Bioinformatic Surveillance Leads to Discovery of Two Novel Putative Bunyaviruses Associated with Black Soldier Fly
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Sequencing
2.2. Metatranscriptome Assembly
2.3. Identification of Conserved Viral Sequences
2.4. Identification of Diverged Viral Fragments
2.5. Phylogenetic Analysis
2.6. Incorporation of Public Data
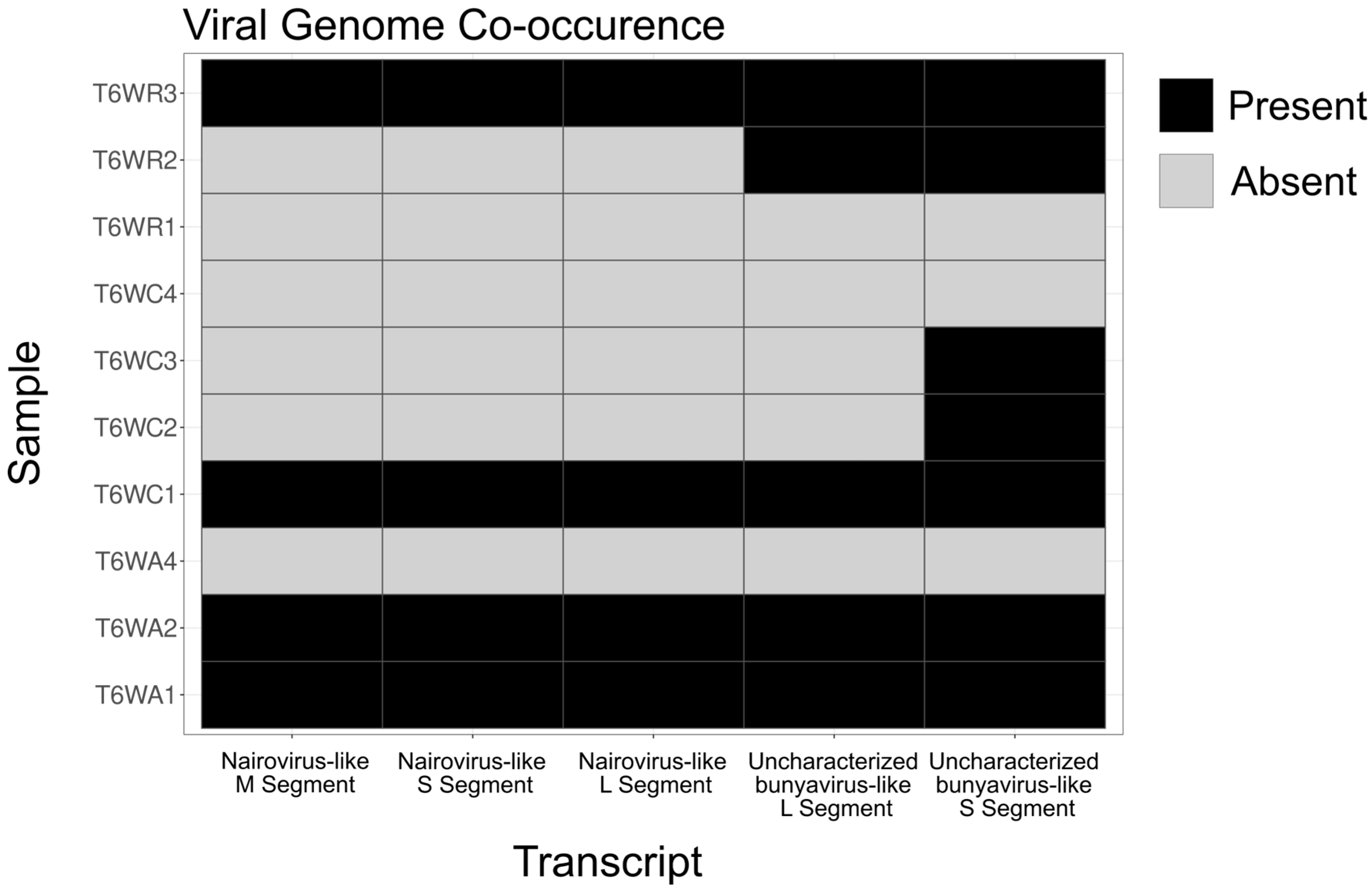
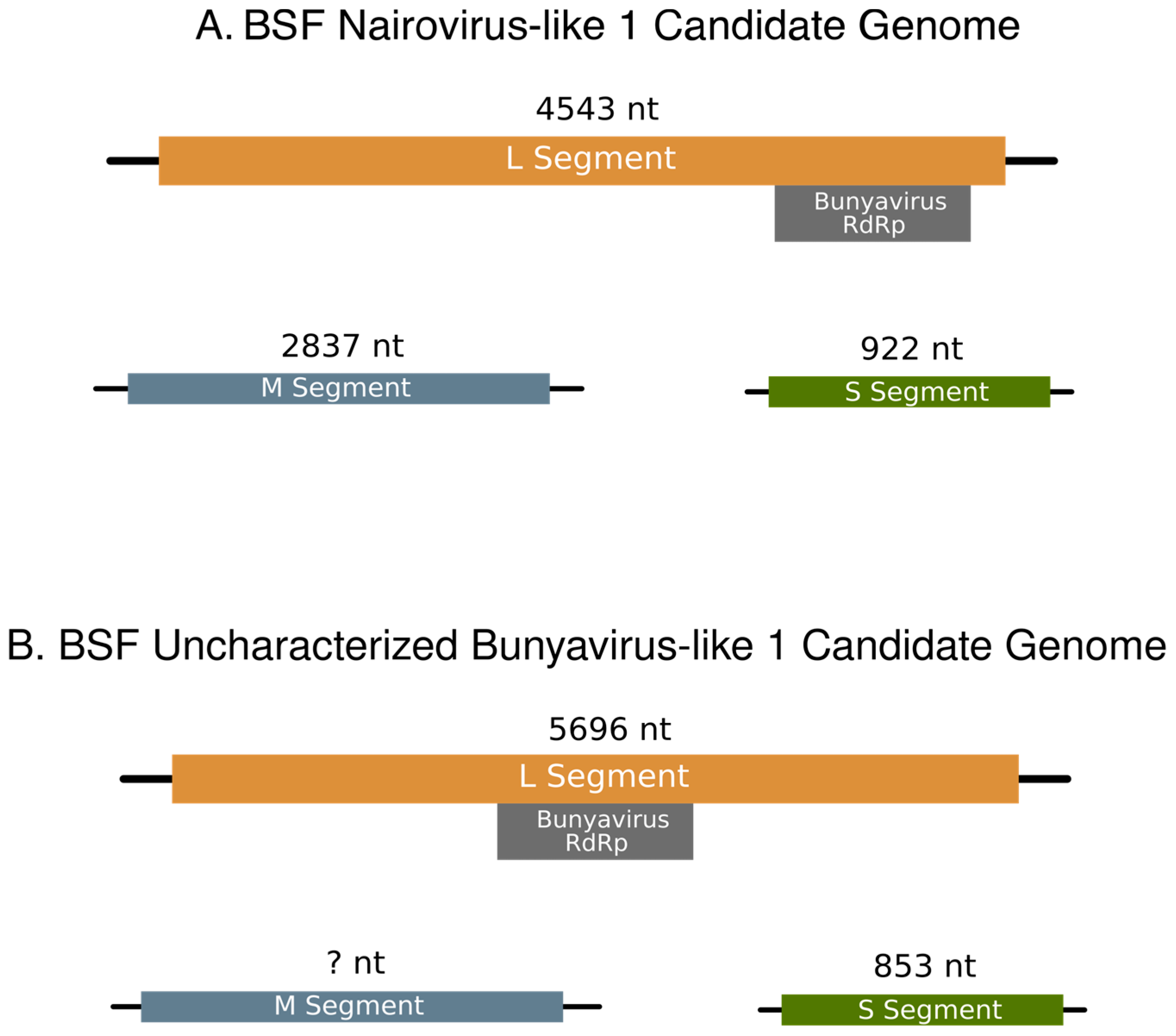
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huis, A. Insects as Food and Feed, a New Emerging Agricultural Sector: A Review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Eilenberg, J.; Vlak, J.M.; Nielsen-LeRoux, C.; Cappellozza, S.; Jensen, A.B. Diseases in Insects Produced for Food and Feed. J. Insects Food Feed. 2015, 1, 87–102. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; van Huis, A. Black Soldier Fly from Pest to “crown Jewel” of the Insects as Feed Industry: An Historical Perspective. J. Insects Food Feed 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Zhu, Z.; Rehman, K.U.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.H.; Cai, M.; Zhang, J.; Yu, Z.; et al. De Novo Transcriptome Sequencing and Analysis Revealed the Molecular Basis of Rapid Fat Accumulation by Black Soldier Fly (Hermetia illucens, L.) for Development of Insectival Biodiesel. Biotechnol. Biofuels 2019, 12, 194. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, Y.; Muhmood, A.; Zeng, B.; Qiu, Y.; Wang, P.; Ren, L. Probing the Antioxidant Activity of Functional Proteins and Bioactive Peptides in Hermetia illucens Larvae Fed with Food Wastes. Sci. Rep. 2022, 12, 2799. [Google Scholar] [CrossRef]
- Joosten, L.; Lecocq, A.; Jensen, A.B.; Haenen, O.; Schmitt, E.; Eilenberg, J. Review of Insect Pathogen Risks for the Black Soldier Fly (Hermetia illucens) and Guidelines for Reliable Production. Entomol. Exp. Appl. 2020, 168, 432–447. [Google Scholar] [CrossRef]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of Antimicrobial Substances from Larvae of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Zhan, S.; Fang, G.; Cai, M.; Kou, Z.; Xu, J.; Cao, Y.; Bai, L.; Zhang, Y.; Jiang, Y.; Luo, X.; et al. Genomic Landscape and Genetic Manipulation of the Black Soldier Fly Hermetia illucens, a Natural Waste Recycler. Cell Res. 2020, 30, 50–60. