The (Re-)Emergence and Spread of Viral Zoonotic Disease: A Perfect Storm of Human Ingenuity and Stupidity
Abstract
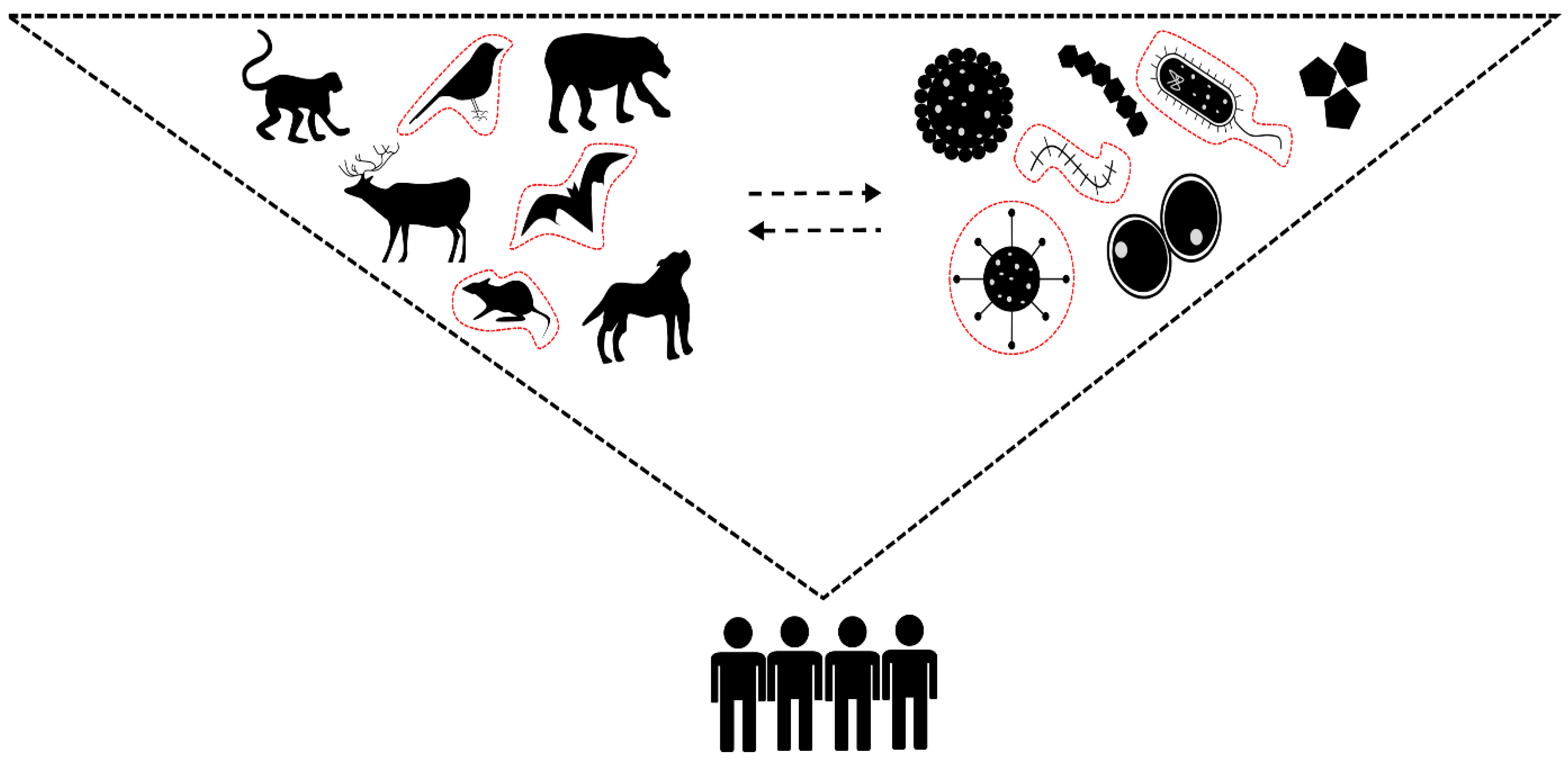
:1. Introduction
2. Are Viruses “Smarter” Than We Think?
3. Human Impactful Drivers in the (Re-)Emergence of Viral Zoonoses
3.1. Land-Use Change and Its Intrinsic Role in the Species–Pathogen Biodiversity Interface
3.2. Wildlife Trade
3.3. Livestock and Domesticated Animals
3.4. Climate Change
4. Human Impactful Drivers Related to the Spread of (Re-)Emerging Viral Zoonotic Disease
4.1. Globalisation
4.2. Geopolitics
4.3. Social Perceptions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, W.L.; Ternova, L.; Parasnis, S.A.; Kovaleva, M.; Nagy, G.J. Climate Change and Zoonoses: A Review of Concepts, Definitions, and Bibliometrics. Int. J. Environ. Res. Public Health 2022, 19, 893. [Google Scholar] [CrossRef]
- Varela, K.; Brown, J.A.; Lipton, B.; Dunn, J.; Stanek, D.; Behravesh, C.B.; Chapman, H.; Conger, T.H.; Vanover, T.; Edling, T.; et al. A Review of Zoonotic Disease Threats to Pet Owners: A Compendium of Measures to Prevent Zoonotic Diseases Associated with Non-Traditional Pets such as Rodents and Other Small Mammals, Reptiles, Amphibians, Backyard Poultry, and Other Selected Animals. Vector Borne Zoonotic Dis. 2022, 22, 303–360. [Google Scholar] [CrossRef]
- Roberts, M.; Dobson, A.; Restif, O.; Wells, K. Challenges in Modelling the Dynamics of Infectious Diseases at the Wildlife–Human Interface. Epidemics 2021, 37, 100523. [Google Scholar] [CrossRef] [PubMed]
- Shanks, S.; van Schalkwyk, M.C.I.; Cunningham, A.A. A Call to Prioritise Prevention: Action is Needed to Reduce the Risk of Zoonotic Disease Emergence. Lancet Reg. Health Eur. 2022, 23, 100506. [Google Scholar] [CrossRef]
- Furuse, Y.; Oshitani, H. Viruses that Can and Cannot Coexist with Humans and the Future of SARS-CoV-2. Front. Microbiol. 2020, 11, 583252. [Google Scholar] [CrossRef]
- Warren, C.J.; Sawyer, S.L. How Host Genetics Dictates Successful Viral Zoonoses. PLoS Biol. 2019, 17, e3000217. [Google Scholar] [CrossRef]
- Weiss, R.A.; Sankaran, N. Emergence of Epidemic Diseases: Zoonoses and Other Origins. Fac. Rev. 2022, 11, 2. [Google Scholar] [CrossRef]
- Bedenham, G.; Kirk, A.; Luhano, U.; Shields, A. The Importance of Biodiversity Risks: Link to Zoonotic Diseases. Br. Actuar. J. 2022, 27, e10. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Littman, R.J.; Littman, M.J. Galen and the Antonine Plague. Am. J. Philol. 1973, 94, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Haileamlak, A. Pandemics will be More Frequent. Ethiop. J. Health Sci. 2022, 32, 228. [Google Scholar] [PubMed]
- Huremović, D. Brief History of Pandemics (Pandemics Throughout History). Psychiatry Pandemics 2019, 16, 7–35. [Google Scholar]
