Abstract
We report a detailed characterization of five thermophilic bacteriophages (phages) that were isolated from compost heaps in Vilnius, Lithuania using Geobacillus thermodenitrificans strains as the hosts for phage propagation. The efficiency of plating experiments revealed that phages formed plaques from 45 to 80 °C. Furthermore, most of the phages formed plaques surrounded by halo zones, indicating the presence of phage-encoded bacterial exopolysaccharide (EPS)-degrading depolymerases. Transmission Electron Microscopy (TEM) analysis revealed that all phages were siphoviruses characterized by an isometric head (from ~63 nm to ~67 nm in diameter) and a non-contractile flexible tail (from ~137 nm to ~150 nm in length). The genome sequencing resulted in genomes ranging from 38,161 to 39,016 bp. Comparative genomic and phylogenetic analysis revealed that all the isolated phages had no close relatives to date, and potentially represent three new genera within siphoviruses. The results of this study not only improve our knowledge about poorly explored thermophilic bacteriophages but also give new insights for further investigation of thermophilic and/or thermostable enzymes of bacterial viruses.
1. Introduction
Geobacillus is a genus of thermophilic, endospore-forming, chemo-organotrophic, and rod-shaped Gram-positive bacteria of the family Bacillaceae [1,2]. Members of this genus are widespread and have been isolated not only from extreme environments such as hot springs, high-temperature oil fields, hydrothermal vents, composts, and greenhouse soil, but have also been extensively found in cold places, much below their minimal growth temperatures, such as soil samples or ocean sediments [2,3]. Geobacillus spp. are broadly exploited in various biotechnological and industrial applications [4,5], and promising results about the antimicrobial potential of these bacteria have been reported [6]. In contrast, the role of the biotic factors, and especially of bacteriophages (phages) affecting Geobacillus spp., remains poorly explored.
A limited number of Geobacillus bacteriophages with completely sequenced genomes have been published to date including phages GVE2 (E2) [7], GVE3 [8], D6E [9], GBK2 [10], GBSV1 [11], BV1 [12], φOH2 [13], TP-84 [14], and GR1 (genome of this phage is deposited in the NCBI database, but has not yet been released to date, accessed on 11 July 2023) [15]. In addition, Geobacillus virus GVE1 has been characterized [16], but the complete genome of this phage has not been published to date (accessed in the NCBI database on 11 July 2023). In addition, a number of bacteriophages infecting Bacillus stearothermophilus strains (according to current classification—G. stearothermophilus) have been reported in the past century, although no complete genomes of these phages have been published to date [17]. Finally, genomes of two Geobacillus phages, which are vB_GthS_PK5.2 (OP341629.1) and vB_GthS_PK2.1 (OP341625.1), have been deposited in the NCBI database by our research group but not characterized to date.
In this study, we present biological characteristics and complete genome analysis of five Geobacillus thermodenitrificans-infecting siphoviruses: vB_GthS_PT9.1, vB_GthS_NIIg9.7, vB_GthS_PK5.1, vB_GthS_PK3.5, and vB_GthS_PK3.6, referred here by their shorter names PT9.1, NIIg9.7, PK5.1, PK3.5 and PK3.6, respectively. All phages show a high-temperature plating profile. Moreover, phages PT9.1, PK5.1, and PK3.6 demonstrate an ability to form plaques even at 80 °C. The phylogenetic analysis indicates that the isolated bacteriophages are phylogenetically the most closely interconnected to each other, but distant from already known viruses, and likely represent three new genera within the siphophages. Thus, the data presented here not only provide information on the morphology, physiology, and genetic diversity of Geobacillus-infecting viruses, but also broaden our understanding of virus–host interactions in dynamic ecosystems, such as compost heaps.
2. Materials and Methods
2.1. Phages and Bacterial Strains
Bacteriophages and bacterial strains were originally isolated from soil samples collected from compost heaps at Vilnius University Botanical Garden, Vingis Park, Vilnius, Lithuania (54.682912, 25.232532). Five Geobacillus bacteriophages are described in this study, namely vB_GthS_PT9.1, vB_GthS_NIIg9.7, vB_GthS_PK5.1, vB_GthS_PK3.5, and vB_GthS_PK3.6. The bacterial strains used in this study are listed in Table S1. Bacterial strains were isolated by using deep agar dilution series. For all of the phage experiments, the bacterial strains were cultivated in Luria–Bertani (LB) broth (Formedium). Solid plates were prepared by adding the appropriate amount of agar (Formedium) or gellan (PanReac Applichem) to the liquid medium. To identify isolated bacterial strains, PCR amplification of 16S rRNA gene fragment was performed by using universal primers woo1 5′-AGAGTTTGATCMTGGCTC-3′ and woo2 5′-GNTACCTTGTTACGACTT-3′ [18]. The BOX element was amplified using the BOXA1R primer 5′-CTACGGCAAGGCGACGCTGACG-3′ [19]. The PCR reaction for each isolate was repeated thrice for reproducibility.
2.2. Phage Isolation, Propagation, and Purification Techniques
Phage isolation was performed by using the technique of enrichment of phages in the source material. Briefly, soil samples of compost heaps (5–10 g) were filled up with 10 mL of LB and incubated for one week at 50 °C, followed by low-speed centrifugation at 2800× g for 15 min. The supernatant was then sequentially filtered through sterile 0.45 and 0.2 µm membrane filters and was assayed for plaque-forming units using the soft agar overlay method described by Adams [20], with minor modifications. Concisely, 0.1 mL of diluted phage suspension was mixed with 0.5 mL of indicator cells (OD600–0.5). The mixture then was added to 3 mL of 0.4% (w/v) soft agar and poured over the 1.2% (w/v) LB agar plate as a uniform layer. The plates were incubated for 24 h at 55 °C before the enumeration of plaques. The efficiency of plating experiments was made at 40–85 °C, and incubation of plates above 75 °C was performed by using 3 mL of 0.1% (w/v) soft gellan and poured over the 0.75% (w/v) gellan plate as a uniform layer. Bacteriophages were purified by performing five consecutive transfers from individual plaques to new bacterial cell lawns. Notably, as isolated thermophilic bacterial strains were growing poorly in the liquid broth, the propagation of bacteriophages was performed using the soft agar overlay method, as described previously by Šimoliūnas et al. [21], with minor modifications. Briefly, phage particles were subsequently collected by adding 5 mL of PB buffer (70 mM NaCl, 10 mM MgSO4, 50 mM Na2HPO4, 30 mM KH2PO4) to the surface of each plate. The top agar was scraped off and the suspension recovered. After 30 min of incubation at 4 °C with mild stirring, the mixture was centrifuged at 2800× g for 15 min at 4 °C. The phage-containing supernatant was collected and filtered through sterile 0.45 µm membrane filters. Phages were concentrated by high-speed centrifugation at 16,000× g for 1 h at 4 °C. The resulting pellets were suspended in PB buffer. To avoid bacterial DNA contamination, DNase I was added to the phage suspensions, and the samples were incubated for 1 h at 37 °C. Further phage purification was performed using a CsCl step gradient [22] as described by Šimoliūnas et al. [23].
2.3. Transmission Electron Microscopy
The CsCl density gradient-purified phage particles were diluted to approximately 1010–1011 PFU/mL with distilled water, and 10 µL of the sample was directly applied onto the carbon-coated nickel grid (Agar Scientific, Essex, UK). After 1 min, the excess liquid was drained with filter paper and stained with two successive drops of 2% uranyl acetate (pH 4.5) for 1 min. The sample was then dried and examined using a Tecnai G2 F20 X-TWIN transmission electron microscope (FEI, Hillsboro, OR, USA).
