Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Ethical Aspects
2.2. Sample Collection and Processing of Biological Material
2.3. Measurement of Cytokine and Chemokine
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
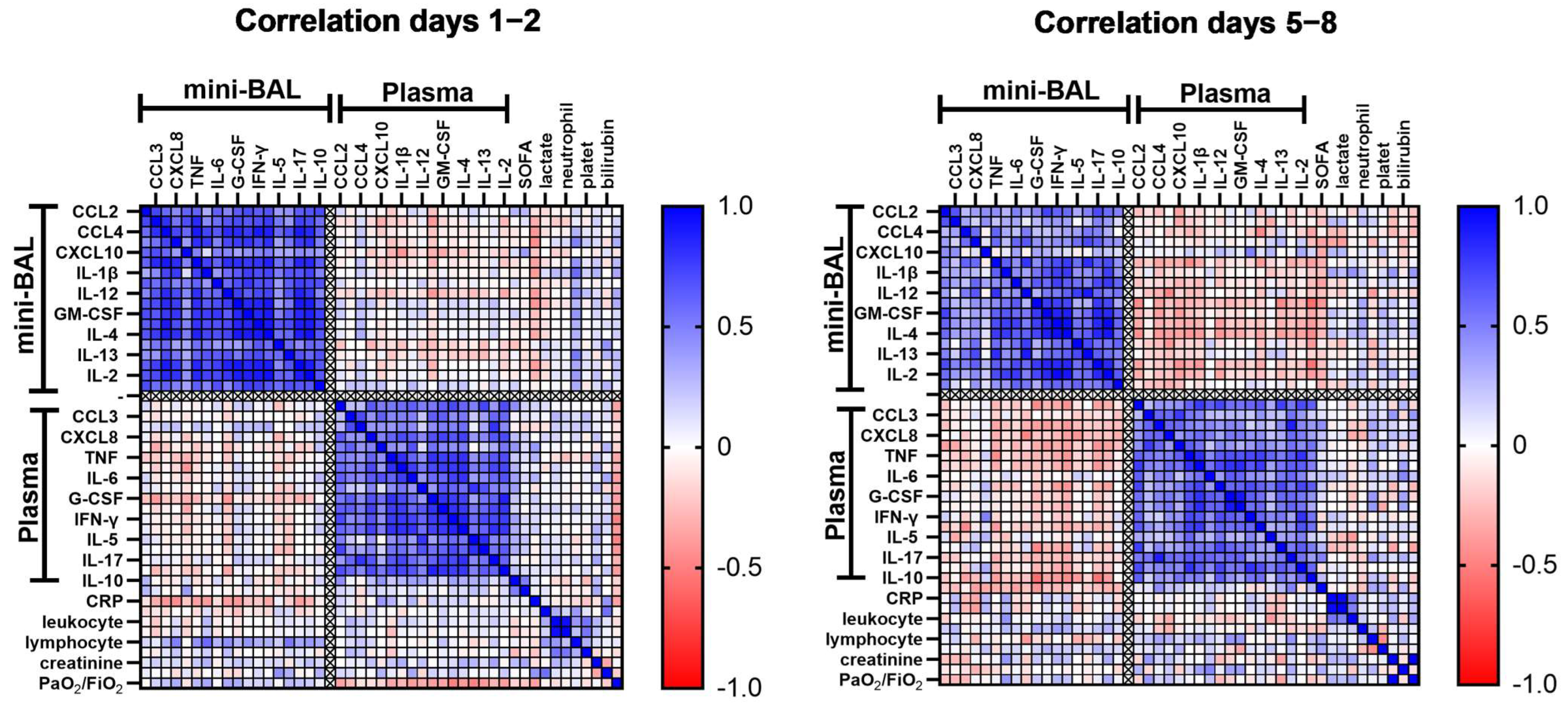
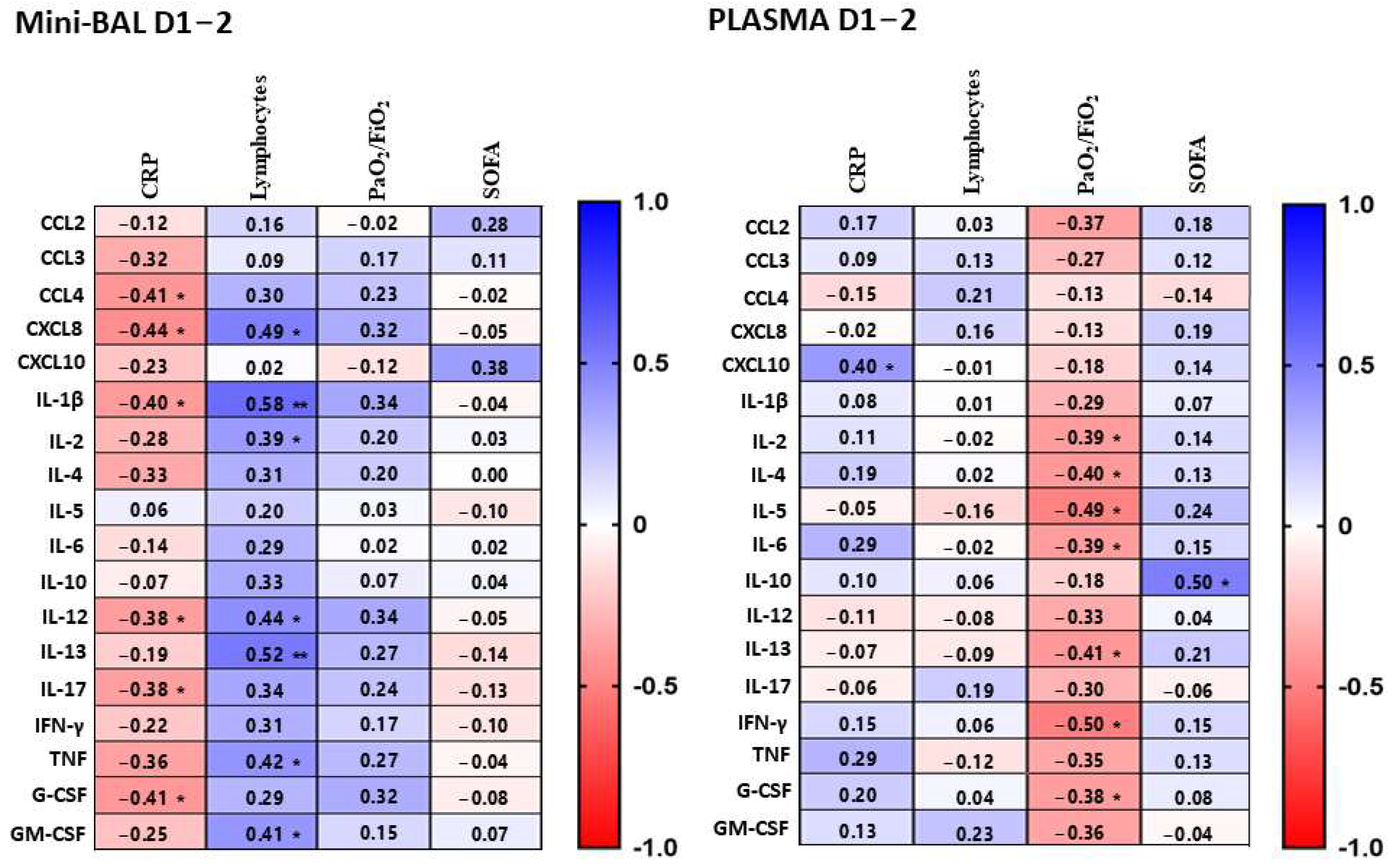
3.2. Compartmentalized Correlation of Cytokines Levels between Lung and Plasma
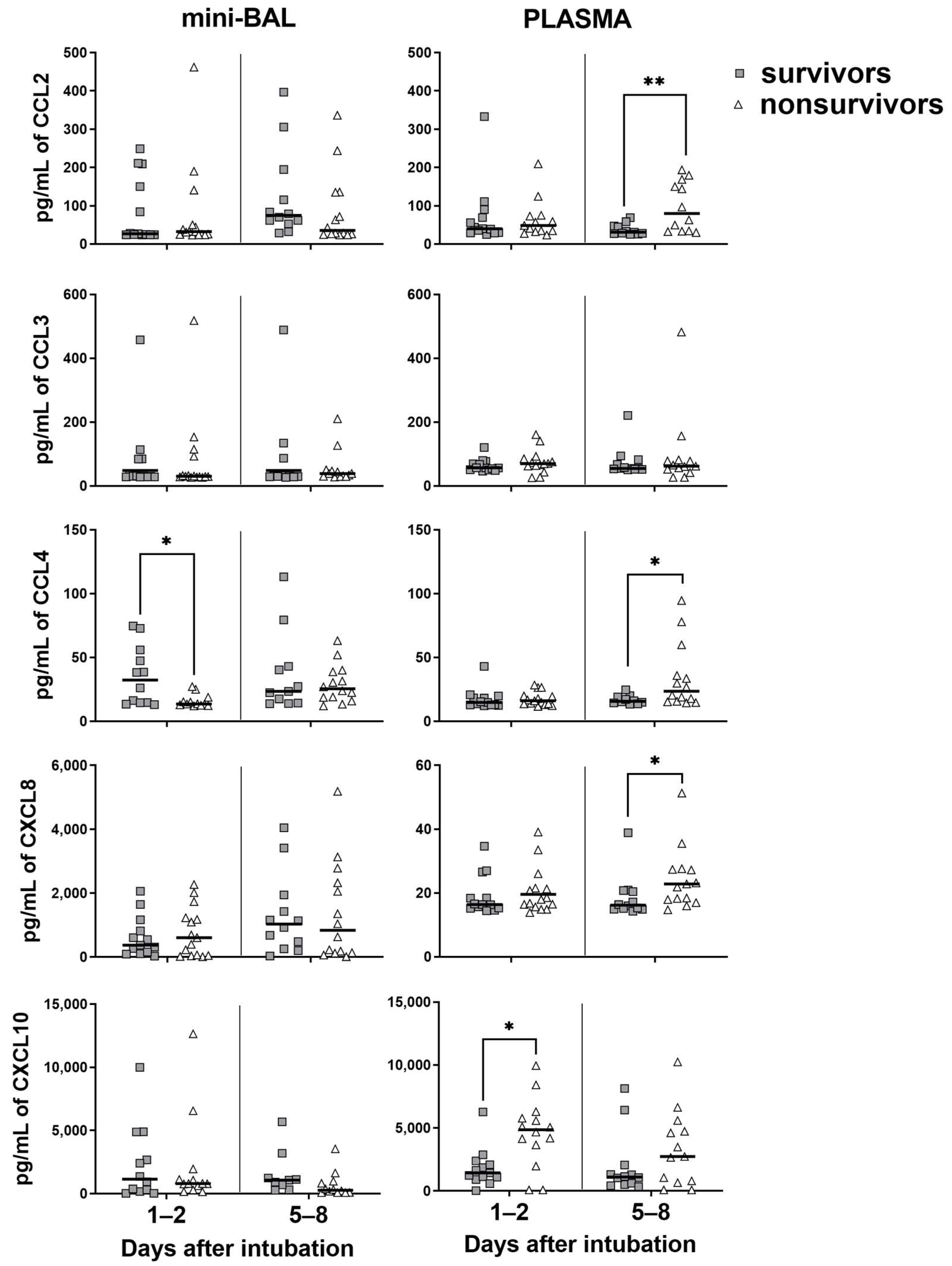
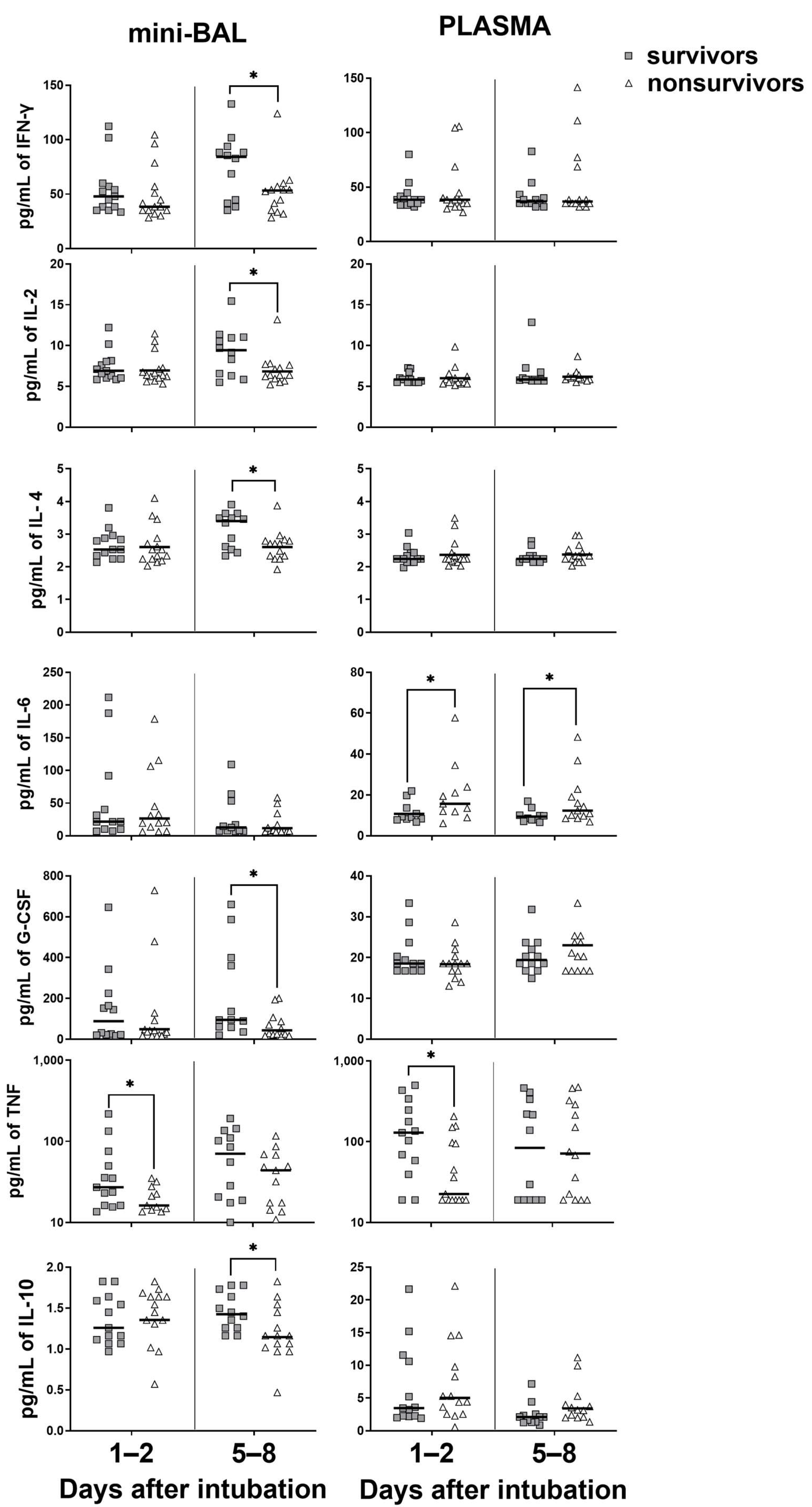
3.3. Survivors Exhibit Higher Levels of Cytokines in the Lung, Whereas Nonsurvivors in the Blood
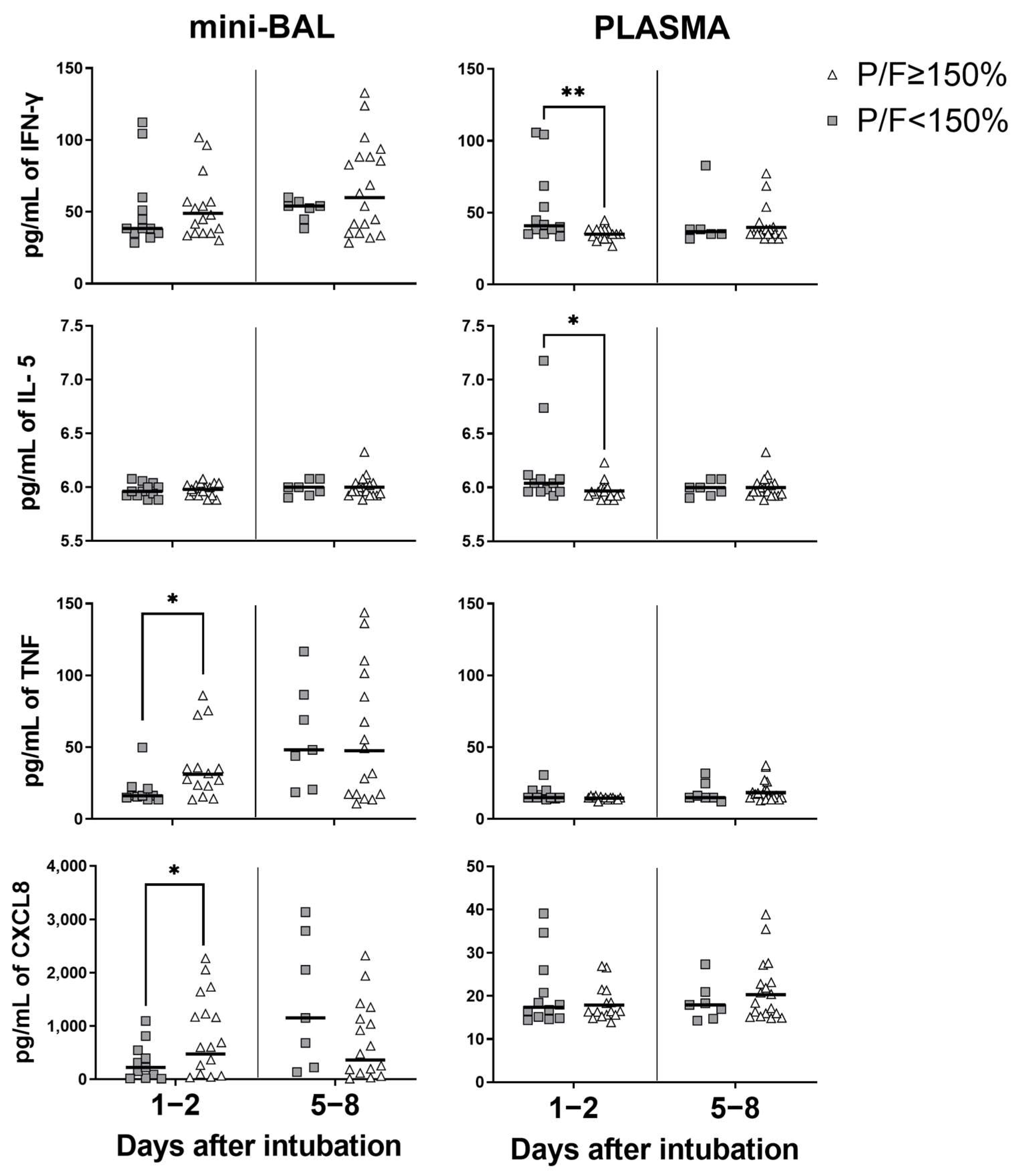
3.4. Systemic Inflammation Is Associated with Worse PaO2/FiO2 Ratio
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jonhs Hopkins University. COVID-19 Dashboard. Available online: https://coronavirus.jhu.edu/map.html (accessed on 3 March 2023).