- Trovato, M.; Sartorius, R.; D’Apice, L.; Manco, R.; De Berardinis, P. Viral Emerging Diseases: Challenges in Developing Vaccination Strategies. Front. Immunol. 2020, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C. Update on Rabies. Res. Rep. Trop. Med. 2011, 2, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 17091. [Google Scholar] [CrossRef]
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The Spread and Evolution of Rabies Virus: Conquering New Frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef]
- Wang, M.K.; Lim, S.-Y.; Lee, S.M.; Cunningham, J.M. Biochemical Basis for Increased Activity of Ebola Glycoprotein in the 2013–16 Epidemic. Cell Host Microbe 2017, 21, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827. [Google Scholar] [CrossRef]
- Magazine, N.; Zhang, T.; Wu, Y.; McGee, M.C.; Veggiani, G.; Huang, W. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses 2022, 14, 640. [Google Scholar] [CrossRef]
- Alleman, M.M.; Jorba, J.; Henderson, E.; Diop, O.M.; Shaukat, S.; Traoré, M.A.; Wiesen, E.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2020–June 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1691–1699. [Google Scholar] [CrossRef]
- Bigouette, J.P.; Henderson, E.; Traoré, M.A.; Wassilak, S.G.F.; Jorba, J.; Mahoney, F.; Bolu, O.; Diop, O.M.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2021–December 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Quadeer, A.A.; Barton, J.P.; Chakraborty, A.K.; McKay, M.R. Deconvolving Mutational Patterns of Poliovirus Outbreaks Reveals its Intrinsic Fitness Landscape. Nat. Commun. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves-Carneiro, D.; Bieniasz, P.D. Mechanisms of Attenuation by Genetic Recoding of Viruses. mBio 2021, 12, e02238-20. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.-M.; Van Ranst, M. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. Attenuation and Degeneration of SARS-CoV-2 Despite Adaptive Evolution. Cureus 2003, 15, e33316. [Google Scholar] [CrossRef]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike Mutation D614G Alters SARS-CoV-2 Fitness. Nature 2021, 592, 116–121. [Google Scholar] [CrossRef]
- Joop, G.; Vilcinskas, A. Coevolution of Parasitic Fungi and Insect Hosts. Zoology 2016, 119, 350–358. [Google Scholar] [CrossRef]
- Kaján, G.L.; Doszpoly, A.; Tarján, Z.L.; Vidovszky, M.Z.; Papp, T. Virus-Host Coevolution with a Focus on Animal and Human DNA Viruses. J. Mol. Evol. 2020, 88, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.K.; Rashid, H. Chapter Six—Land Use Change and Coastal Management. In Climatic Hazards in Coastal Bangladesh; Butterworth-Heinemann: Oxford, UK, 2017; pp. 183–207. [Google Scholar]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Rulli, M.C.; D’Odorico, P.; Galli, N.; Hayman, D.T.S. Land-Use Change and the Livestock Revolution Increase the Risk of Zoonotic Coronavirus Transmission from Rhinolophid Bats. Nat. Food 2021, 2, 409–416. [Google Scholar] [CrossRef]