2.4. DNA Isolation
The aliquots of high-titer (1011–1012 PFU/mL) phage suspensions were subjected to phenol/chloroform extraction and ethanol precipitation, as described by Carlson and Miller [24]. The isolated phage DNA was subsequently used for PCR, or it was subjected to genome sequencing.
2.5. Genome Sequencing and Analysis
The complete genome sequences of the bacteriophages were determined using Illumina DNA sequencing technology at Macrogen (Amsterdam, The Netherlands). DNA libraries were prepared using TruSeq DNA PCR Free (350) library preparation. The 151 bp length paired-end sequence reads were generated using the NovaSeq (6000) platform.
FASTQ read sequence files were generated using bcl2fastq version 2.20 (Illumina, San Diego, CA, USA). The quality of the raw reads was evaluated using FASTQC quality control tool version 0.11.9 [25] (available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, accessed on 11 July 2023). Low-quality bases and adapters were trimmed using TrimGalore version 0.6.6 [26] (available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/, accessed on 11 July 2023) using standard parameters. Samples were downsampled using reformat.sh tool from BBMAP package version 38.96 (available online: https://sourceforge.net/projects/bbmap/files/, accessed on 11 July 2023) up to approx. 70M reads each. The quality of the reads was improved using BayesHammer [27] bundled with the SPAdes package and genomes were assembled using SPAdes packages version 3.13.1 [28]. The BBMAP package (v38.96) was used to evaluate the mapping rate and coverage. The reads of the bacteriophages were assembled into single linear contigs ranging from 38,238 (phage PK5.1) to 39,093 (phage NIIg9.7) bp with an average coverage from 537.144 (phage NIIg9.7) to 546.829 (phage PT9.1) (Table S2). The ends of the contigs were confirmed using PCR, followed by Sanger sequencing reactions at Macrogen (Amsterdam, The Netherlands). PCR fragments were obtained by the amplification of phage wild-type DNA using the primers presented in Table S2. PhageTerm [29] was used for the determination of phage termini. No known packaging mechanisms were identified in any of the genomes, and to preserve gene contiguity, the genome start points were selected from the predicted terminase small subunit gene.
The open reading frames (ORFs) were predicted with Geneious Prime version 2023.1 (available online: http://www.geneious.com/, accessed on 11 July 2023) using a minimum ORF size of 60 nt. The analysis of the genome sequences was performed using BLASTp, Fasta-Protein, Fasta-Nucleotide, Transeq (available online: http://www.ebi.ac.uk/Tools/st/emboss_transeq/, accessed on 11 July 2023), Clustal Omega (available online: http://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 11 July 2023), and DNA sequence editor available online: http://www.biocourseware.com/iphone/dnaseqeditor/index.htm/, accessed on 11 July 2023), as well as HHPred and HHblits [30,31]. The tRNAscan-SE 2.0 (available online: http://lowelab.ucsc.edu/tRNAscan-SE/, accessed on 11 July 2023) and ARAGORN (available online: http://www.ansikte.se/ARAGORN/, accessed on 11 July 2023) were used to search for tRNAs. Neighbor-joining phylogenetic tree analysis was conducted using MEGA version 5 [32]. ViPTree [33] version 3.6 was used for the total proteome comparisons (available online: https://www.genome.jp/viptree/, accessed on 15 June 2023). The overall nucleotide sequence identity was calculated using VIRIDIC (intergenomic distance calculator) [34].
2.6. Analysis of Structural Proteins
An analysis of the structural proteins of phage virions was performed using a modified filter-aided sample preparation (FASP) protocol, followed by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) analysis, as described by Šimoliūnas et al. [21].
2.7. Nucleotide Sequence Accession Numbers
The complete genome sequences of Geobacillus bacteriophages were deposited in the EMBL nucleotide sequence database under the following accession numbers: vB_GthS_PT9.1 (OP341630), vB_GthS_NIIg9.7 (OP341624), vB_GthS_PK5.1 (OP341628), vB_GthS_PK3.5 (OP341626), and vB_GthS_PK3.6 (OP341627). The accession numbers of the PCR-amplified 16S rRNA gene sequences of Bacillus-group bacteria isolated during this study are presented in Table S1.
3. Results
3.1. Identification and Characterization of the Bacterial Isolates
In total, 41 different bacterial strains were isolated during this study based on the results of morphological, physiological, and genetic analysis (Table S1). Genetic analysis was based on the comparison of PCR-amplified 16S rRNA gene fragment sequences. In addition, BOX-PCR, which can be applied for molecular typing within the Geobacillus genus [35], was performed (Figure 1). It was demonstrated that all isolated thermophilic bacteria are the members of phylogenetically closely related genera: Geobacillus (nineteen strains), Aeribacillus (nine strains), Parageobacillus (eight strains), Ureibacillus (three strains) and Brevibacillus (two strains).
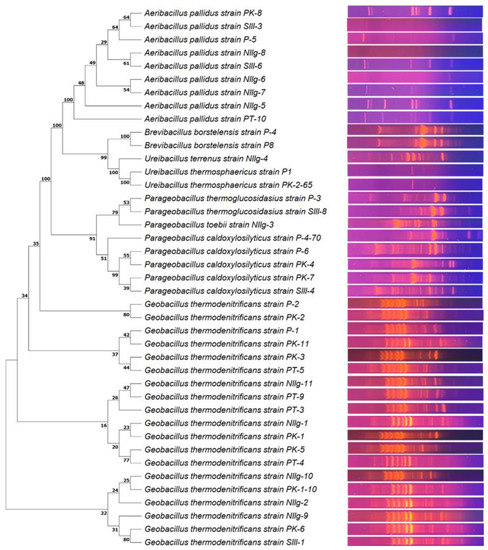
Figure 1.
Neighbor-joining phylogenetic tree analysis based on the alignment of the 16S rRNA gene fragment nucleotide sequences (left) and the BOX-PCR electrophoresis pattern (right) of isolated thermophilic bacterial strains. Neighbor-joining bootstrap values are indicated at each branch.
Isolated bacteria formed colonies of various morphology (Figure S1) and demonstrated different growth temperature ranges (Figure S2). Brevibacillus borstelensis strains P8 and P-4 demonstrated the lowest growth temperature (from 22 to 55 °C) whereas six Geobacillus thermodenitrificans strains (NIIg-1, NIIg-2, NIIg-9, NIIg-11, PK-1-10, and PT-4) propagated even at 80 °C under investigated conditions. In addition, all tested strains were able to grow on LB agar/gellan medium but demonstrate limited growth in liquid LB medium. Isolated bacterial strains were used as hosts for phage isolation and propagation experiments. Bacteriophages were recovered from the same compost samples from which the bacteria were isolated.
3.2. Host Range, Morphology, and Physiological Characteristics of the Phages
Bacteriophages PT9.1, NIIg9.7, PK5.1, PK3.5, and PK3.6 were isolated by using the technique of enrichment of phages in the source material as previously described (Section 2.2), using the local isolates PT9, NIIg9, PK5, and PK3 of Geobacillus thermodenitrificans, accordingly, as hosts. In further experiments, 46 thermophilic bacterial strains closely related by clades were used to explore the host range of the isolated Geobacillus bacteriophages (Table S3). With the exception of Geobacillus thermodenitrificans strains, the other tested Geobacillus spp., as well as all of the tested strains of Aeribacillus, Brevibacillus, Parageobacillus, Peribacillus, and Ureibacillus spp., were found to be resistant to the isolated phages. However, phages demonstrated different host ranges: NIIg9.7 and PT9.1 were active against nine, PK3.5 lysed four, whereas PK3.6 and PK5.1 infected only two of the tested strains.
