Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes
Abstract
:1. Introduction
2. Mpox Virus Pathophysiology
3. Mpox Clades and Differences in Pathogenicity
4. Pregnancy with Clade I Mpox Virus Infection
5. Pregnancy with Clade IIa Mpox Virus Infection
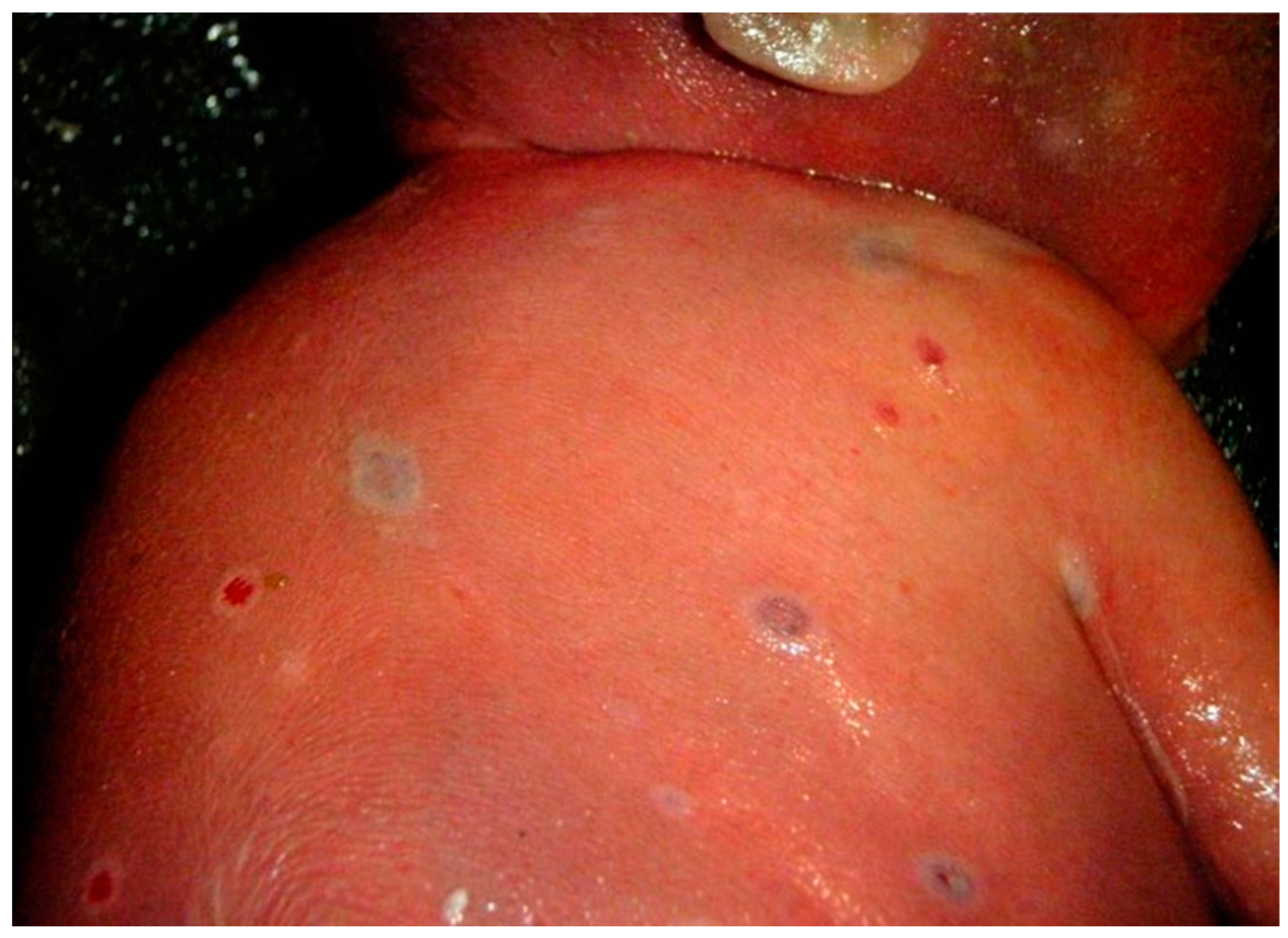
6. Pregnancy with Clade IIb Mpox Virus Infection (2022–2023 Global Mpox Outbreak)
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Titanji, B.K.; Tegomoh, B.; Nematollahi, S.; Konomos, M.; Kulkarni, P.A. Monkeypox: A Contemporary Review for Healthcare Professionals. Open Forum Infect. Dis. 2022, 9, ofac310. [Google Scholar] [CrossRef]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Meaney-Delman, D.M.; Galang, R.R.; Petersen, B.W.; Jamieson, D.J. A Primer on Monkeypox Virus for Obstetrician-Gynecologists: Diagnosis, Prevention, and Treatment. Obstet. Gynecol. 2022, 140, 391–397. [Google Scholar] [CrossRef]
- Dashraath, P.; Alves, M.P.; Schwartz, D.A.; Nielsen-Saines, K.; Baud, D. Potential mechanisms of intrauterine transmission of monkeypox virus. Lancet Microbe 2023, 4, e14. [Google Scholar] [CrossRef] [PubMed]
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef]
- Swiss Institute of Bioinformatics (SIB). Orthopoxvirus. Viral Zone. Available online: https://viralzone.expasy.org/149?outline=all_by_species (accessed on 8 June 2023).
- Mucker, E.M.; Thiele-Suess, C.; Baumhof, P.; Hooper, J.W. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol. Ther. Nucleic Acids 2022, 28, 847–858. [Google Scholar] [CrossRef]