- Goldstein, J.E.; Budiman, I.; Canny, A.; Dwipartidrisa, D. Pandemics and the Human-Wildlife Interface in Asia: Land Use Change as a Driver of Zoonotic Viral Outbreaks. Environ. Res. Lett. 2022, 17, 063009. [Google Scholar] [CrossRef]
- García-Peña, G.E.; Rubio, A.V.; Mendoza, H.; Fernández, M.; Milholland, M.T.; Aguirre, A.A.; Suzán, G.; Zambrana-Torrelio, C. Land-Use Change and Rodent-Borne Diseases: Hazards on the Shared Socioeconomic Pathways. Phil. Trans. R. Soc. B 2021, 376, 20200362. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Field, H.E.; Smith, C.; Divljan, A.; Palmer, C.; Tabor, G.; Daszak, P.; Foley, J.E. Reproduction and Nutritional Stress are Risk Factors for Hendra Virus Infection in Little Red Flying Foxes (Pteropus scapulatus). Proc. Biol. Sci. 2008, 275, 861–869. [Google Scholar] [CrossRef]
- Keesing, F.; Ostfeld, R.S. Impacts of Biodiversity and Biodiversity Loss on Zoonotic Diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Keatts, L.O.; Robards, M.; Olson, S.H.; Hueffer, K.; Insley, S.J.; Joly, D.O.; Kutz, S.; Lee, D.S.; Chetkiewicz, C.-L.B.; Lair, S.; et al. Implications of Zoonoses from Hunting and Use of Wildlife in North American Arctic and Boreal Biomes: Pandemic Potential, Monitoring, and Mitigation. Front. Public Health 2021, 9, 627654. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Biodiversity Series: The Function of Biodiversity in the Ecology of Vector-Borne Zoonotic Diseases. Can. J. Zool. 2000, 78, 2061–2078. [Google Scholar] [CrossRef]
- Occhibove, F.; Kenobi, K.; Swain, M.; Risley, C. An Eco-Epidemiological Modeling Approach to Investigate Dilution Effect in Two Different Tick-Borne Pathosystems. Ecol. Appl. 2022, 32, e2550. [Google Scholar] [CrossRef]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, C.N.; Sauer, E.L.; Sehgal, T.; Young, S.; et al. Biodiversity Inhibits Parasites: Broad Evidence for the Dilution Effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef]
- Khalil, H.; Ecke, F.; Evander, M.; Magnusson, M.; Hörnfeldt, B. Declining Ecosystem Health and the Dilution Effect. Sci. Rep. 2016, 6, 31314. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.G.; Heesterbeek, J.A.P. Quantifying the Dilution Effect for Models in Ecological Epidemiology. J. R. Soc. Interface 2018, 15, 20170791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, F.W.; Rohr, J.R.; Laine, A.-L. Biodiversity Loss Underlies the Dilution Effect of Biodiversity. Ecol. Lett. 2020, 23, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Espira, L.M.; Brouwer, A.F.; Han, B.A.; Foufopoulos, J.; Eisenberg, J.N.S. Dilution of Epidemic Potential of Environmentally Transmitted Infectious Diseases for Species with Partially Overlapping Habitats. Am. Nat. 2022, 199, 43–56. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Lin, Z.; Wang, X.; Hu, K.; Liu, M.; Zhao, Y.; Qi, Y.; Zhou, S. Spatial Scale-Dependent Dilution Effects of Biodiversity on Plant Diseases in Grasslands. Ecology 2023, 104, e3944. [Google Scholar] [CrossRef]
