Oxygen-Sensing Protein Cysteamine Dioxygenase from Mandarin Fish Involved in the Arg/N-Degron Pathway and Siniperca chuatsi Rhabdovirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell, Fish, and Hypoxic Exposure
2.2. Virus and Infection
2.3. Antibodies and Reagents
2.4. Molecular Cloning of Mandarin Fish ADO and RGS4 cDNAs
2.5. Sequence Analysis
2.6. Tissue Distribution Analysis
2.7. Quantitative Reverse Transcription PCR (RT–qPCR)
2.8. Cell Transfection
2.9. Immunofluorescence Assay (IFA)
2.10. Western Blot
2.11. TCID50 Assay
3. Results
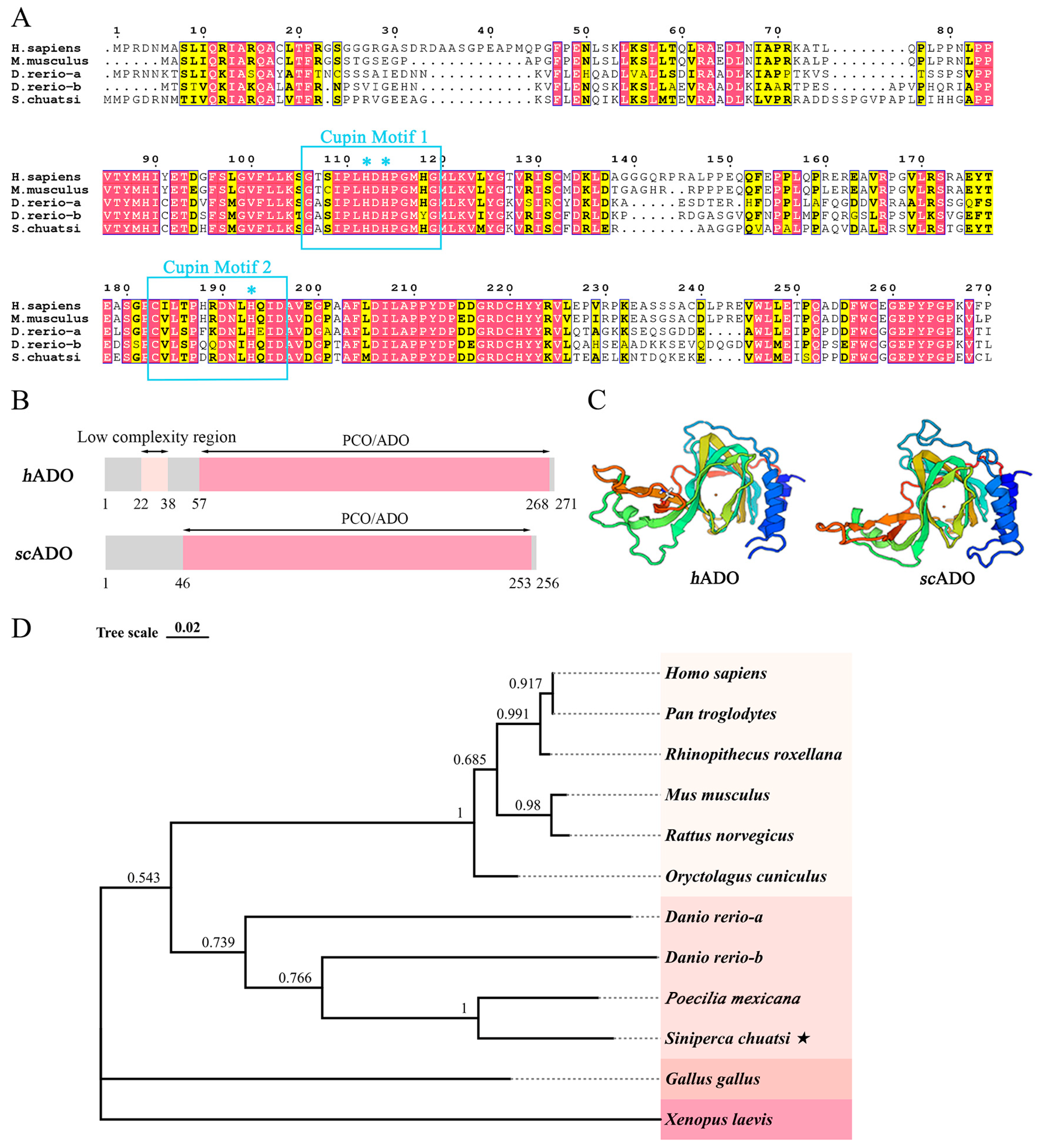
3.1. Bioinformatics Analysis of scADO
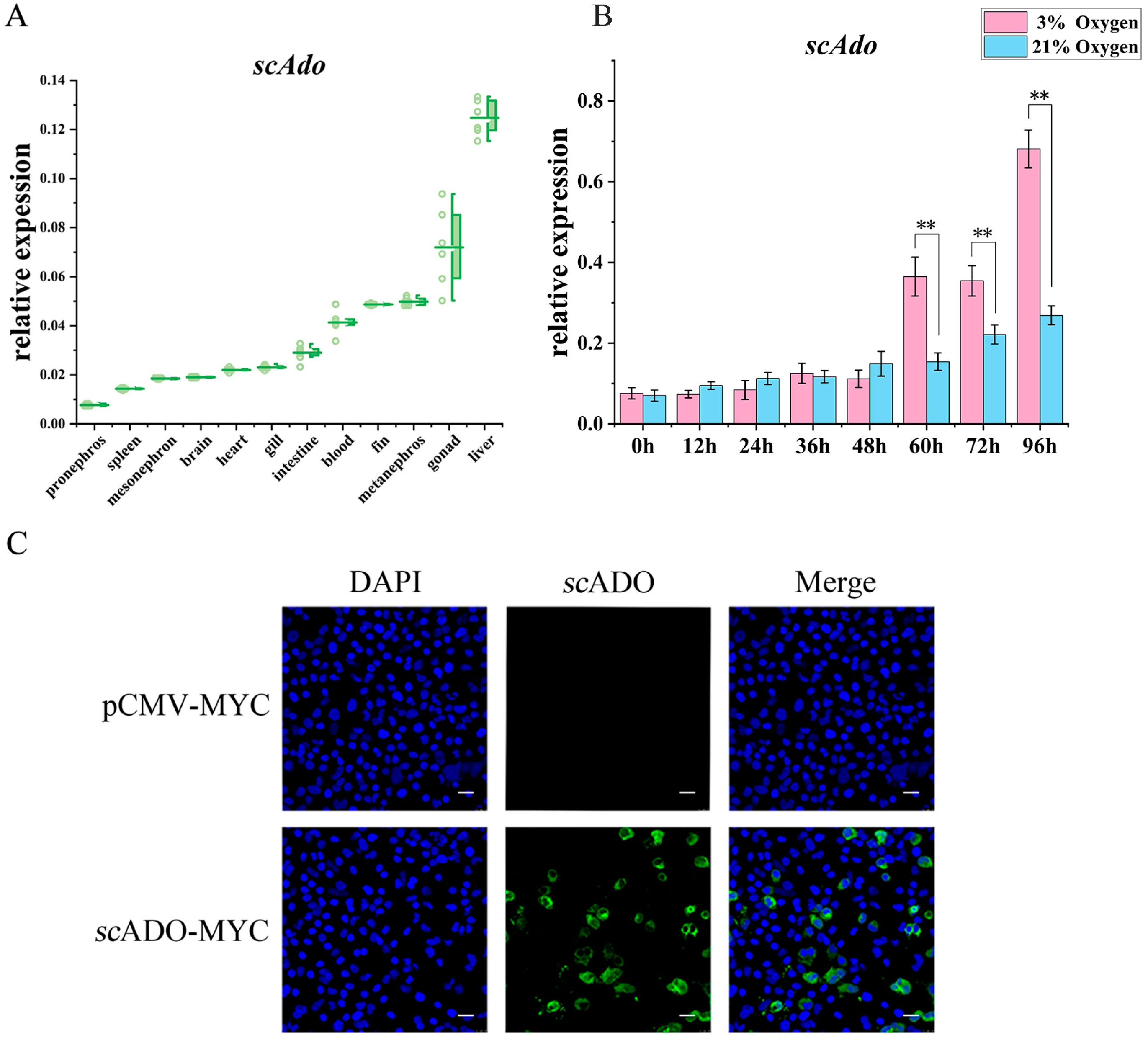
3.2. Expression Patterns and Subcellular Localization of scAdo
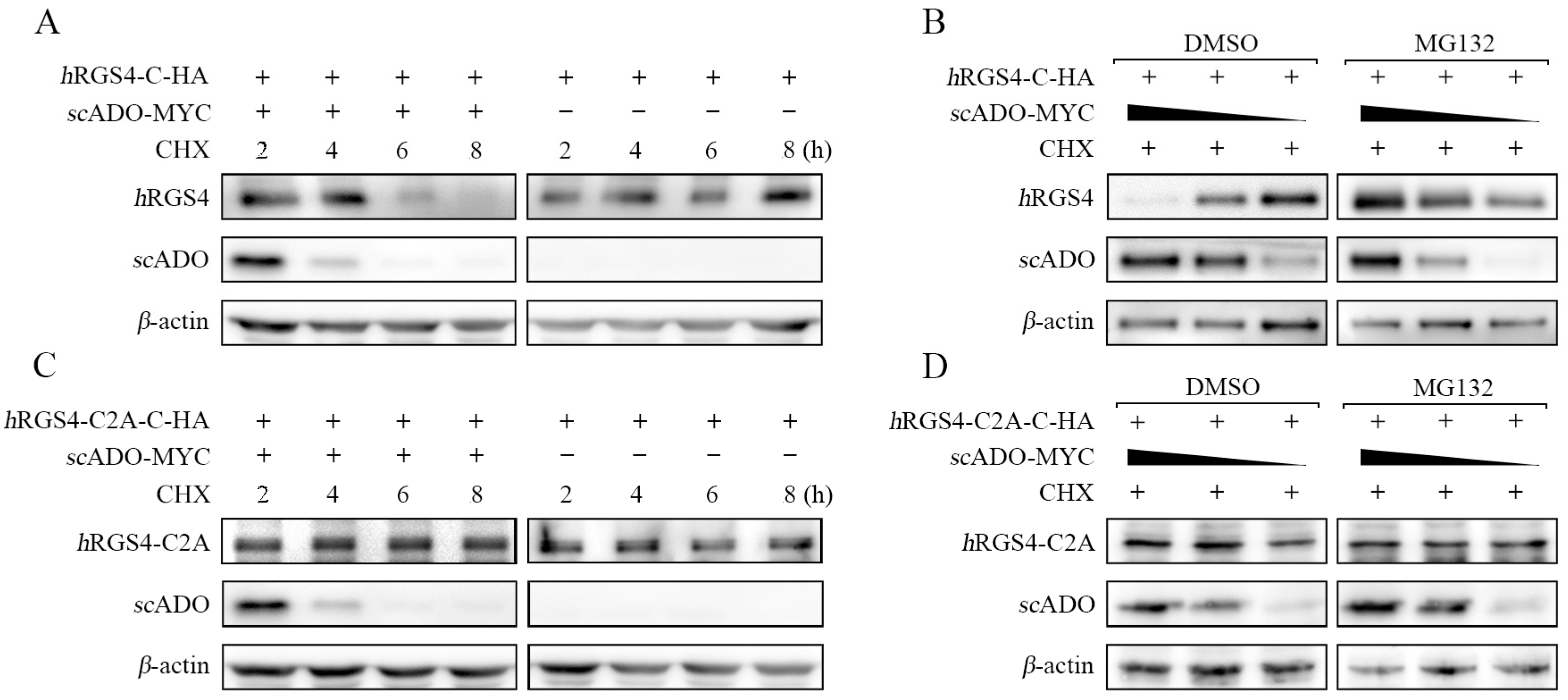
3.3. scADO Could Regulate the Stability of hRGS4
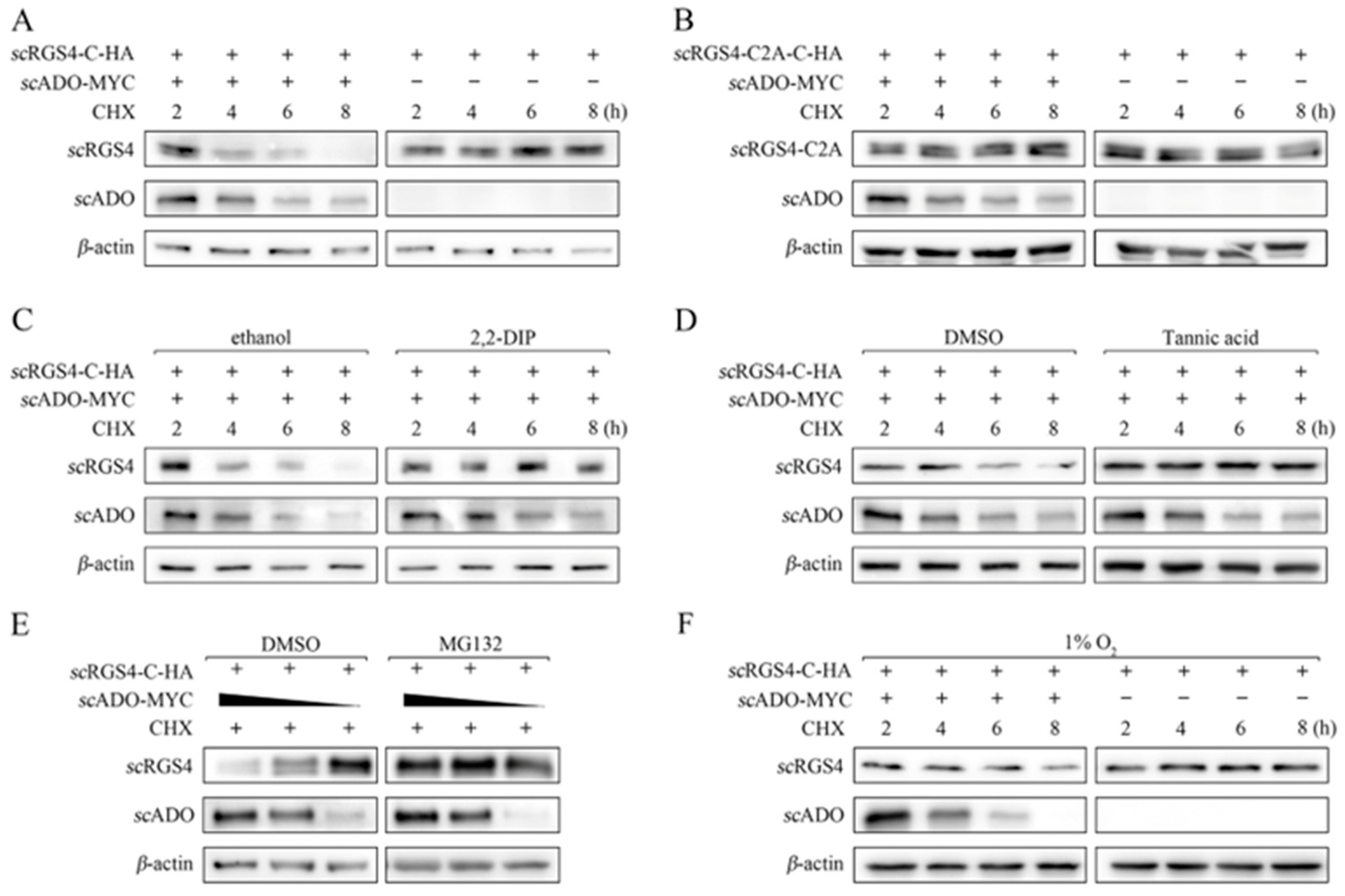
3.4. scADO Regulated the Stability of scRGS4 through the Cys Branch of the Arg/N-Degron Pathway
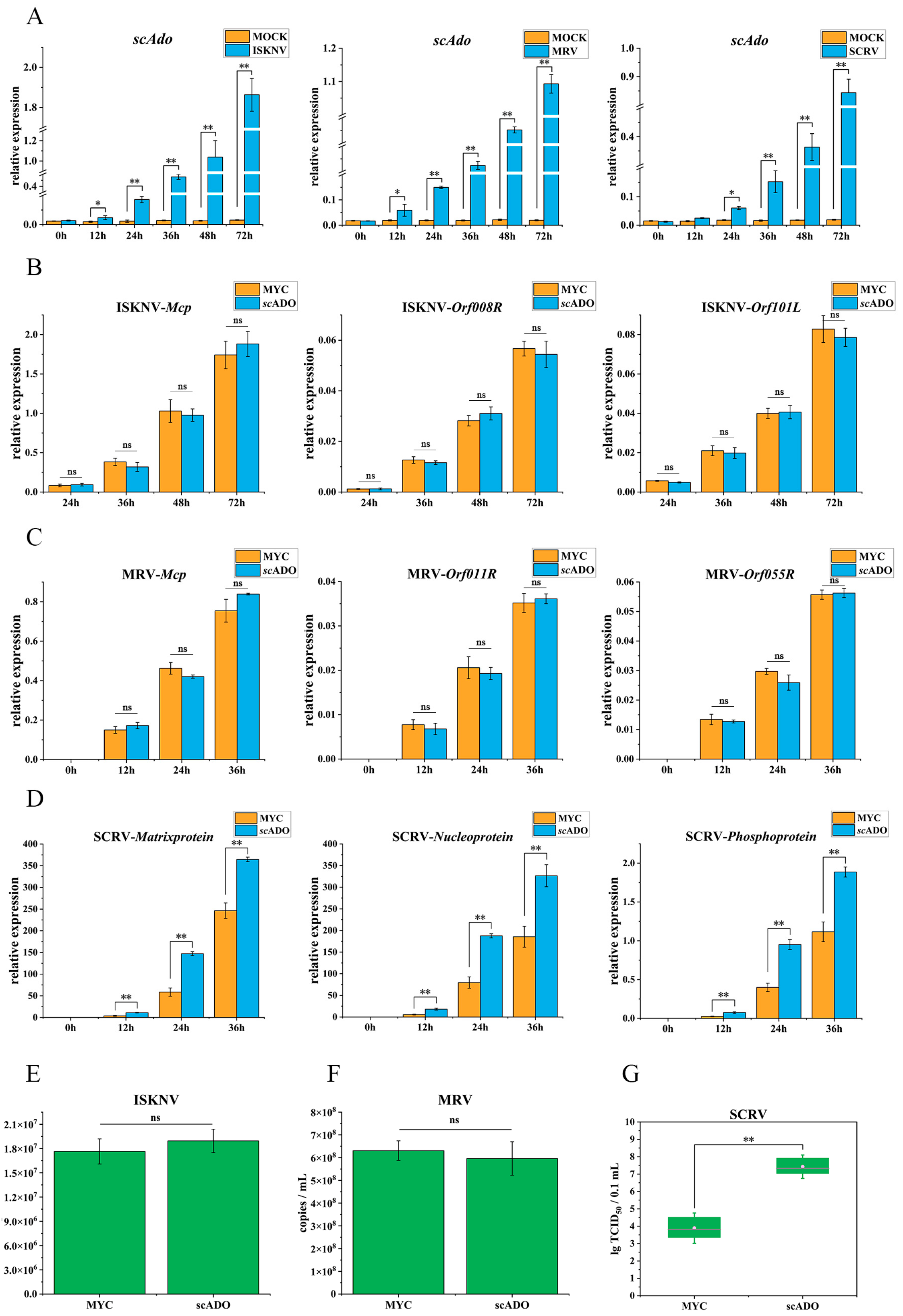
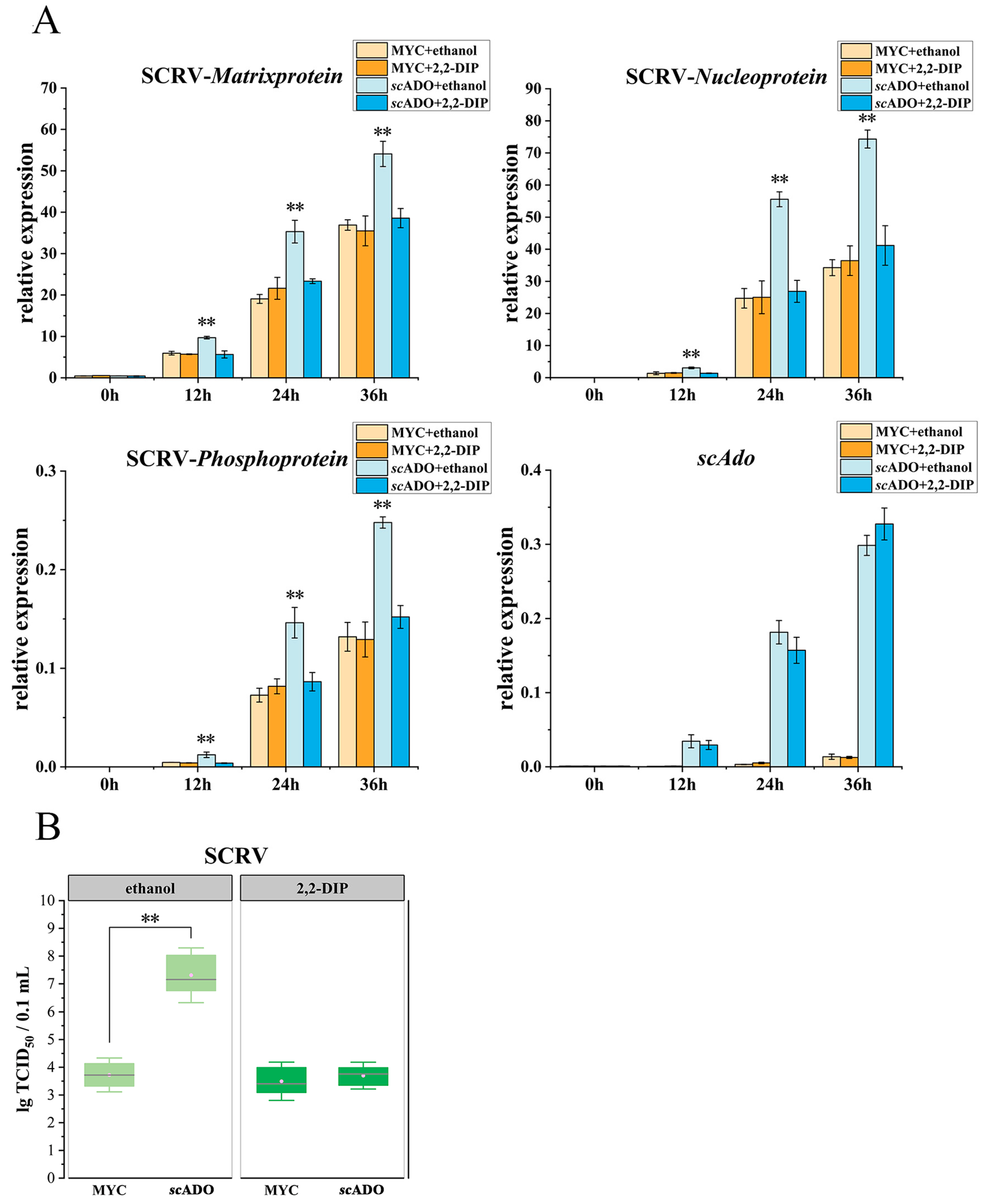
3.5. scADO Promoted SCRV Replication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Bunn, H.F. Hypoxia-inducible factor and its biomedical relevance. J. Biol. Chem. 2003, 278, 19575–19578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinowitz, M.H. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: Tricking the body into mounting orchestrated survival and repair responses. J. Med. Chem. 2013, 56, 9369–9402. [Google Scholar]