- Realegeno, S.; Priyamvada, L.; Kumar, A.; Blackburn, J.B.; Hartloge, C.; Puschnik, A.S.; Sambhara, S.; Olson, V.A.; Carette, J.E.; Lupashin, V.; et al. Conserved Oligomeric Golgi (COG) complex proteins facilitate orthopoxvirus entry, fusion and spread. Viruses 2020, 12, 707. [Google Scholar] [CrossRef]
- Realegeno, S.; Puschnik, A.S.; Kumar, A.; Goldsmith, C.; Burgado, J.; Sambhara, S.; Olson, V.A.; Carroll, D.; Damon, I.; Hirata, T.; et al. Monkeypox virus host factor screen using haploid cells identifies essential role of GARP complex in extracellular virus formation. J. Virol. 2017, 91, e00011–e00017. [Google Scholar] [CrossRef]
- Magnus, P.V.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, Q.; Dong, X.; Wang, M.; Liu, Z.; Li, Z. Mpox, Caused by the MPXV of the Clade IIb Lineage, Goes Global. Trop. Med. Infect. Dis. 2023, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mpox (Monkeypox). Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 8 June 2023).
- European Centre for Disease Prevention and Control. Factsheet for Health Professionals on Mpox (Monkeypox). Available online: https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals (accessed on 5 June 2023).
- Ulaeto, D.; Agafonov, A.; Burchfield, J.; Carter, L.; Happi, C.; Jakob, R.; Krpelanova, E.; Kuppalli, K.; Lefkowitz, E.J.; Mauldin, M.R.; et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect. Dis. 2023, 23, 273–275. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommends New Name for Monkeypox Disease. Available online: https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease (accessed on 6 June 2023).