- Arish, M.; Qian, W.; Narasimhan, H.; Sun, J. COVID-19 immunopathology: From acute diseases to chronic sequelae. J. Med. Virol. 2023, 95, e28122. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.-E.; Rovina, N.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021, 22, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Pan, H.; Li, R.; He, K.; Zhang, H.; Liu, L. Increased Circulating Cytokines Have a Role in COVID-19 Severity and Death with a More Pronounced Effect in Males: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 802228. [Google Scholar] [CrossRef]
- Gonçalves, J.J.; da Mata, C.P.S.M.; Lourenço, A.A.; Ribeiro, L.; Ferreira, G.M.; Fraga-Silva, T.F.d.C.; de Souza, F.M.; Almeida, V.E.S.; Batista, I.A.; D’avila-Mesquita, C.; et al. Timeline Kinetics of Systemic and Airway Immune Mediator Storm for Comprehensive Analysis of Disease Outcome in Critically Ill COVID-19 Patients. Front. Immunol. 2022, 13, 903903. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chen, L.-D.; Zhang, Z.-Y.; Wei, X.-J.; Cai, Y.-Q.; Yao, W.-Z.; Wang, M.-H.; Huang, Q.-F.; Zhang, X.-B. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir. Res. 2020, 21, 201. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef]