Transmission electron microscopy observations of the phages PT9.1, NIIg9.7, PK5.1, PK3.5, and PK3.6 (Figure 2) revealed particles that all fit the B1 morphotype in Bradley’s classification [36,37]. Based on the morphological characteristics, all five phages were siphoviruses characterized by isometric heads and non-contractile tails (Figure 2). The phages PT9.1 and PK5.1 possessed the smallest heads (diameter of 62.72 ± 2.34 nm and 62.91 ± 3.20 nm, respectively), whereas the phage PK3.6 was the largest of the viruses examined in this study, with a head diameter of 66.93 ± 4.24 nm. Phages PK3.5 and PK3.6 contained the longest (149.98 ± 15.01 nm) and the shortest (137.32 ± 5.75 nm) tails, accordingly. Notably, no short or long tail fibers were clearly visible using TEM. The morphological features of bacteriophages described in this study are presented in Table 1.
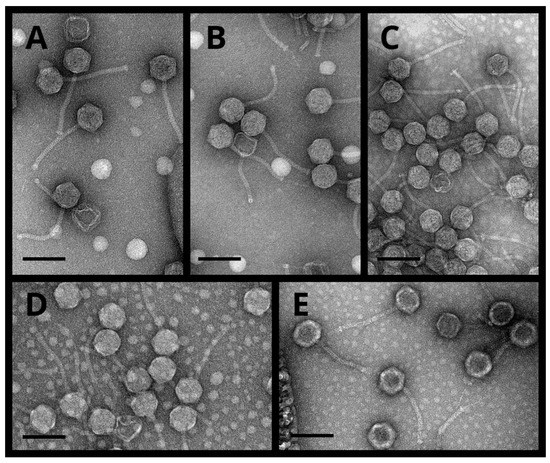
Figure 2.
Electron micrographs of bacteriophages PT9.1 (A), NIIg9.7 (B), PK5.1 (C), PK3.5 (D), and PK3.6 (E). Scale bar represents 100 nm.

Table 1.
Morphological features and plaque morphology of isolated thermophilic bacteriophages.
To determine the optimal conditions for phage propagation, the effect of temperature on the efficiency of plating (e.o.p.) was examined in the temperature range of 40–85 °C (Figure 3A). It was demonstrated that phages NIIg9.7 and PK3.5 infected their host cells from 50 to 78 °C. Moreover, PT9.1, PK5.1, and PK3.6 formed plaques even at 80 °C, and the lowest temperatures for their infectivity are 45, 48, and 50 °C, accordingly. In addition, all phages formed plaques with a clear center surrounded by an opaque halo zone (Figure 3B). Phage NIIg9.7 formed plaques with the largest halo zones; after one day of incubation at 55 °C, these were up to 11.42 ± 0.75 mm in diameter (Table 1, Figure 3B). As it was mentioned previously, all tested host strains demonstrated limited growth in liquid LB medium; thus, it was not possible to perform the adsorption test and/or single-step experiments under the investigated conditions.
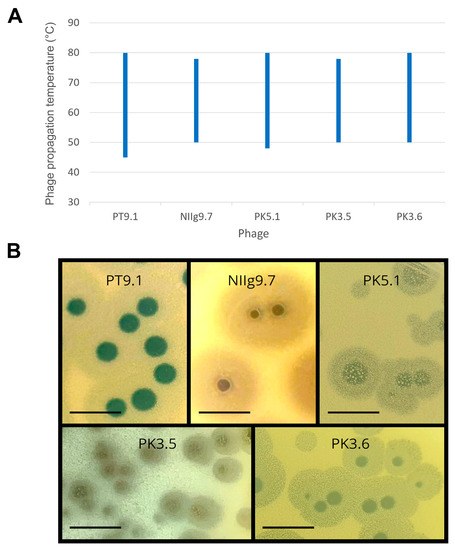
Figure 3.
Propagation temperature range (A) and morphology of plaques (B) of the thermophilic bacteriophages. Determination of phage propagation temperature range was examined by using Geobacillus thermodenitrificans strains PT-9 (phage PT9.1), NIIg-9 (phage NIIg9.7), PK-5 (phage PK5.1), and PK-3 (phages PK3.5 and PK3.6) as the hosts. The morphology of the plaque-forming units was monitored after 24 h of incubation at 55 °C by using the same hosts as for phage propagation temperature range experiments. Scale bar represents 1 cm.
3.3. Overview of Viral Genomes
The summary of general genomic features of the isolated phages is shown in Table 2. All bacteriophages contained double-stranded DNA genomes varying from 38,161 bp (phage PK5.1) to 39,016 bp (phage NIIg9.7). Phages possessed genomes with a GC content from 43.5% (phage PK3.5) to 44.8% (phage PK3.6), which were insignificantly lower than that (48.8–53.1%) observed for Geobacillus species [38]. Similar to other dsDNA bacteriophages, the genomes of thermophilic phages were close packed—92.0% (phage PK3.6) to 94.2% (phage PT9.1) of the genomes were coding. The analysis of the genome sequences revealed that a number of predicted ORFs encoding for proteins ranged from 64 (phage PK5.1) to 76 (phages NIIg9.7 and PK3.5), but no open reading frames encoding for tRNAs were identified (Table 2; Tables S4–S8). Notably, an apparent asymmetry in the distribution of the genes on the two DNA strands of phages was observed. With the exception of ORF40 encoding a ribbon–helix–helix domain-containing protein from phage NIIg9.7, all other ORFs have been predicted to be transcribed from the same DNA strands (Figure 4).

Table 2.
Genomic characteristics of isolated thermophilic bacteriophages.
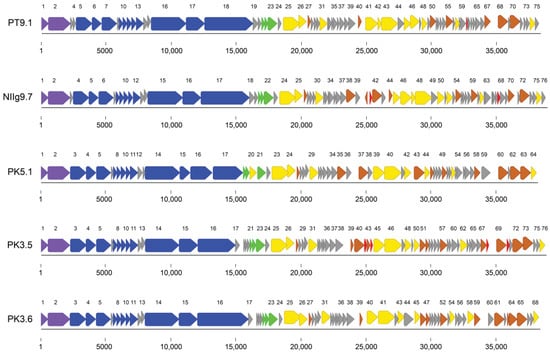
Figure 4.
Functional genome maps of Geobacillus bacteriophages. The coding capacity of the genomes is shown. Numbers indicate ORF position in genome, and functions are assigned according to the characterized ORFs in the NCBI database and HHpred analysis. The color code is as follows: yellow—DNA replication, recombination, and repair; blue—structural proteins; purple—DNA packaging; brown—transcription, translation, nucleotide metabolism; green—lysis, phage–host interaction; grey—conserved hypothetical proteins; red—hypothetical proteins with no reliable identity when compared to database entries.
Based on homology to biologically defined proteins, the percentage of ORFs after a putative functional annotation ranges from 45% (34 out of 75 PT9.1 ORFs) to 58% (37 out of 64 PK5.1 ORFs). As was observed in other siphoviruses, the genomes of Geobacillus phages appeared to have a modular organization, with genes for DNA packaging, structure/morphogenesis, host lysis, replication/regulation, transcription/translation, and nucleotide metabolism clustered together (Figure 4). Notably, none of the predicted gene products showed sequence homology with antibiotic resistance determinants or integration-related proteins.