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages Between Environmental Issues and Zoonotic Diseases: With Reference to COVID-19 Pandemic. Environ. Sustain. 2021, 4, 455–467. [Google Scholar] [CrossRef]
- Tapia-Ramírez, G.; Lorenzo, C.; Navarrete, D.; Carrillo-Reyes, A.; Retana, O.; Carrasco-Hernández, R. A Review of Mammarenaviruses and Rodent Reservoirs in the Americas. EcoHealth 2022, 19, 22–39. [Google Scholar] [CrossRef]
- Dacheux, L.; Cervantes-Gonzalez, M.; Guigon, G.; Thiberge, J.-M.; Vandenbogaert, M.; Maufrais, C.; Caro, V.; Bourhy, H. A Preliminary Study of Viral Metagenomics of French Bat Species in Contact with Humans: Identification of New Mammalian Viruses. PLoS ONE 2014, 9, e87194. [Google Scholar] [CrossRef]
- French, R.; Charon, J.; Le Lay, C.; Muller, C.; Holmes, E.C. Human Land Use Impacts Viral Diversity and Abundance in a New Zealand River. Virus Evol. 2022, 8, veac032. [Google Scholar] [CrossRef]
- Rulli, M.C.; Santini, M.; Hayman, D.T.S.; D’Odorico, P. The Nexus Between Forest Fragmentation in Africa and Ebola Virus Disease Outbreaks. Sci. Rep. 2017, 7, 41613. [Google Scholar] [CrossRef] [Green Version]
- Rush, E.R.; Dale, E.; Aguirre, A.A. Illegal Wildlife Trade and Emerging Infectious Diseases: Pervasive Impacts to Species, Ecosystems and Human Health. Animals 2021, 11, 1821. [Google Scholar] [CrossRef]
- Shivaprakash, K.N.; Sen, S.; Paul, S.; Kiesecker, J.M.; Bawa, K.S. Mammals, Wildlife Trade, and the Next Global Pandemic. Curr. Biol. 2021, 31, 3671–3677. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, P.A.; O’Regan, K.C. Wildlife Trade, COVID-19, and Other Zoonotic Diseases; Congressional Research Service: Washington, DC, USA, 2021. [Google Scholar]
- Wyatt, T.; van Uhm, D.; Nurse, A. Differentiating Criminal Networks in the Illegal Wildlife Trade: Organized, Corporate and Disorganized Crime. Trends Organ. Crime 2020, 23, 350–366. [Google Scholar] [CrossRef]
- Mozer, A.; Prost, S. An Introduction to Illegal Wildlife Trade and its Effects on Biodiversity and Society. Forensic Sci. Int. Anim. Environ. 2023, 3, 100064. [Google Scholar] [CrossRef]
- Liu, J.; Mucker, E.M.; Chapman, J.L.; Babka, A.M.; Gordon, J.M.; Bryan, A.V.; Raymond, J.L.W.; Bell, T.M.; Facemire, P.R.; Goff, A.J.; et al. Retrospective Detection of Monkeypox Virus in the Testes of Nonhuman Primate Survivors. Nat. Microbiol. 2022, 7, 1980–1986. [Google Scholar] [CrossRef]
- Centre for Disease Control. Update: Multistate Outbreak of Monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb. Mortal Wkly. Rep. 2003, 52, 642–646. [Google Scholar]
- Wang, W.; Qi, W.; Liu, J.; Du, H.; Zhao, L.; Zheng, Y.; Wang, G.; Pan, Y.; Huang, B.; Feng, Z.; et al. First Human Infection Case of Monkey B Virus Identified in China, 2021. China CDC Weekly 2021, 3, 632–633. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Tse, H.; Chan, W.-M.; Choi, G.K.Y.; Zhang, A.J.X.; Sridhar, S.; Wong, S.C.Y.; Chan, J.F.W.; Chan, A.S.F.; Woo, P.C.Y.; et al. A Novel Psittacine Adenovirus Identified During an Outbreak of Avian Chlamydiosis and Human Psittacosis: Zoonosis Associated with Virus-Bacterium Coinfection in Birds. PLoS Negl. Trop. Dis. 2014, 8, e3318. [Google Scholar] [CrossRef] [Green Version]