- Pandolfi, L.; Fossali, T.; Frangipane, V.; Bozzini, S.; Morosini, M.; D’amato, M.; Lettieri, S.; Urtis, M.; Di Toro, A.; Saracino, L.; et al. Broncho-alveolar inflammation in COVID-19 patients: A correlation with clinical outcome. BMC Pulm. Med. 2020, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Guillamet, C.V.; Day, A.; Borcherding, N.; Guillamet, R.V.; Choreño-Parra, J.A.; House, S.L.; O’halloran, J.A.; Zúñiga, J.; Ellebedy, A.H.; et al. Comprehensive Immunologic Evaluation of Bronchoalveolar Lavage Samples from Human Patients with Moderate and Severe Seasonal Influenza and Severe COVID-19. J. Immunol. 2021, 207, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Doré, É.; Dubuc, I.; Archambault, A.-S.; Flamand, O.; Laviolette, M.; Flamand, N.; Boilard, É.; Flamand, L. Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19. J. Allergy Clin. Immunol. 2021, 148, 368–380. [Google Scholar] [CrossRef]
- Arifa, R.D.N.; Gonçalves-Pereira, M.H.; Santiago, L.; Ravetti, C.G.; Vassallo, P.F.; Oliveira, F.d.F.S.; Sabino, A.d.P.; Teixeira, M.M.; Nobre, V.; Santiago, H.d.C.; et al. Reduction in the number of neutrophils in the broncho-alveolar aspirate is associated with worse prognosis in elderly patients with severe COVID-19. Inflamm. Res. 2023, 72, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, M.H.; Santiago, L.; Ravetti, C.G.; Vassallo, P.F.; de Andrade, M.V.M.; Vieira, M.S.; Oliveira, F.d.F.S.d.; Carobin, N.V.; Li, G.; Sabino, A.d.P.; et al. Dysfunctional phenotype of systemic and pulmonary regulatory T cells associate with lethal COVID-19 cases. Immunology 2023, 168, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Bock, K.R.; Richards, R.D.; Hearns, M.L. The safety and diagnostic accuracy of minibronchoalveolar lavage in patients with suspected ventilator-associated pneumonia. Ann. Intern. Med. 1995, 122, 743–748. [Google Scholar] [CrossRef]