3.4. Structural Proteins and Proteomic Analysis
A bioinformatics analysis of the genome sequences of isolated bacteriophages allowed for the identification of a number of genes coding for proteins involved in virion structure and assembly (Figure 4; Tables S4–S8). Eleven structural genes, including those coding for the head (portal protein, Clp protease, major capsid protein, head-tail connector, and head closure protein), tail (two putative tail components, major tail protein, tape measure protein, distal tail protein), and tail fiber (tail fiber protein), were identified in the genomes of phages PT9.1, NIIg9.7, PK3.5, and PK3.6. Phage PK5.1 contained twelve structural proteins, including those eleven mentioned above and a structural protein encoded by ORF16, which shares the highest identity (27% amino acid sequence identity; E-value of 3 × 10–17) to the putative tail fiber protein (Sequence ID: VEV89172.1) from Staphylococcus phage Stab22. The PT9.1 gp18, NIIg9.7 gp17, PK5.1 gp17, PK3.5 gp16, and PK3.6 gp16 were identified as tail fiber proteins. Based on the results of BLASTp analysis, the identity at the aa level of PT9.1 gp18 vs. NIIg9.7 gp17 was 97.57% (query cov. 100%; E-value, 0.0), and PK3.5 gp16, vs. PK3.6 gp16 was 85.31% (query cov. 100%; E-value, 0.0). In contrast, PT9.1 gp18 shared only 33.88% (query cov. 48%; E-value, 1 × 10−61) and 34.50% (query cov. 48%; E-value, 4 × 10−61) aa identity with PK3.5 gp16 and PK3.6 gp16, respectively. Similarly, NIIg9.7 gp17 was only 33.95% (query cov. 48%; E-value, 1 × 10−61) and 34.57% (query cov. 48%; E-value, 1 × 10−61) identical to PK3.5 gp16 and PK3.6 gp16, respectively. PK5.1 gp17 shared 79.17% (query cov. 15%; E-value, 3 × 10−51) and 78.33% (query cov. 15%; E-value, 4 × 10−50) aa identity with PT9.1 gp18 and NIIg9.7 gp17, respectively. Meanwhile, no significant similarity of PK5.1 gp17 to PK3.5 gp16 and PK3.6 gp16 was found.
FASP followed by LC-MS/MS confirmed that a number of the aforementioned structural proteins were present in the virions of thermophilic bacteriophages (Table S9). Three structural proteins were identified in the virions of phages PT9.1 (major capsid protein, tape measure protein, and distal tail protein) and PK3.6 (major capsid protein, tape measure protein, and putative tail fiber protein). Four structural proteins were detected in the virions of phages NIIg9.7 (major capsid protein, tape measure protein, distal tail protein, and tail fiber protein) and PK5.1 (Clp protease, major capsid protein, tape measure protein, and structural protein). Finally, five structural proteins, including major capsid protein (gp05), putative tail component (gp09), tape measure protein (gp14), distal tail protein (gp15), and putative tail fiber protein (gp16), were identified in the virions of PK3.5. Indetermination of potential structural proteins, which were identified by bioinformatics approaches but not detected by proteomics analysis, might be due to the incompatibility of these proteins with sample preparation procedures and/or because of their low abundance in virions.
3.5. Packaging
The packaging machine of tailed bacteriophages usually consists of two essential components: a terminase complex and a portal ring [39]. Most characterized terminases consist of a small subunit (TerS) involved in DNA recognition and a large terminase subunit (TerL) containing the ATPase and the endonuclease activities [40]. The genes associated with DNA packaging of isolated thermophilic bacteriophages include all three aforementioned proteins: the TerS and TerL were encoded by ORF01 and ORF02, respectively, and the portal protein is encoded by ORF03 (phages PK5.1, PK3.5, and PK3.6), ORF04 (phage NIIg9.7), and ORF05 (phage PT9.1). All phages possessed TerS containing conserved Terminase_4 (pfam05119) domain and TerL containing conserved Terminase_1 (pfam03354) domain. Also, all phages had a portal protein with conserved Phage_portal (pfam04860) domain.
3.6. DNA Replication, Recombination, and Repair
The bioinformatics analysis revealed that the genes associated with DNA replication, recombination, and repair (DNA RRR) of isolated bacteriophages included those coding for an FtsK/SpoIIIE family protein, a replication/relaxation protein (dnaD), an ERF family protein, a replicative DNA helicase, a single-stranded DNA binding (SSB) protein, and a Holliday junction resolvase. In addition, phages PT9.1 and NIIg9.7 possessed a loader and inhibitor of replicative helicase encoded by ORF42 and ORF47, respectively. Moreover, with the exception of NIIg9.7 and PK3.5, each phage encoded three HNH endonucleases–site-specific DNA endonucleases that promote the lateral transfer of their own coding region and flanking DNA between genomes by a recombination-dependent process termed homing [41]. Phage PK3.5 contained two HNH endonucleases, and phage NIIg9.7 possessed two HNH endonucleases and a putative endonuclease (gp45) containing MTES_1575 (pfam18741) conserved domain. However, the genomes of thermophilic bacteriophages contained no homologs to characterize DNA polymerase genes, suggesting that these phages most likely use DNA polymerase of the host cell.
3.7. Transcription, Translation, Nucleotide Metabolism, and DNA Modification
Based on the amino acid sequence similarity, a set of genes encoding products potentially involved in transcription, translation, nucleotide metabolism, and DNA modification were present in the genomes of thermophilic bacteriophages. The phages contained transcriptional regulators belonging to families XRE, ArpU, Rha, and RinA (Tables S4–S8) as well as putative transcriptional regulators encoded by ORF45, ORF53, and ORF47 of phages PK5.1, PK3.5 and PK3.6, respectively. In addition, one antirepressor protein encoded by ORF40 of phage PK3.5 was detected. All phages contain metallophosphoesterase with a conserved COG1407 superfamily domain. With the exception of PK5.1, all phages also encoded dUTP diphosphatase containing dUTPase_2 (pfam08761) conserved domain. On the other hand, phage PK5.1 was the only one containing phosphoadenosine phosphosulfate reductase and nucleoside triphosphate pyrophosphohydrolase encoded by ORF43 and ORF49, respectively. Moreover, thymidylate synthase was encoded by ORF38 and ORF35 of phages NIIg9.7 and PK5.1, respectively. With the exception of PK5.1, all phages possessed DNA N-6-adenine-methyltransferase containing Dam (pfam05869) conserved domain. In addition, DNA methyltransferase containing the N6_N4_Mtase (pfam01555) conserved domain was encoded twice in the genomes of phages PK3.5, PK3.6, and PK5.1, and once in phage PT9.1, but it was not detected in the genome of phage NIIg9.7.