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Clinical Features of 282 Patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Neglected Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Iizuka, I.; Shiota, T.; Sakai, K.; Ogata, M.; et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 2009, 90, 2266–2271. [Google Scholar] [CrossRef]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef]
- Lopera, J.G.; Falendysz, E.A.; Rocke, T.E.; Osorio, J.E. Attenuation of monkeypox virus by deletion of genomic regions. Virology 2015, 475, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Leung, M.K.; Hauhart, R.; Buller, R.M.; Bertram, P.; Wang, X.; Rosengard, A.M.; Kotwal, G.J.; Atkinson, J.P. Structure and regulatory profile of the monkeypox inhibitor of complement: Comparison to homologs in vaccinia and variola and evidence for dimer formation. J. Immunol. 2006, 76, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Sklenovská, N.; Van Ranst, M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Mwamba, D.K.; Kebela, B.I.; Shongo, R.L.; Pukuta, E.; Kayembe, N.J.M. Profil épidemiologique du monkeypox en RDC, 2010–2014. Ann. Afr. Med. 2014, 8, 1855–1860. [Google Scholar]
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef]
- World Health Organization. Organization. Regional Office for Africa. Weekly Bulletin on Outbreak and Other Emergencies: Week 41: 05–11 October 2020. 2020. Available online: https://apps.who.int/iris/handle/10665/336026 (accessed on 5 June 2023).
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.T. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Pittman, P.R.; Martin, J.W.; Kingebeni, P.M.; Tamfum, J.M.; Mwema, G.; Wan, Q.; Ewala, P.; Alonga, J.; Bilulu, G.; Reynolds, M.G.; et al. Clinical characterization and placental pathology of mpox infection in hospitalized patients in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2023, 17, e0010384. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Pittman, P.R. Monkeypox and pregnancy: Correspondence. Am. J. Obstet. Gynecol. 2023, 228, 365. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Ha, S.; Dashraath, P.; Baud, D.; Pittman, P.R.; Waldorf, K.A. Monkeypox Virus in Pregnancy, the Placenta and Newborn: An Emerging Poxvirus with Similarities to Smallpox and Other Orthopoxvirus Agents Causing Maternal and Fetal Disease. Arch. Pathol. Lab. Med. 2023, 147, 746–757. [Google Scholar] [CrossRef]
- Jezek, Z.; Fenner, F. Human Monkeypox. Monographs in Virology; Karger: Basel, Switzerland, 1988; Volume 17. [Google Scholar]
- Kisalu, N.K.; Mokili, J.L. Toward Understanding the Outcomes of Monkeypox Infection in Human Pregnancy. J. Infect. Dis. 2017, 216, 795–797. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical course and outcome of human monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, e210–e214. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multi-Country Outbreak of Mpox, External Situation Report #25–24 June 2023, Edition 24. Available online: https://reliefweb.int/report/world/multi-country-outbreak-mpox-monkeypox-external-situation-report-25-published-24-june-2023 (accessed on 7 July 2023).
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Jessen, H.; Wyen, C.; Grunwald, S.; Noe, S.; Teichmann, J.; Krauss, A.S.; Kolarikal, H.; Scholten, S.; Schuler, C.; et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: A large outbreak cohort in Germany. HIV Med. 2023, 24, 389–397. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: An observational analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Luna, N.; Ramírez, A.L.; Muñoz, M.; Ballesteros, N.; Patiño, L.H.; Castañeda, S.A.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.; Ramírez, J.D. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel. Med. Infect. Dis. 2022, 49, 102402. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Palich, R.; Ghosn, J.; Walmsley, S.; Moschese, D.; Cortes, C.P.; Galliez, R.M.; Garlin, A.B.; Nozza, S.; Mitja, O.; et al. Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: A global case series. Lancet 2022, 400, 1953–1965. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Amer, F.A. Monkeypox virus infection in women and non-binary people: Uncommon or neglected? Lancet 2022, 400, 1903–1905. [Google Scholar] [CrossRef]
- World Health Organization. 2022-23 Mpox (Monkeypox) Outbreak: Global Trends. 13 June 2023. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 19 June 2023).
- Tin, A.U.S.; Spots First Monkeypox Case in a Pregnant Woman as Cases Climb. CBS News. 26 July 2022. Available online: https://www.cbsnews.com/news/monkeypox-pregnant-woman-baby-cases (accessed on 21 May 2023).