- Khan, M.N.; Bhattacharyya, T.; Andrikopoulos, P.; Esteban, M.A.; Barod, R.; Connor, T.; Ashcroft, M.; Maxwell, P.H.; Kiriakidis, S. Factor inhibiting HIF (FIH-1) promotes renal cancer cell survival by protecting cells from HIF-1α-mediated apoptosis. Br. J. Cancer 2011, 104, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.D.; Klecker, M.; Hopkinson, R.J.; Weits, D.A.; Mueller, C.; Naumann, C.; O’Neill, R.; Wickens, J.; Yang, J.; Brooks-Bartlett, J.C.; et al. Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nat. Commun. 2017, 8, 14690. [Google Scholar] [CrossRef] [Green Version]
- Giuntoli, B.; Perata, P. Group VII Ethylene Response Factors in Arabidopsis: Regulation and Physiological Roles. Plant Physiol. 2018, 176, 1143–1155. [Google Scholar] [CrossRef] [Green Version]
- Dominy, J.E., Jr.; Simmons, C.R.; Hirschberger, L.L.; Hwang, J.; Coloso, R.M.; Stipanuk, M.H. Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J. Biol. Chem. 2007, 282, 25189–25198. [Google Scholar] [CrossRef] [Green Version]
- Masson, N.; Keeley, T.P.; Giuntoli, B.; White, M.D.; Puerta, M.L.; Perata, P.; Hopkinson, R.J.; Flashman, E.; Licausi, F.; Ratcliffe, P.J. Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science 2019, 365, 65–69. [Google Scholar] [CrossRef]