- Chomel, B.B.; Belotto, A.; Meslin, F.-X. Wildlife, Exotic Pets, and Emerging Zoonoses. Emerg. Infect. Dis. 2007, 13, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.L.; Barry, P.A. B-Virus (Cercopithecine herpesvirus 1) Infection in Humans and Macaques: Potential for Zoonotic Disease. Emerg. Infect. Dis. 2003, 9, 246–250. [Google Scholar] [CrossRef]
- Kock, R.; Caceres-Escobar, H. Situation Analysis on the Roles and Risks of Wildlife in the Emergence of Human Infectious Diseases; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of Major Human Infectious Diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef]
- Düx, A.; Lequime, S.; Patrono, L.V.; Vrancken, B.; Boral, S.; Gogarten, J.F.; Hilbig, A.; Horst, D.; Merkel, K.; Prepoint, B.; et al. Measles Virus and Rinderpest Virus Divergence Dated to the Sixth Century BCE. Science 2020, 368, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Kelly, J.T.; Graham, S.C.; Birch, J.; Gonçalves-Carneiro, D.; Mitchell, T.; Thompson, R.N.; Lythgoe, K.A.; Logan, N.; Hosie, M.J.; et al. Structure Guided Identification of a Nonhuman Morbillivirus with Zoonotic Potential. J. Virol. 2018, 92, e01248-18. [Google Scholar] [CrossRef] [Green Version]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef]
- Mena1, I.; Nelson, M.I.; Quezada-Monroy, F.; Dutta, J.; Cortes-Fernández, R.; Lara-Puente, J.H.; Castro-Peralta, F.; Cunha, L.F.; Trovaõ, N.S.; Lozano-Dubernard, B.; et al. Origins of the 2009 H1N1 Influenza Pandemic in Swine in Mexico. eLife 2016, 5, e16777. [Google Scholar] [CrossRef]
- Graham, J.P.; Leibler, J.H.; Price, L.B.; Otte, J.M.; Pfeiffer, D.U.; Tiensin, T.; Silbergeld, E.K. The Animal-Human Interface and Infectious Disease in Industrial Food Animal Production: Rethinking Biosecurity and Biocontainment. Public Health Rep. 2008, 123, 282–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, K.B.; Chua, B.H.; Wang, C.W. Anthropogenic Deforestation, El Niño and the Emergence of Nipah Virus in Malaysia. Malays. J. Pathol. 2002, 24, 15–21. [Google Scholar] [PubMed]
- Kuehnert, P.A.; Stefan, C.P.; Badger, C.V.; Ricks, K.M. Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A Silent but Widespread Threat. Curr. Trop. Med. Rep. 2021, 8, 141–147. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Chomel, B.B.; Martínez-López, B. Climate Change and Zoonoses: A Review of the Current Status, Knowledge Gaps, and Future Trends. Acta Trop. 2022, 226, 106225. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). State of the Global Climate in 2022. Available online: https://public.wmo.int/en/our-mandate/climate/wmo-statement-state-of-global-climate (accessed on 10 May 2023).