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A Bioinformatic Study of Antimicrobial Peptides Identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Pienaar, R.D.; Gilbert, C.; Belliardo, C.; Herrero, S.; Herniou, E.A. First Evidence of Past and Present Interactions between Viruses and the Black Soldier Fly, Hermetia illucens. Viruses 2022, 14, 1274. [Google Scholar] [CrossRef] [PubMed]
- Batson, J.; Dudas, G.; Haas-Stapleton, E.; Kistler, A.L.; Li, L.M.; Logan, P.; Ratnasiri, K.; Retallack, H. Single Mosquito Metatranscriptomics Identifies Vectors, Emerging Pathogens and Reservoirs in One Assay. Elife 2021, 10, e68353. [Google Scholar] [CrossRef]
- Semberg, E.; de Miranda, J.R.; Low, M.; Jansson, A.; Forsgren, E.; Berggren, Å. Diagnostic Protocols for the Detection of Acheta Domesticus Densovirus (AdDV) in Cricket Frass. J. Virol. Methods 2019, 264, 61–64. [Google Scholar] [CrossRef]
- Andrews, S. FASTQC. A Quality Control Tool for High Throughput Sequence Data. 2010. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Generalovic, T.N.; McCarthy, S.A.; Warren, I.A.; Wood, J.M.D.; Torrance, J.; Sims, Y.; Quail, M.; Howe, K.; Pipan, M.; Durbin, R.; et al. A High-Quality, Chromosome-Level Genome Assembly of the Black Soldier Fly (Hermetia illucens L.). G3 Genes Genomes Gene. 2021, 11, jkab085. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Merkel, D. Docker: Lightweight Linux Containers for Consistent Development and Deployment. Linux J. 2014, 2014, 2. [Google Scholar]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive Protein Alignments at Tree-of-Life Scale Using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein Domains Identifier. Nucleic Acids Res. 2005, 33, W116–W120. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of Branch Support Methods Demonstrates Accuracy, Power, and Robustness of Fast Likelihood-Based Approximation Schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Vasimuddin, M.; Misra, S.; Li, H.; Aluru, S. Efficient Architecture-Aware Acceleration of BWA-MEM for Multicore Systems. In Proceedings of the 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS), Rio de Janeiro, Brazil, 20–24 May 2019; pp. 314–324. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative Genomics Viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Callanan, J.; Stockdale, S.R.; Shkoporov, A.; Draper, L.A.; Ross, R.P.; Hill, C. RNA Phage Biology in a Metagenomic Era. Viruses 2018, 10, 386. [Google Scholar] [CrossRef]
- Müller, T.; Vingron, M. Modeling Amino Acid Replacement. J. Comput. Biol. 2000, 7, 761–776. [Google Scholar] [CrossRef]
- Wang, D.; Fu, S.; Wu, H.; Cao, M.; Liu, L.; Zhou, X.; Wu, J. Discovery and Genomic Function of a Novel Rice Dwarf-Associated Bunya-like Virus. Viruses 2022, 14, 1183. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Shi, M.; Eden, J.-S.; Holmes, E.C.; Hesson, J.C. Meta-Transcriptomic Comparison of the RNA Viromes of the Mosquito Vectors Culex Pipiens and Culex Torrentium in Northern Europe. Viruses 2019, 11, 1033. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the Invertebrate RNA Virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Widen, S.G.; Wood, T.G.; Guzman, H.; Tesh, R.B.; Vasilakis, N. A Global Genomic Characterization of Nairoviruses Identifies Nine Discrete Genogroups with Distinctive Structural Characteristics and Host-Vector Associations. Am. J. Trop. Med. Hyg. 2016, 94, 1107–1122. [Google Scholar] [CrossRef] [PubMed]

| Sample | Number of Reads | % Mapped to BSF Genome | Sample Type |
|---|---|---|---|
| SG1 | 35,728 | 0.59% | Spent Grain |
| T3LA2 | 23,447 | 32.26% | Larvae |
| T3LA4 | 1,017,935 | 85.79% | Larvae |
| T3LC1 | 18,192 | 15.88% | Larvae |
| T3LC2 | 22,027 | 38.51% | Larvae |
| T3LC4 | 162,829 | 86.39% | Larvae |
| T3LR1 | 1,137,578 | 85.40% | Larvae |
| T3LR3 | 12,247 | 24.13% | Larvae |
| T3WA1 | 604 | 1.90% | Frass |
| T3WA2 | 7373 | 2.10% | Frass |
| T3WA4 | 7613 | 1.83% | Frass |
| T3WC1 | 17,569 | 0.73% | Frass |