- Bouchnak, I.; van Wijk, K.J. N-Degron Pathways in Plastids. Trends Plant Sci. 2019, 24, 917–926. [Google Scholar] [CrossRef]
- Dougan, D.A.; Truscott, K.N.; Zeth, K. The bacterial N-end rule pathway: Expect the unexpected. Mol. Microbiol. 2010, 76, 545–558. [Google Scholar] [CrossRef]
- Varshavsky, A. The N-end rule: Functions, mysteries, uses. Proc. Natl. Acad. Sci. USA 1996, 93, 12142–12149. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.G.; Sheng, J.; Qi, X.; Xu, Z.; Takahashi, T.T.; Varshavsky, A. The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 2005, 437, 981–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timms, R.T.; Koren, I. Tying up loose ends: The N-degron and C-degron pathways of protein degradation. Biochem. Soc. Trans. 2020, 48, 1557–1567. [Google Scholar] [CrossRef]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; Wilkie, T.M.; Gilman, A.G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell 1996, 86, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Tasaki, T.; Moroi, K.; An, J.Y.; Kimura, S.; Davydov, I.V.; Kwon, Y.T. RGS4 and RGS5 are in vivo substrates of the N-end rule pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 15030–15035. [Google Scholar] [CrossRef]
- Chun, Y. Preliminary study on asphyxiation point and oxygen consumption rate of Siniperca Chuatsi. Acta Agric. Jiangxi 1998, 10, 96–98. [Google Scholar]
- Ding, W.; Cao, L.; Cao, Z.; Bing, X. Transcriptomic responses of the liver of mandarin fish (Siniperca chuatsi) under hypoxic stress. J. Fish. Biol. J. Fish Biol. 2023, 103, 44–58. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Zeng, K.; Weng, S.P.; Chan, S.M. Experimental transmission, pathogenicity and physical–chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 2002, 204, 11–24. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Weng, S.; He, J. Occurrence of a lethal ranavirus in hybrid mandarin (Siniperca scherzeri × Siniperca chuatsi) in Guangdong, South China. Vet. Microbiol. 2017, 203, 28–33. [Google Scholar] [CrossRef]
- Tao, J.; Gui, J.; Zhang, Q. Isolation and characterization of a rhabdovirus from co-infection of two viruses in mandarin fish. Aquaculture 2007, 262, 1–9. [Google Scholar] [CrossRef]
- Ma, D.; Deng, G.; Bai, J.; Li, S.; Yu, L.; Quan, Y.; Yang, X.; Jiang, X.; Zhu, Z.; Ye, X. A strain of Siniperca chuatsi rhabdovirus causes high mortality among cultured Largemouth Bass in South China. J. Aquat. Anim. Health 2013, 25, 197–204. [Google Scholar] [CrossRef]