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, K.A.; et al. Over Half of Known Human Pathogenic Diseases can be Aggravated by Climate Change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Rocklöv, J.; Dubrow, R. Climate Change: An Enduring Challenge for Vector-Borne Disease Prevention and Control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef]
- Colón-González, F.J.; Sewe, M.O.; Tompkins, A.M.; Sjödin, H.; Casallas, A.; Rocklöv, J.; Caminade, C.; Lowe, R. Projecting the Risk of Mosquito-Borne Diseases in a Warmer and More Populated World: A Multi-Model, Multi-Scenario Intercomparison Modelling Study. Lancet Planet. Health 2021, 5, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Shocket, M.S.; Verwillow, A.B.; Numazu, M.G.; Slamani, H.; Cohen, J.M.; Moustaid, F.E.; Rohr, J.; Johnson, L.R.; Mordecai, E.A. Transmission of West Nile and Five Other Temperate Mosquito-Borne Viruses Peaks at Temperatures Between 23 °C and 26 °C. eLife 2020, 9, e58511. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D. The Ecology of Climate Change and Infectious Diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Ryan, S.J.; McNally, A.; Johnson, L.R.; Mordecai, E.A.; Ben-Horin, T.; Paaijmans, K.; Lafferty, K.D. Mapping Physiological Suitability Limits for Malaria in Africa Under Climate Change. Vector Borne Zoonotic Dis. 2015, 15, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, C.; Michalski, D.; Münch, J.; Petros, S.; Bergs, S.; Trawinski, H.; Lübbert, C.; Uwe, G.L. Autochthonous West Nile Virus Infection Outbreak in Humans, Leipzig, Germany. Euro Surveill. 2020, 25, 46. [Google Scholar]
- Ryan, S.J.; Lippi, C.A.; Villena, O.C.; Singh, A.; Murdock, C.C.; Johnson, L.R. Mapping Current and Future Thermal Limits to Suitability for Malaria Transmission by the Invasive Mosquito Anopheles stephensi. Malaria J. 2023, 22, 104. [Google Scholar] [CrossRef]
- Bett, B.; Kiunga, P.; Gachohi, J.; Sindato, C.; Mbotha, D.; Robinson, T.; Lindahl, J.; Grace, D. Effects of Climate Change on the Occurrence and Distribution of Livestock Diseases. Prev. Vet. Med. 2017, 137, 119–129. [Google Scholar] [CrossRef]
- Fouque, F.; Reeder, J.C. Impact of Past and On-Going Changes on Climate and Weather on Vector-Borne Diseases Transmission: A Look at the Evidence. Infect. Dis. Poverty 2019, 8, 51. [Google Scholar] [CrossRef]
- Thompson, M.C.; Stanberry, L.R. Climate Change and Vectorborne Diseases. N. Engl. J. Med. 2022, 387, 1969–1978. [Google Scholar] [CrossRef]
- Anyamba, A.; Damoah, R.; Kemp, A.; Small, J.L.; Rostal, M.K.; Bagge, W.; Cordel, C.; Brand, R.; Karesh, W.B.; Paweska, J.T. Climate Conditions During a Rift Valley Fever Post-Epizootic Period in Free State, South Africa, 2014–2019. Front. Vet. Sci. 2022, 8, 730424. [Google Scholar] [CrossRef]
- Nili, S.; Khanjani, N.; Jahani, Y.; Bakhtiari, B. The Effect of Climate Variables on the Incidence of Crimean Congo Hemorrhagic Fever (CCHF) in Zahedan, Iran. BMC Public Health 2020, 20, 1893. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Estrada-Peña, A.; Garrison, A.R.; Schmaljohn, C.; Spiropoulou, C.F.; Bergeron, E.; Bente, D.A. A Chronological Review of Experimental Infection Studies of the Role of Wild Animals and Livestock in the Maintenance and Transmission of Crimean-Congo Hemorrhagic Fever Virus. Antiviral Res. 2016, 135, 31–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, R.M.; Manica, A.; Mora, C. Shifts in Global Bat Diversity Suggest a Possible Role of Climate Change in the Emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total Environ. 2021, 767, 145413. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yu, P.; Cazelles, B.; Xu, L.; Tan, H.; Yang, J.; Huang, S.; Xu, B.; Cai, J.; Ma, C.; et al. Interannual Cycles of Hantaan Virus Outbreaks at the Human–Animal Interface in Central China are Controlled by Temperature and Rainfall. Proc. Natl. Acad. Sci. USA 2017, 114, 8041–8046. [Google Scholar] [CrossRef]