3.8. Lysis Cassette
All dsDNA phages accomplish host lysis using a muralytic enzyme (known as an endolysin) and a holin, a small membrane protein that permeabilizes the membrane at a programmed time [42]. The lysis cassette of all isolated thermophilic bacteriophages consisted of a hemolysin containing XhlA (pfam10779) conserved domain, a holin, and an endolysin (N-acetylmuramoyl-L-alanine amidase) containing N-terminal MurNAc-LAA (cd02696) and C-terminal SPOR (pfam05036) conserved domains. With the exception of PK5.1, which encoded an HNH endonuclease (gp20) inserted between holin (gp19) and endolysin (gp21), all three lysis proteins were encoded in canonical order (Figure 4). Hemolysins with the XhlA family motif are cell-surface-associated proteins that lyse insect granulocytes and plasmatocytes, as well as rabbit and horse erythrocytes [43]. However, it was demonstrated that proteins similar to XhlA, which were encoded by the Bacillus phage SPP1 and prophage PBSX, functioned as holins [44]. The presence of holin and XhlA domain-containing proteins had also been described in the case of other Bacillus-group bacteria infecting phages [7,45,46]. It is likely that activation of more than one holin may facilitate the lysis of cells grown in different conditions or coming from different phage hosts [44].
3.9. Phylogenetic Analysis of the Phages
In order to determine the phylogenetic relationship among isolated thermophilic bacteriophages and their closest relatives to date, a comparison of the individual genes most often used for the analysis of the evolutionary relationships among bacteriophages [47] was carried out. The phylogenetic trees based on the alignment of the PT9.1, NIIg9.7, PK3.5, PK3.6, and PK5.1 major capsid protein, terminase large subunit, tape measure protein, replicative helicase, and amino acid sequences with those returned by BLASTP homology searches were constructed (Figure 5). It was demonstrated that, in most cases, isolated Geobacillus bacteriophages were phylogenetically the most closely interconnected to each other but distant from other phages and occupied a somewhat intermediate position among unclassified siphoviruses within the class Caudoviricetes.
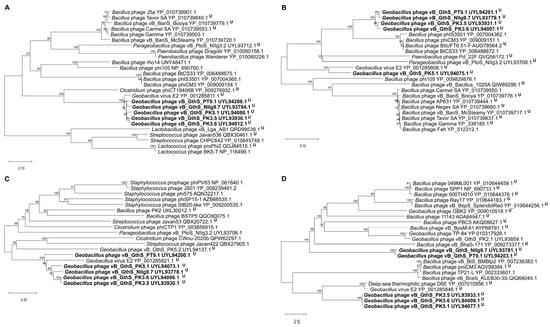
Figure 5.
Neighbor-joining tree analysis based on the alignment of the amino acid sequences of isolated thermophilic bacteriophages: (A) major capsid protein, (B) terminase large subunit, (C) tape measure protein (TMP), and (D) helicase. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches. U—unclassified bacteriophages within the class Caudoviricetes.
To obtain a more detailed picture of the phylogenetic relationships of PT9.1, NIIg9.7, PK3.5, PK3.6, PK5.1, and their closest relatives, a comparative total proteome comparison was performed using the ViPTree web service. Based on the whole-proteome alignment of isolated Geobacillus phages and their closest relatives, it was demonstrated that phage PT9.1 is the most closely related to NIIg9.7, whereas the closest relative of PK3.5 was PK3.6. Phage PK5.1 was in between PT9.1-NIIg9.7 and PK3.5-PK3.6 (Figure 6). Bacteriophages of this study were the most closely related to unclassified Geobacillus virus GVE2 (NC_009552). However, the phylogenetic relationship between phages characterized in this study and virus GVE2 was distant, suggesting that PT9.1, NIIg9.7, PK3.5, PK3.6, and PK5.1 formed a not yet identified cluster of phages within the siphoviruses.
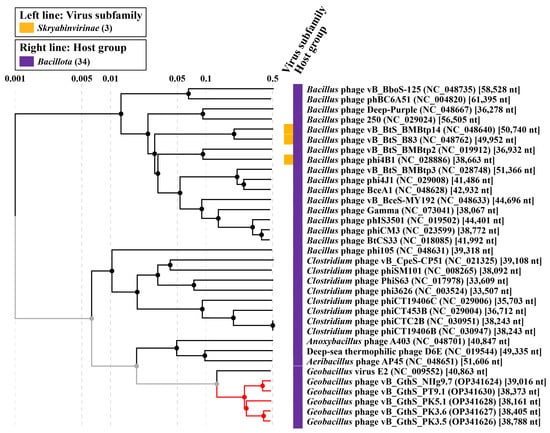
Figure 6.
ViPTree-generated proteomic tree of isolated thermophilic bacteriophages and dsDNA viruses represented in the rectangular view. The tree is constructed by BIONJ based on genomic distance matrixes, and mid-point rooted. Branch lengths are logarithmically scaled from the root of the entire proteomic tree. The numbers at the top represent the log-scaled branch lengths based on the SG (normalized tBLASTx scores) values.
To determine the most homologous regions in the genomes of isolated thermophilic phages, genome alignment was performed by using ViPTree. Genomes of all bacteriophages shared several regions of nucleotide similarity that covered the essential structural and virion morphogenesis protein-encoding genes, as well as genes related to lysis and DNA metabolism and modification (Figure 7).
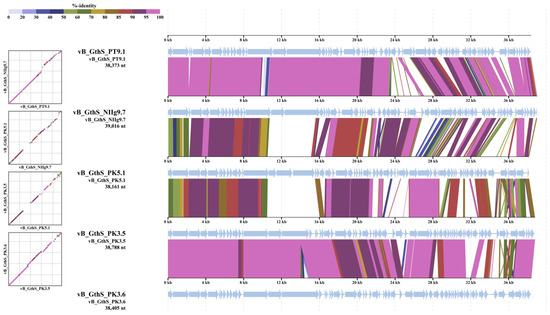
Figure 7.
ViPTree-generated whole-proteome alignment of isolated thermophilic bacteriophages. Colored lines in the alignment indicate tBLASTx results (E-value < 0.01). Positions of each sequence are automatically adjusted (i.e., circularly permuted and reverse-stranded) for clear representation of collinearity among genomes.
Nevertheless, the nucleotide-based virus overall nucleotide sequence identity in-between isolated Geobacillus phages and their closest relatives was calculated using VIRIDIC (Figure 8). It was demonstrated that the highest identity (84.2%) was between phages PK3.5 and PK3.6. Similarly, the identity of PT9.1 vs. NIIg9.7 was 82.5%, whereas phage PK5.1 demonstrated 57.9%, 56.8%, 54.4%, and 48.5% identity with PK3.5, PK3.6, NIIg9.7, and PT9.1, accordingly. In addition, of all the phages studied in this work, PK5.1 shared the highest overall nucleotide identity with other viruses sequenced to date, which is 31.0% identity with Geobacillus virus GVE2. Thus, it seemed that the bacteriophages of this study were phylogenetically the most closely related to each other and potentially represent a new cluster of thermophilic siphoviruses.
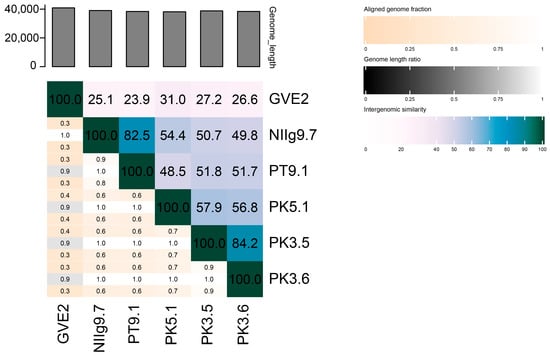
Figure 8.
