM_009900) | |
| R: AACAAATGCGACATCTGGCAC | 59.4 | ||||
| ** S100B | F: GGTGACAAGCACAAGCTGAA | 58.4 | 139 | (NM_009115) | |
| R: ACTCCCCATCCCCATCTTCG | 62.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengifo, A.C.; Rivera, J.; Álvarez-Díaz, D.A.; Naizaque, J.; Santamaria, G.; Corchuelo, S.; Gómez, C.Y.; Torres-Fernández, O. Morphological and Molecular Changes in the Cortex and Cerebellum of Immunocompetent Mice Infected with Zika Virus. Viruses 2023, 15, 1632. https://doi.org/10.3390/v15081632
Rengifo AC, Rivera J, Álvarez-Díaz DA, Naizaque J, Santamaria G, Corchuelo S, Gómez CY, Torres-Fernández O. Morphological and Molecular Changes in the Cortex and Cerebellum of Immunocompetent Mice Infected with Zika Virus. Viruses. 2023; 15(8):1632. https://doi.org/10.3390/v15081632
Chicago/Turabian StyleRengifo, Aura Caterine, Jorge Rivera, Diego Alejandro Álvarez-Díaz, Julián Naizaque, Gerardo Santamaria, Sheryll Corchuelo, Claudia Yadira Gómez, and Orlando Torres-Fernández. 2023. "Morphological and Molecular Changes in the Cortex and Cerebellum of Immunocompetent Mice Infected with Zika Virus" Viruses 15, no. 8: 1632. https://doi.org/10.3390/v15081632
APA StyleRengifo, A. C., Rivera, J., Álvarez-Díaz, D. A., Naizaque, J., Santamaria, G., Corchuelo, S., Gómez, C. Y., & Torres-Fernández, O. (2023). Morphological and Molecular Changes in the Cortex and Cerebellum of Immunocompetent Mice Infected with Zika Virus. Viruses, 15(8), 1632. https://doi.org/10.3390/v15081632