- Ferro, I.; Bellomo, C.M.; López, W.; Coelho, R.; Alonso, D.; Bruno, A.; Córdoba1, F.E.; Martinez, V.P. Hantavirus Pulmonary Syndrome Outbreaks Associated with Climate Variability in Northwestern Argentina, 1997–2017. PLoS Negl. Trop. Dis. 2020, 14, e0008786. [Google Scholar] [CrossRef]
- Douglas, K.O.; Payne, K.; Sabino-Santos, G., Jr.; Agard, J. Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: A Systematic Review. Pathogens 2022, 11, 15. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Park, A.W.; Kramer, A.M.; Han, B.A.; Alexander, L.W.; Drake, J.M. Spatiotemporal Fluctuations and Triggers of Ebola Virus Spillover. Emerg. Infect. Dis. 2017, 23, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Jiafeng, W. Some Reflections of Modernization Theory and Globalization Theory. Chin. Stud. His. 2009, 43, 72–98. [Google Scholar] [CrossRef]
- Ahmad, S.; Arshed, N.; Salem, S.; Khan, Y.A.; Hameed, K.; Kam, S. Role of Globalization Defining the Incidence of Entrepreneurship. PLoS ONE 2022, 17, e0265757. [Google Scholar] [CrossRef]
- Zimmermann, K.F.; Karabulut, G.; Bilgin, M.H.; Doker, A.S. Inter-Country Distancing, Globalisation and the Coronavirus Pandemic. World Econ. 2020, 43, 1484–1498. [Google Scholar] [CrossRef]
- Frenk, J.; Gómez-Dantés, O.; Knaul, F.M. Globalization and Infectious Diseases. Infect. Dis. Clin. N. Am. 2011, 25, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Littman, R.J. The Plague of Athens: Epidemiology and Paleopathology. Mt. Sinai. J. Med. 2009, 76, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Townsend, L.; Zembe, Y.; Cheyip, M.; Guttmacher, S.; Carter, R.; Mathews, C. HIV Prevalence and Risk Factors Among Male Foreign Migrants in Cape Town, South Africa. AIDS Behav. 2014, 18, 2020–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, N.R.; Rambaut, A.; Suchard, M.A.; Baele, G.; Bedford, T.; Ward, M.J.; Tatem, A.J.; Sousa, J.D.; Arinaminpathy, N.; Pepin, J.; et al. HIV Epidemiology. The Early Spread and Epidemic Ignition of HIV-1 in Human Populations. Science 2014, 346, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Perrings, C.; Kinzig, A.; Collins, J.P.; Minteer, B.A.; Daszak, P. Economic Growth, Urbanization, Globalization, and the Risks of Emerging Infectious Diseases in China: A Review. Ambio 2017, 46, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Rourke, E.-T.M.; Phelan, G.L. Policy Opportunities to Enhance Sharing for Pandemic Research. Science 2020, 368, 716–718. [Google Scholar] [CrossRef]
- Elbe, S. Who Owns a Deadly Virus? Viral Sovereignty, Global Health Emergencies, and the Matrix of the International. Int. Political Sociol. 2022, 16, olab037. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Massad, E.; Burattini, M.N.; Khan, K.; Struchiner, C.J.; Coutinho, F.A.B.; Wilder-Smith, A. On the Origin and Timing of Zika Virus Introduction in Brazil. Epidemiol. Infect. 2017, 145, 2303–2312. [Google Scholar] [CrossRef] [Green Version]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First Report of Autochthonous Transmission of Zika Virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Belderok, S.-M.; Rimmelzwaan, G.F.; van den Hoek, A.; Sonder, G.J.B. Effect of Travel on Influenza Epidemiology. Emerg. Infect. Dis. 2013, 19, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, D.-H.; Shin, S.-S.; Kang, C.; Kim, J.S.; Jun, B.Y.; Lee, J.-K. In-Flight Transmission of Novel Influenza A (H1N1). Epidemiol. Health 2010, 32, e2010006. [Google Scholar] [CrossRef] [PubMed]
- Kickbusch, I.; Holzscheiter, A. Can Geopolitics Derail the Pandemic Treaty? BMJ 2021, 375, e069129. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-Borne Virus Diversity, Spillover and Emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Larkan, F.; Ryan, C.; Kevany, S. The Geopolitics of Ebola and Global Health Security: Why Anthropology Matters. Irish J. Anthropol. 2015, 18, 9–14. [Google Scholar]