The whole-genome comparison and clustering of phages PT9.1, NIIg9.7, PK5.1, PK3.5, PK3.6, and their closest relative—Geobacillus virus GVE2. The comparison and clustering were performed with the use of VIRIDIC. Different shades of blue in the right half of the heatmap represent different intergenomic similarities (%) between the genomes of each pair compared, as indicated above the heatmap and specified by numbers. The left half of the heatmap shows three indicator values for each genome pair: aligned fraction of genome one for the genome in this row (top value), genome length ratio for the two genomes in this pair (middle value), and aligned fraction of genome two for the genome in this column (bottom value).
According to the Bacterial and Archaeal Viruses Subcommittee (BAVS) of the International Committee on Taxonomy of Viruses (ICTV), two phages are assigned to the same species if their genomes are more than 95% identical, while a genus is described as a cohesive group of viruses sharing a high degree (>70%) of nucleotide identity of the full genome length [48]. Following this and based on the results of the comparative genome sequence analysis performed during this study, we considered that all bacteriophages of this study represent new species. Moreover, PK3.5 together with PK3.6, as well as PT9.1 together with NIIg9.7, and a singleton PK5.1 could not be classified to any genus currently recognized by ICTV, and likely represent three new genera within the siphoviruses.
4. Discussion
Geobacillus spp. are widely distributed throughout environments, and the high potential of these bacteria and/or their enzymes in various biotechnological, industrial, or medical applications had been reported [4,5,6]. On the other hand, G. stearothermophilus can cause flat sour spoilage in low-acid canned foods [49]. In addition, G. stearothermophilus, G. thermoleovorans, and G. thermodenitrificans often form biofilms on different abiotic surfaces, which cause significant financial losses in the food industry [50,51,52]. Thus, a number of publications on lytic enzymes from Geobacillus phages, which demonstrated antimicrobial activity against their hosts and other detrimental bacterial strains, have been reported. The vast majority of these reports characterized viral endolysins [15,53,54,55,56,57].
Genomic analysis of Geobacillus bacteriophages PT9.1, NIIg9.7, PK5.1, PK3.5, and PK3.6 revealed that the lysis cassette of all these phages consisted of three lytic enzymes: two potential holins and one endolysin. Endolysins of bacteriophages of this study were N-acetylmuramoyl-L-alanine amidases containing N-terminal MurNAc-LAA and C-terminal SPOR superfamily conserved domains, and one of their closest BLASTp homologs was a well-characterized endolysin (YP_001285830.1) from Geobacillus virus GVE2. It was demonstrated that the GVE2 recombinant endolysin was thermostable, exhibited good tolerances at acid pH values, and played important roles in the lysis process of the host cells [53,54]. Bioinformatics analysis revealed that the identity in the amino acid level of endolysins from phages of this study to GVE2 endolysin ranged from 59.15 to 60.41% (phages PK3.6 and PK5.1, accordingly). Thus, the endolysins from phages of this study could also be attractive tools in applications in molecular biology as well as industries, although more detailed studies would be needed to confirm this.
In contrast to endolysins, to our knowledge, only one depolymerase from Geobacillus phages, which is a thermostable capsule depolymerase from phage TP-84 [58], has been characterized in detail to date. Phage-encoded depolymerases are collectively referred to as enzymes able to degrade polymeric substances found in the bacterial cell surface, such as polysaccharides, or present in bacterial biofilms [59]. The depolymerase activity is commonly identified by a constantly increasing halo zone surrounding the phage plaques [60,61]. The vast majority of phage depolymerases are encoded in the same open reading frame of phage structural proteins (mostly on tail spikes, tail fibers, and base plates) or in close proximity to those genes, and thus are considered structural proteins [59,62].
The morphology of plaques (clear central part surrounded by a turbid halo zone) formed by Geobacillus phages PT9.1, NIIg9.7, PK5.1, PK3.5, and PK3.6 suggested that all viruses of this study possessed depolymerase activity. Bioinformatics analysis revealed that potential candidates of this activity were tail fiber proteins encoded by PT9.1 ORF18, NIIg9.7 ORF17, PK5.1 ORF17, PK3.5 ORF16, and PK3.6 ORF16. BLASTp analysis demonstrated that all aforementioned proteins contain N-terminal Prophage_tail (pfam06605) superfamily domain presented in phage tail proteins that are probably acting as endopeptidases, whereas diverse conserved domains were encoded in the C-terminus of the aforementioned proteins (Figure S3). Potential depolymerases of phages PT9.1, NIIg9.7, and PK5.1 possessed C-terminal Peptidase_S74 (pfam13884) domain, which is specific for depolymerases acting as sialidases [59]. In contrast, gp16 of phage PK3.5 and gp16 of phage PK3.6 encoded central tolA_full (TIGR02794) and C-terminal choice_anch_G (NF033766) and (PHA02515), respectively, conserved domains, which had never been characterized as domains specific to depolymerases. However, HHpred analysis demonstrated that gp16 of PK3.5 and gp16 of PK3.6 had the best hits to tail-associated lysin (6V8I_CE) of Staphylococcus phage 80alpha (probability 99.98% and E-value 7.6 × 10−21; probability 99.88% and E-value 1.5 × 10−20, respectively). Thus, it is likely that all five aforementioned tail fiber proteins of the isolated Geobacillus phages could have potential depolymerase activity, although experimental validation would be needed to confirm this hypothesis.
The results of the host range determination experiments confirmed that isolated thermophilic bacteriophages, especially PK3.6 and PK5.1, were very specific for their hosts (considering that 41 out of the 46 tested bacterial strains were isolated from the same environment and at the same time as the phages, and that all tested strains belonged to the Bacillus-group bacteria, including 20 strains of G. thermodenitrificans). While many well-studied model phages seemed to exhibit a narrow host range, recent ecological and metagenomics studies indicated that phages may have specificities that ranged from narrow to broad [63]. However, it is still an open question what factors determine this specificity, especially in the case of thermophilic bacteriophages. It is known that viral host range could be determined by a number of molecular mechanisms [63,64], and it is likely that the initial stage of phage infection could be one of the most important.
The PT9.1 gp18, NIIg9.7 gp17, PK5.1 gp17, PK3.5 gp16, and PK3.6 gp16 were tail fiber proteins, which, in our prediction, could play a significant role in the initial steps of host recognition and adsorption. Summarizing the results of the bioinformatics analysis, PT9.1 gp18 shared a high identity with NIIg9.7 gp17, as well as PK3.5 gp16 with PK3.6 gp16; meanwhile, PK5.1 gp17 had no close homology at the amino acid level to the aforementioned proteins. In comparison, the host range determination experiments revealed that phages PT9.1 and NIIg9.7 were active against nine G. thermodenitrificans strains (including seven strains that are the same for both phages), whereas PK5.1 and PK3.6 infected only two G. thermodenitrificans strains (including one strain that is the same for both phages). On the other hand, despite the high similarity of PK3.5 gp16 and PK3.6 gp16, phage PK3.5 infected four G. thermodenitrificans strains. Thus, it was likely that different profiles of phage hosts may be determined by small differences in the amino acid sequences of tail fiber proteins and/or by other viral proteins potentially included in phage–host interaction or even later stages of the phage replication cycle. Further studies, however, would be needed to gain a more comprehensive understanding of the interactions between thermophilic Geobacillus phages and their hosts.