- Infectious Disease Society of America. CDC/IDSA Monkeypox: Updates on Testing, Vaccination & Treatment. 23 July 2022. Available online: https://www.idsociety.org/multimedia/cliniciancalls/cdcidsa-monkeypox-updates-on-testing-vaccination--treatment (accessed on 11 May 2023).
- Oakley, L.P.; Hufstetler, K.; O’Shea, J.; Sharpe, J.D.; McArdle, C.; Neelam, V.; Roth, N.M.; Olsen, E.O.; Wolf, M.; Pao, L.Z.; et al. Mpox Cases among Cisgender Women and Pregnant Persons—United States, 11 May–7 November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 9–14. [Google Scholar] [CrossRef]
- Khalil, A.; Samara, A.; O’Brien, P.; Coutinho, C.M.; Duarte, G.; Quintana, S.M.; Ladhani, S.N. Monkeypox in pregnancy: Update on current outbreak. Lancet Infect. Dis. 2022, 22, 1534–1535. [Google Scholar] [CrossRef]
- Government of Brazil. Monkeypox Epidemiological Bulletin No 8 (Emergency Operations Center). 26 August 2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/variola-dos-macacos/boletim-epidemiologico-de-monkeypox-no-8-coe/view (accessed on 15 June 2023).
- Campinas Confirma Varíola dos Macacos em Gestante de 37 anos. Acidade on Campinas. 5 August 2022. Available online: https://www.acidadeon.com/campinas/cotidiano/Campinas-confirma-variola-dos-macacos-em-gestante-de-37-anos-20220805-0031.html (accessed on 19 June 2023).
- Alvarenga, L. Primeira Mulher Com Varíola dos Macacos em Minas Recebe Alta e Deixa Hospital. Otempo. 16 August 2022. Available online: https://www.otempo.com.br/o-tempo-betim/primeira-mulher-com-variola-dos-macacos-em-minas-recebe-alta-e-deixa-hospital-1.2717116 (accessed on 15 June 2023).
- Brazil Ministry of Health. Boletim Epidemiológico de Monkeypox nº 22 (COE). 23 May 2023. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/variola-dos-macacos/boletim-epidemiologico-de-monkeypox-no-22-coe/view (accessed on 19 June 2023).
- Pan American Health Organization. Situation Report on Monkeypox Multi-Country Outbreak Response—Region of the Americas. N.7—3 March 2023. Available online: https://www.paho.org/en/documents/situation-report-monkeypox-multi-country-outbreak-response-region-americas-n7-3-march (accessed on 17 June 2023).
- Antonello, V.S.; Cornelio, P.E.; Dallé, J. Disseminated Neonatal Monkeypox Virus Infection: Case Report in Brazil. Pediatr. Infect. Dis. J. 2023, 42, e152–e153. [Google Scholar] [CrossRef]
- Ramnarayan, P.; Mitting, R.; Whittaker, E.; Marcolin, M.; O’Regan, C.; Sinha, R.; Bennett, A.; Moustafa, M.; Tickner, N.; Gilchrist, M.; et al. Neonatal Monkeypox Virus Infection. N. Engl. J. Med. 2022, 387, 1618–1620. [Google Scholar] [CrossRef]
- Mukit, F.A.; Louie, E.M.; Cape, H.T.; Bohn, S.N. A Suspected Case of a Neonatal Monkeypox Infection with Ocular Involvement. Cureus 2023, 15, e38819. [Google Scholar] [CrossRef]
- Soares, M.L.C.; Teixeira, D.C.; Pin, W.R.; Coelho, I.A.; Matos, J.C.; Romanelli, R.M.C. Newborn Exposed to the Monkeypox Virus: A Brief Report. Pediatr. Infect. Dis. J. 2023, 42, e316. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.E.F.; Ng, O.T.; Ho, Z.J.M.; Mak, T.M.; Marimuthu, K.; Vasoo, S.; Yeo, T.W.; Ng, Y.K.; Cui, L.; Ferdous, Z.; et al. Imported Monkeypox, Singapore. Emerg. Infect. Dis. 2020, 26, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’Connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509. [Google Scholar] [CrossRef]
- Rao, A.K.; Schulte, J.; Chen, T.H.; Hughes, C.M.; Davidson, W.; Neff, J.M.; Markarian, M.; Delea, K.C.; Wada, S.; Liddell, A.; et al. Monkeypox in a Traveler Returning from Nigeria—Dallas, Texas, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Parvin, R.; Ali, A.; Nagy, A.; Zhu, Z.; Zhao, S.; Neuhaus, J.; Paul, A.K.; Hafez, H.M.; Shehata, A.A. Monkeypox virus: A comprehensive review of taxonomy, evolution, epidemiology, diagnosis, prevention, and control regiments so far. Ger. J. Microbiol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- World Health Organization. Surveillance, Case Investigation and Contact Tracing for Mpox (Monkeypox): Interim Guidance. 22 December 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4 (accessed on 22 July 2023).