| T3WC2 | 21,706 | 1.21% | Frass |
| T3WC4 | 52,404 | 0.53% | Frass |
| T3WR1 | 315,689 | 0.90% | Frass |
| T3WR3 | 47,411 | 0.47% | Frass |
| T6LA1 | 388,220 | 86.20% | Larvae |
| T6LA2 | 1,264,636 | 90.31% | Larvae |
| T6LA3 | 212,178 | 77.46% | Larvae |
| T6LA4 | 125 | 9.20% | Larvae |
| T6LC1 | 89,711 | 60.09% | Larvae |
| T6LC2 | 551 | 26.23% | Larvae |
| T6LC3 | 1,500,632 | 86.55% | Larvae |
| T6LC4 | 833,147 | 93.56% | Larvae |
| T6LR1 | 66 | 0.76% | Larvae |
| T6LR2 | 210 | 30.71% | Larvae |
| T6LR3 | 75 | 53.33% | Larvae |
| T6LR4 | 456,745 | 95.04% | Larvae |
| T6WA1 | 995,664 | 5.14% | Frass |
| T6WA2 | 956,959 | 2.84% | Frass |
| T6WA4 | 10,006 | 1.82% | Frass |
| T6WC1 | 684,749 | 2.26% | Frass |
| T6WC2 | 737,122 | 3.18% | Frass |
| T6WC3 | 93,147 | 15.04% | Frass |
| T6WC4 | 79,076 | 1.98% | Frass |
| T6WR1 | 20,437 | 1.34% | Frass |
| T6WR2 | 1,130,727 | 2.12% | Frass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walt, H.K.; Kooienga, E.; Cammack, J.A.; Tomberlin, J.K.; Jordan, H.R.; Meyer, F.; Hoffmann, F.G. Bioinformatic Surveillance Leads to Discovery of Two Novel Putative Bunyaviruses Associated with Black Soldier Fly. Viruses 2023, 15, 1654. https://doi.org/10.3390/v15081654
Walt HK, Kooienga E, Cammack JA, Tomberlin JK, Jordan HR, Meyer F, Hoffmann FG. Bioinformatic Surveillance Leads to Discovery of Two Novel Putative Bunyaviruses Associated with Black Soldier Fly. Viruses. 2023; 15(8):1654. https://doi.org/10.3390/v15081654
Chicago/Turabian StyleWalt, Hunter K., Emilia Kooienga, Jonathan A. Cammack, Jeffery K. Tomberlin, Heather R. Jordan, Florencia Meyer, and Federico G. Hoffmann. 2023. "Bioinformatic Surveillance Leads to Discovery of Two Novel Putative Bunyaviruses Associated with Black Soldier Fly" Viruses 15, no. 8: 1654. https://doi.org/10.3390/v15081654
APA StyleWalt, H. K., Kooienga, E., Cammack, J. A., Tomberlin, J. K., Jordan, H. R., Meyer, F., & Hoffmann, F. G. (2023). Bioinformatic Surveillance Leads to Discovery of Two Novel Putative Bunyaviruses Associated with Black Soldier Fly. Viruses, 15(8), 1654. https://doi.org/10.3390/v15081654