5. Conclusions
In conclusion, we showed that Geobacillus-infecting bacteriophages PT9.1, NIIg9.7, PK5.1, PK3.5, and PK3.6 are thermophilic siphoviruses possessing potential depolymerase activity and diverse host profiles. In addition, our results indicate that the isolated phages are substantially distinct from all of the previously described phages and may be considered representatives of three novel genera within the siphoviruses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15081691/s1, Figure S1: the morphology of colonies of isolated bacterial strains after 24 h of incubation on LB agar medium at 55 °C; Figure S2: bacterial growth temperature ranges; Figure S3: comparison of conserved domains in potential depolymerase genes of thermophilic bacteriophages PT9.1, NIIg9.7, PK5.1, PK3.5, and PK3.6; Table S1: bacterial strains used in this study to determine the host range of the bacteriophages; Table S2: the host range of the Geobacillus bacteriophages; Table S3: summarized information of assembled viral contigs and nucleotide sequences of primers used to confirm the completeness of the assembled contigs.; Table S4: PT-9.1 ORFs with homologs in other viruses or cellular organisms; Table S5: NIIg9.7 ORFs with homologs in other viruses or cellular organisms; Table S6: PK5.1 ORFs with homologs in other viruses or cellular organisms; Table S7: PK3.5 ORFs with homologs in other viruses or cellular organisms; Table S8: PK3.6 ORFs with homologs in other viruses or cellular organisms; Table S9: viral structural proteins identified by Mass Spectrometry.
Author Contributions
E.Š. and M.Š. conceived and designed the experiments; E.Š., M.Š., G.L., M.S. and A.K. performed the experiments; E.Š., M.Š., G.L., A.K. and K.K. analyzed the data; R.M., M.V. and N.K. contributed reagents/materials/analysis tools; E.Š. wrote the paper; R.M. and N.K. reviewed and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [European Social Fund] (project No. 09.3.3-LMT-K-712-19-0102) under a grant agreement with the Research Council of Lithuania (LMTLT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The complete genome sequences of Geobacillus bacteriophages of this study are available in the EMBL nucleotide sequence database under accession numbers OP341630 (phage vB_GthS_PT9.1), OP341624 (phage vB_GthS_NIIg9.7), OP341628 (phage vB_GthS_PK5.1), OP341626 (phage vB_GthS_PK3.5), and OP341627 (phage vB_GthS_PK3.6). The sequences of the PCR-amplified 16S rRNA gene fragments of Bacillus-group bacteria isolated during this study are available in the EMBL nucleotide sequence database under accession numbers presented in Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McMullan, G.; Christie, J.M.; Rahman, T.J.; Banat, I.M.; Ternan, N.G.; Marchant, R. Habitat, applications and genomics of the aerobic, thermophilic genus Geobacillus. Biochem. Soc. Trans. 2004, 32, 214–217. [Google Scholar] [CrossRef]
- Najar, I.N.; Thakur, N. A systematic review of the genera Geobacillus and Parageobacillus: Their evolution, current taxonomic status and major applications. Microbiology 2020, 166, 800–816. [Google Scholar] [CrossRef]
- Zeigler, D.R. The Geobacillus paradox: Why is a thermophilic bacterial genus so prevalent on a mesophilic planet? Microbiology 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Hussein, A.H.; Lisowska, B.K.; Leak, D.J. The genus Geobacillus and their biotechnological potential. Adv. Appl. Microbiol. 2015, 92, 1–48. [Google Scholar]
- Wada, K.; Suzuki, H. Chapter 15 Biotechnological platforms of the moderate thermophiles, Geobacillus species: Notable properties and genetic tools. In Physiological and Biotechnological Aspects of Extremophiles; Salwan, R., Sharma, V., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 195–218. [Google Scholar]
- Zebrowska, J.; Witkowska, M.; Struck, A.; Laszuk, P.E.; Raczuk, E.; Ponikowska, M.; Skowron, P.M.; Zylicz-Stachula, A. Antimicrobial potential of the genera Geobacillus and Parageobacillus, as well as endolysins biosynthesized by their bacteriophages. Antibiotics 2022, 11, 242. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X. Deep-sea thermophilic Geobacillus bacteriophage GVE2 transcriptional profile and proteomic characterization of virions. Appl. Microbiol. Biotechnol. 2008, 80, 697–707. [Google Scholar] [CrossRef]
- van Zyl, L.J.; Sunda, F.; Taylor, M.P.; Cowan, D.; Trindade, M.I. Identification and characterization of a novel Geobacillus thermoglucosidasius bacteriophage, GVE3. Arch. Virol. 2015, 160, 2269–2282. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X. Genome analysis of deep-sea thermophilic bacteriophage D6E. Appl. Environ. Microbiol. 2010, 76, 7861–7866. [Google Scholar] [CrossRef]
- Marks, T.J.; Hamilton, P.T. Characterization of a thermophilic bacteriophage of Geobacillus kaustophilus. Arch. Virol. 2014, 159, 2771–2775. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, F.; Wu, S.; Xu, Y.; Zhang, X. Genomic and proteomic characterization of a thermophilic Geobacillus bacteriophage GBSV1. Res. Microbiol. 2009, 16, 166–171. [Google Scholar] [CrossRef]
- Liu, B.; Wu, S.; Xie, L. Complete genome sequence and proteomic analysis of a thermophilic bacteriophage BV1. Acta Oceanol. Sin. 2010, 29, 84–89. [Google Scholar] [CrossRef]
- Doi, K.; Mori, K.; Martono, H.; Nagayoshi, Y.; Fujino, Y.; Tashiro, K.; Kuhara, S.; Ohshima, T. Draft genome sequence of Geobacillus kaustophilus GBlys, a lysogenic strain with bacteriophage φOH2. Genome Announc. 2013, 1, e00634-13. [Google Scholar] [CrossRef]
- Skowron, P.M.; Kropinski, A.M.; Zebrowska, J.; Janus, L.; Szemiako, K.; Czajkowska, E.; Maciejewska, N.; Skowron, M.; Łoś, J.; Łoś, M.; et al. Sequence, genome organization, annotation and proteomics of the thermophilic, 47.7-kb Geobacillus stea-rothermophilus bacteriophage TP-84 and its classification in the new Tp84virus genus. PLoS ONE 2018, 13, e0195449. [Google Scholar]
- Choi, D.; Kong, M. LysGR1, a novel thermostable endolysin from Geobacillus stearothermophilus bacteriophage GR1. Front. Microbiol. 2023, 14, 1178748. [Google Scholar] [CrossRef]
- Liu, B.; Wu, S.; Song, Q.; Zhang, X.; Xie, L. Two novel bacteriophages of thermophilic bacteria isolated from deep-sea hydrothermal fields. Curr. Microbiol. 2006, 53, 163–166. [Google Scholar] [CrossRef]
- Łubkowska, B.; Jeżewska-Frąckowiak, J.; Sobolewski, I.; Skowron, P.M. Bacteriophages of thermophilic ‘Bacillus group’ bacteria—A review. Microorganisms 2021, 9, 1522. [Google Scholar] [CrossRef]
- Godon, J.J.; Zumstein, E.; Dabert, P.; Habouzit, F.; Moletta, R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 1997, 63, 2802–2813. [Google Scholar] [CrossRef]