- He, J.; Yu, Y.; Li, Z.M.; Liu, Z.X.; Weng, S.P.; Guo, C.J.; He, J.G. Hypoxia triggers the outbreak of infectious spleen and kidney necrosis virus disease through viral hypoxia response elements. Virulence 2022, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Shi, N.; He, J. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Guo, Z.; Zhao, Y.; Luo, S.; Yu, T.; Zhang, D.; Wang, G. Identification of the Nrf2 in the fathead minnow muscle cell line: Role for a regulation in response to H2O2 induced the oxidative stress in fish cell. Fish Physiol. Biochem. 2020, 46, 1699–1711. [Google Scholar] [CrossRef]
- Qin, X.W.; Luo, Z.Y.; Pan, W.Q.; He, J.; Li, Z.M.; Yu, Y.; Liu, C.; Weng, S.P.; He, J.G.; Guo, C.J. The Interaction of Mandarin Fish DDX41 with STING Evokes type I Interferon Responses Inhibiting Ranavirus Replication. Viruses 2023, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xie, T.L.; Li, X.; Yu, Y.; Zhan, Z.P.; Weng, S.P.; Guo, C.J.; He, J.G. Molecular cloning of Y-Box binding protein-1 from mandarin fish and its roles in stress-response and antiviral immunity. Fish Shellfish Immunol. 2019, 93, 406–415. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Zhan, Z.P.; Qin, X.W.; Pan, W.Q.; Liang, M.C.; Li, C.R.; Weng, S.P.; He, J.G.; Guo, C.J. Interaction of Teleost Fish TRPV4 with DEAD Box RNA Helicase 1 Regulates Iridovirus Replication. J. Virol. 2023, 97, e0049523. [Google Scholar] [CrossRef]