- Panag, D.S.; Jain, N.; Katagi, D.; De Jesus Cipriano Flores, G.; Silva Dutra Macedo, G.D.; Rodrigo Díaz Villa, G.; Yèche, M.; Velázquez Mérida, S.Y.; Kapparath, S.; Sert, Z.; et al. Variations in national surveillance reporting for Mpox virus: A comparative analysis in 32 countries. Front. Public. Health 2023, 11, 1178654. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; French, D.; O’Sullivan, D.M.; Moran-Gilad, J.; Zumla, A. Monkeypox: Another test for PCR. Eurosurveillance 2022, 27, 2200497. [Google Scholar] [CrossRef] [PubMed]
- Tomori, O. Monkeypox in Nigeria: Why the Disease Needs Intense Management. The Conversation. 25 July 2021. Available online: https://theconversation.com/monkeypox-in-nigeria-why-the-disease-needs-intense-management-165022 (accessed on 16 June 2023).
- D’Antonio, F.; Pagani, G.; Buca, D.; Khalil, A. Monkeypox infection in pregnancy: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100747. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Mulkey, S.B.; Roberts, D.J. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: Clinical-pathologic correlations. Am. J. Obstet. Gynecol. 2023, 228, 261–269. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency from COVID-19 Causes Stillbirth and Neonatal Death from Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Khalil, A.; Samara, A.; Ladhani, S.; O’Brien, P. Monkeypox and pregnancy: Time for global surveillance and prevention strategies. Lancet 2022, 400, 1193. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Pregnant Women, Vaccine Development for Emerging and Epidemic Viral Infections and Maternal Immunization: Human Rights and the Global Survival of Mothers and Infants. Curr. Trop. Med. Rep. 2019, 6, 179–185. [Google Scholar] [CrossRef]
- Schwartz, D.A. Clinical Trials and Administration of Zika Virus Vaccine in Pregnant Women: Lessons (that Should Have Been) Learned from Excluding Immunization with the Ebola Vaccine during Pregnancy and Lactation. Vaccines 2018, 6, 81. [Google Scholar] [CrossRef]
- Schwartz, D.A. Being Pregnant during the Kivu Ebola Virus Outbreak in DR Congo: The rVSV-ZEBOV Vaccine and Its Accessibility by Mothers and Infants during Humanitarian Crises and in Conflict Areas. Vaccines 2020, 8, 38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, D.A.; Pittman, P.R. Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes. Viruses 2023, 15, 1649. https://doi.org/10.3390/v15081649
Schwartz DA, Pittman PR. Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes. Viruses. 2023; 15(8):1649. https://doi.org/10.3390/v15081649
Chicago/Turabian StyleSchwartz, David A., and Phillip R. Pittman. 2023. "Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes" Viruses 15, no. 8: 1649. https://doi.org/10.3390/v15081649
APA StyleSchwartz, D. A., & Pittman, P. R. (2023). Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes. Viruses, 15(8), 1649. https://doi.org/10.3390/v15081649