- Versalovic, J.; Schneider, M.; de Bruijn, F.J.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence based PCR (rep-PCR). Methods Cell. Mol. Biol. 1994, 5, 25–40. [Google Scholar]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Šimoliūnas, E.; Kaliniene, L.; Stasilo, M.; Truncaitė, L.; Zajančkauskaitė, A.; Staniulis, J.; Nainys, J.; Kaupinis, A.; Valius, M.; Meškys, R. Isolation and characterization of vB_ArS-ArV2—First Arthrobacter sp. infecting bacteriophage with completely sequenced genome. PLoS ONE 2014, 9, e111230. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Šimoliūnas, E.; Kaliniene, L.; Truncaitė, L.; Zajančkauskaitė, A.; Staniulis, J.; Kaupinis, J.; Ger, M.; Valius, M.; Meškys, R. Klebsiella phage vB_KleM-RaK2—A giant singleton virus of the family Myoviridae. PLoS ONE 2013, 8, e60717. [Google Scholar] [CrossRef]
- Carlson, K.; Miller, E. Experiments in T4 genetics. In Bacteriophage T4; Karam, J.D., Ed.; ASM Press: Washington, DC, USA, 1994; pp. 419–483. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 11 July 2023).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Nikolenko, S.I.; Korobeynikov, A.I.; Alekseyev, M.A. BayesHammer: Bayesian clustering for error correction in single-cell sequencing. BMC Genom. 2013, 14, S7. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes de novo assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Alva, V.; Nam, S.Z.; Söding, J.; Lupas, A.N. The MPI bioinformatics toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res. 2016, 44, W410–W415. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.Z.; Rau, D.; Kubler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A completely reimplemented mpi bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. Viptree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-a novel tool to calculate the intergenomic similarities of prokaryote-infecting viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Meintanis, C.; Chalkou, K.I.; Kormas, K.A.; Lymperopoulou, D.S.; Katsifas, E.A.; Hatzinikolaou, D.G.; Karagouni, A.D. Application of rpoB sequence similarity analysis, REP-PCR and BOX-PCR for the differentiation of species within the genus Geobacillus. Lett. Appl. Microbiol. 2008, 46, 395–401. [Google Scholar] [CrossRef]
- Bradley, D.E. Ultrastructure of bacteriophages and bacteriocins. Bacteriol. Rev. 1967, 31, 230–314. [Google Scholar] [CrossRef]
- Ackermann, H.W.; Eisenstark, A. The present state of phage taxonomy. Intervirology 1974, 3, 201–219. [Google Scholar] [CrossRef]
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Phylogenomic re-assessment of the thermophilic genus Geobacillus. Syst. Appl. Microbiol. 2016, 39, 527–533. [Google Scholar] [CrossRef]
- Rao, V.B.; Feiss, M. The bacteriophage DNA packaging motor. Annu. Rev. Genet. 2008, 42, 647–681. [Google Scholar] [CrossRef]
- Rao, V.B.; Feiss, M. Mechanisms of DNA packaging by large double stranded DNA viruses. Annu. Rev. Virol. 2015, 2, 351–378. [Google Scholar] [CrossRef]
- Edgell, D.R.; Gibb, E.A.; Belfort, M. Mobile DNA elements in T4 and related phages. Virol. J. 2010, 7, 290. [Google Scholar] [CrossRef]
- Young, I.; Wang, I.; Roof, W.D. Phages will out: Strategies of host cell lysis. Trends Microbiol. 2000, 8, 120–128. [Google Scholar] [CrossRef]
- Cowles, K.N.; Goodrich-Blair, H. Expression and activity of a Xenorhabdus nematophila haemolysin required for full virulence towards Manduca sexta insects. Cell. Microbiol. 2005, 7, 209–219. [Google Scholar] [CrossRef]
- Fernandes, S.; São-José, C. Probing the function of the two holin-like proteins of bacteriophage SPP1. Virology 2017, 500, 184–189. [Google Scholar] [CrossRef]
- Halgasova, N.; Ugorcakova, J.; Gerova, M.; Timko, J.; Bukovska, G. Isolation and characterization of bacteriophage PhiBP from Paenibacillus polymyxa CCM 7400. FEMS Microbiol. Lett. 2010, 305, 128–135. [Google Scholar] [CrossRef]
- Nakonieczna, A.; Rutyna, P.; Fedorowicz, M.; Kwiatek, M.; Mizak, L.; Łobocka, M. Three novel bacteriophages, J5a, F16Ba, and z1a, specific for Bacillus anthracis, define a new clade of historical Wbeta phage relatives. Viruses 2022, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Cowan, D.A. Using signature genes as tools to assess environmental viral ecology and diversity. Appl. Environ. Microbiol. 2014, 80, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A roadmap for genome-based phage taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Wells-Bennik, M.H.J.; Janssen, P.W.M.; Klaus, V.; Yang, C.; Zwietering, M.H.; den Besten, H.M.W. Heat resistance of spores of 18 strains of Geobacillus stearothermophilus and impact of culturing conditions. Int. J. Food Microbiol. 2019, 291, 161–172. [Google Scholar] [CrossRef]
- Andre, S.; Zuber, F.; Remize, F. Thermophilic spore-forming bacteria isolated from spoiled canned food and their heat resistance. Results of a French ten-year survey. Int. J. Food Microbiol. 2013, 165, 134–143. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; Yuan, L.; Li, Y.; Liu, T.J.; He, G.Q. Propensity for biofilm formation by aerobic mesophilic and thermophilic spore forming bacteria isolated from chinese milk powders. Int. J. Food Microbiol. 2017, 262, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Karaca, B.; Buzrul, S.; Cihan, A.C. Anoxybacillus and Geobacillus biofilms in the dairy industry: Effects of surface material, incubation temperature and milk type. Biofouling 2019, 35, 551–560. [Google Scholar] [CrossRef]
- Ye, T.; Zhang, X. Characterization of a lysin from deep-sea thermophilic bacteriophage GVE2. Appl. Microbiol. Biotechnol. 2008, 78, 635–641. [Google Scholar] [CrossRef]
- Jin, M.; Ye, T.; Zhang, X. Roles of bacteriophage GVE2 endolysin in host lysis at high temperatures. Microbiology 2013, 159, 1597–1605. [Google Scholar] [CrossRef]
- Swift, S.M.; Reid, K.P.; Donovan, D.M.; Ramsay, T.G. Thermophile lytic enzyme fusion proteins that target Clostridium perfringens. Antibiotics 2019, 8, 214. [Google Scholar] [CrossRef]
- Miller, E.; Warek, U.; Xu, D. Endolysin from Bacteriophage against Geobacillus and Methods of Using. International Patent Application WIPO/PCT WO 2016/123425 Al, 4 August 2016. [Google Scholar]
- Żebrowska, J.; Żołnierkiewicz, O.; Ponikowska, M.; Puchalski, M.; Krawczun, N.; Makowska, J.; Skowron, P. Cloning and characterization of a thermostable endolysin of bacteriophage TP-84 as a potential disinfectant and biofilm-removing biological agent. Int. J. Mol. Sci. 2022, 23, 7612. [Google Scholar] [CrossRef] [PubMed]
- Łubkowska, B.; Czajkowska, E.; Stodolna, A.; Sroczyński, M.; Zylicz-Stachula, A.; Ireneusz, S.; Skowron, M.P. A novel thermostable TP-84 capsule depolymerase: A method for rapid polyethyleneimine processing of a bacteriophage-expressed proteins. Microb. Cell Factories 2023, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Oliveira, H.; Melo, L.D.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.; Ceyssens, P.J.; Krylov, V.N.; Noben, J.P.; Volckaert, G.; Lavigne, R. Identification of EPS-degrading activity within the tail spikes of the novel Pseudomonas putida phage AF. Virology 2012, 434, 251–256. [Google Scholar] [CrossRef]
- Harper, D.; Parracho, H.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and function of phage encoded depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).