- van Heyningen, E. A Tool for Modernisation? The Boer Concentration Camps of the South African War, 1900–1902. S. Afr. J. Sci. 2010, 106, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shanks, G.D.; Hu, Z.; Waller, M.; Lee, S.; Terfa, D.; Howard, A.; van Heyningen, E.; Brundage, J.F. Measles Epidemics of Variable Lethality in the Early 20th Century. Am. J. Epidemiol. 2013, 179, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Morens, D.M.; Taubenberger, J.K. A Forgotten Epidemic that Changed Medicine: Measles in the US Army, 1917–1918. Lancet Infect. Dis. 2015, 15, 852–861. [Google Scholar] [CrossRef]
- Liang, S.T.; Liang, L.T.; Rosen, J.M. COVID-19: A Comparison to the 1918 Influenza and How We Can Defeat it. Postgrad Med. J. 2021, 97, 273–274. [Google Scholar] [CrossRef]
- Molgaard, C.A. Military Vital Statistics the Spanish Flu and the First World War. J. R. Stat. Soc. 2019, 16, 32–37. [Google Scholar] [CrossRef]
- Byerly, C.R. The U.S. Military and the Influenza Pandemic of 1918–1919. Public Health Rep. 2010, 125, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, C.J.; Farrell, M.J.; Grange, Z.; Han, B.A.; Mollentze, N.; Phelan, A.L.; Rasmussen, A.L.; Albery, G.F.; Bett, B.; Brett-Major, D.M.; et al. The Future of Zoonotic Risk Prediction. Phil. Trans. R. Soc. B 2021, 376, 20200358. [Google Scholar] [CrossRef] [PubMed]
- Tabbaa, D. Emerging Zoonoses: Responsible Communication with the Media—Lessons Learned and Future Perspectives. Int. J. Antimicrob. Agents 2010, 36, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Agley, J.; Xiao, Y. Misinformation About COVID-19: Evidence for Differential Latent Profiles and a Strong Association with Trust in Science. BMC Public Health 2021, 21, 89. [Google Scholar] [CrossRef]
- Islam, M.S.; Sarkar, T.; Khan, S.H.; Kamal, A.-H.M.; Hasan, S.M.M.; Kabir, A.; Yeasmin, D.; Islam, M.A.; Chowdhury, K.I.A.; Anwar, K.S.; et al. COVID-19–Related Infodemic and its Impact on Public Health: A Global Social Media Analysis. Am. J. Trop. Med. Hyg. 2010, 103, 1621–1629. [Google Scholar] [CrossRef]
- Gisondi, M.A.; Barber, R.; Faust, J.S.; Raja, A.; Strehlow, M.C.; Westafer, L.M.; Gottlieb, M. A Deadly Infodemic: Social Media and the Power of COVID-19 Misinformation. J. Med. Internet Res. 2022, 24, e35552. [Google Scholar] [CrossRef]
- Al-Ramahi, M.; Elnoshokaty, A.; El-Gayar, O.; Nasralah, T.; Wahbeh, A. Public Discourse Against Masks in the COVID-19 Era: Infodemiology Study of Twitter Data. JMIR Pub. Health Surveill. 2021, 7, e26780. [Google Scholar] [CrossRef]
- Tenkorang, E.Y. Effect of Knowledge and Perceptions of Risks on Ebola-Preventive Behaviours in Ghana. Int. Health 2018, 10, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Yamanis, T.; Nolan, E.; Shepler, S. Fears and Misperceptions of the Ebola Response System During the 2014-2015 Outbreak in Sierra Leone. PLoS Negl. Trop. Dis. 2016, 10, e0005077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duesberg, P. HIV is Not the Cause of AIDS. Science 1988, 241, 514. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. AIDS Researchers Decry Mbeki’s Views on HIV. Science 2000, 288, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Chigwedere, P.; Seage, G.R., III; Gruskin, S.; Lee, T.-H.; Esses, M. Estimating the Lost Benefits of Antiretroviral Drug Use in South Africa. J. Acquir. Immune Defic. Syndr. 2008, 49, 410–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marie, V.; Gordon, M.L. The (Re-)Emergence and Spread of Viral Zoonotic Disease: A Perfect Storm of Human Ingenuity and Stupidity. Viruses 2023, 15, 1638. https://doi.org/10.3390/v15081638
Marie V, Gordon ML. The (Re-)Emergence and Spread of Viral Zoonotic Disease: A Perfect Storm of Human Ingenuity and Stupidity. Viruses. 2023; 15(8):1638. https://doi.org/10.3390/v15081638
Chicago/Turabian StyleMarie, Veronna, and Michelle L. Gordon. 2023. "The (Re-)Emergence and Spread of Viral Zoonotic Disease: A Perfect Storm of Human Ingenuity and Stupidity" Viruses 15, no. 8: 1638. https://doi.org/10.3390/v15081638
APA StyleMarie, V., & Gordon, M. L. (2023). The (Re-)Emergence and Spread of Viral Zoonotic Disease: A Perfect Storm of Human Ingenuity and Stupidity. Viruses, 15(8), 1638. https://doi.org/10.3390/v15081638