- Yu, Y.; He, J.; Liu, W.H.; Li, Z.M.; Weng, S.P.; He, J.G.; Guo, C.J. Molecular Characterization and Functional Analysis of Hypoxia-Responsive Factor Prolyl Hydroxylase Domain 2 in Mandarin Fish (Siniperca chuatsi). Animals 2023, 13, 1556. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer reihenversuche. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1931, 4, 480–483. [Google Scholar] [CrossRef]
- Zeng, R.Y.; Pan, W.Q.; Lin, Y.F.; He, J.; Luo, Z.Y.; Li, Z.M.; Weng, S.P.; He, J.G.; Guo, C.J. Development of a gene-deleted live attenuated candidate vaccine against fish virus (ISKNV) with low pathogenicity and high protection. iScience 2021, 24, 102750. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Culham, A.; Carter, C.E.; Sosa-Aguirre, C.R.; Goodenough, P.W. Evolution of functional diversity in the cupin superfamily. Trends Biochem. Sci. 2001, 26, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shin, I.; Li, J.; Liu, A. Crystal structure of human cysteamine dioxygenase provides a structural rationale for its function as an oxygen sensor. J. Biol. Chem. 2021, 297, 101176. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; van Dongen, J.T.; Licausi, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014, 5, 3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Yu, Y.; Qin, X.W.; Zeng, R.Y.; Wang, Y.Y.; Li, Z.M.; Mi, S.; Weng, S.P.; Guo, C.J.; He, J.G. Identification and functional analysis of the Mandarin fish (Siniperca chuatsi) hypoxia-inducible factor-1α involved in the immune response. Fish Shellfish Immunol. 2019, 92, 141–150. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequences (5′-3′) |
|---|---|
| scAdo-F-EcoR I | GGAATTCGGATGCCAGGTGACAGAAACATG |
| scAdo-R-Kpn I | GGGGTACCTCAGAGGCAGACCTCCGGGCC |
| hRgs4-F-Bgl II | GGGAATTCATGTGCTCCAAACTCGTAGAGATG |
| hRgs4-C2A-F-SalI | GCGTCGACATGGCTAAAGGGCTTGCAGGTC |
| hRgs4-R-Kpn I | GGGGTACCGGCACACTGAGGGACCAGGGA |
| scRgs4-F-EcoR I | GGGAATTCATGTGTAAAGGACTTGCAACA |
| scRgs4-C2A-F-EcoR I | GGGAATTCATGGCTAAAGGACTTGCAACA |
| scRgs4-R-Kpn I | CCGGTACCGGCACCGCCAGTTAACGCCTG |
| Name | Sequences (5′-3′) |
|---|---|
| scAdo-F | GCGTTCATGGACATCCT |
| scAdo-R | CCTCCGCACCAGAAAT |
| ISKNV-Mcp-F | CAATGTAGCACCCGCACTGACC |
| ISKNV-Mcp-R | ACCTCACGCTCCTCACTTGTC |
| ISKNV-Orf008R-F | TGACCTGTGGCCTAGATGATAAC |
| ISKNV-Orf008R-R | AGAGGCAGAGCAGCAGCATGTAGAGT |
| ISKNV-Orf101L-F | AAGCCGAGGACCCCAAGAAGT |
| ISKNV-Orf101L-R | GTCCTGACCGCCCACCAGTAT |
| MRV-Mcp-F | ATCTCGCCACTTATGACAG |
| MRV-Mcp-R | CAAGAGTTGAGCACATAGTC |
| MRV-Orf011R-F | ACGCAAGAAGTTAGAGCATA |
| MRV-Orf011R-R | CCTGGTAGAATAGAGGTGATT |
| MRV-Orf055R-F | ACAGTGGATCTAGTCAACAT |
| MRV-Orf055R-R | GTACGCAGTCACAGTCAG |
| SCRV-Matrixprotein-F | CGGTTGCCATCTCTTATGA |
| SCRV-Matrixprotein-R | CCTCTGCTTCTGCTATCTG |
| SCRV-Nucleoprotein-F | TCGCATCATTCACTGGATT |
| SCRV-Nucleoprotein-R | TGGCAGAGTAAGGAGACA |
| SCRV-Phosphoprotein -F | ACAGCAGAGGTCTCAAGA |
| SCRV-Phosphoprotein-R | ATTAGCATCCGCAGAAGG |
| β-actin-F | CCCTCTGAACCCCAAAGCCA |
| β-actin-R | CAGCCTGGATGGCAACGTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; He, J.; Li, Z.; Weng, S.; Guo, C.; He, J. Oxygen-Sensing Protein Cysteamine Dioxygenase from Mandarin Fish Involved in the Arg/N-Degron Pathway and Siniperca chuatsi Rhabdovirus Infection. Viruses 2023, 15, 1644. https://doi.org/10.3390/v15081644
Liu W, He J, Li Z, Weng S, Guo C, He J. Oxygen-Sensing Protein Cysteamine Dioxygenase from Mandarin Fish Involved in the Arg/N-Degron Pathway and Siniperca chuatsi Rhabdovirus Infection. Viruses. 2023; 15(8):1644. https://doi.org/10.3390/v15081644
Chicago/Turabian StyleLiu, Wenhui, Jian He, Zhimin Li, Shaoping Weng, Changjun Guo, and Jianguo He. 2023. "Oxygen-Sensing Protein Cysteamine Dioxygenase from Mandarin Fish Involved in the Arg/N-Degron Pathway and Siniperca chuatsi Rhabdovirus Infection" Viruses 15, no. 8: 1644. https://doi.org/10.3390/v15081644