- Erden, V.; Basaranoglu, G.; Beycan, I.; Delatioglu, H.; Hamzaoglu, N.S. Reproducibility of mini-BAL culture results using 10 mL or 20 mL instilled fluid. Intensive Care Med. 2003, 29, 1856. [Google Scholar] [CrossRef]
- Pizzichini, E.; Pizzichini, M.M.; Efthimiadis, A.; Evans, S.; Morris, M.M.; Squillace, D.; Gleich, G.J.; Dolovich, J.; Hargreave, F.E. Indices of airway inflammation in induced sputum: Reproducibility and validity of cell and fluid-phase measurements. Am. J. Respir. Crit. Care Med. 1996, 154, 308–317. [Google Scholar] [CrossRef]
- Den, G.; Yin, M.; Chen, X.; Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care 2020, 24, 179. [Google Scholar]
- Wu, D.; Yang, X.O. Dysregulation of Pulmonary Responses in Severe COVID-19. Viruses 2021, 13, 957. [Google Scholar] [CrossRef]
- Chang, Y.; Bai, M.; You, Q. Associations between Serum Interleukins (IL-1beta, IL-2, IL-4, IL-6, IL-8, and IL-10) and Disease Severity of COVID-19: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2022, 2022, 2755246. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B.; Network, A.R.D.S.; National Institutes of Health. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Quah, P.; Li, A.; Phua, J. Mortality rates of patients with COVID-19 in the intensive care unit: A systematic review of the emerging literature. Crit. Care 2020, 24, 285. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, D.; Torres Arias, M. Immunouniverse of SARS-CoV-2. Immunol. Med. 2022, 45, 186–224. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.J.; Holter, J.C.; Christensen, E.E.; Schjalm, C.; Tonby, K.; Pischke, S.E.; Jenum, S.; Skeie, L.G.; Nur, S.; Lind, A.; et al. Increased interleukin-6 and macrophage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci. Rep. 2020, 10, 21697. [Google Scholar] [CrossRef]
- Unterman, A.; Sumida, T.S.; Nouri, N.; Yan, X.; Zhao, A.Y.; Gasque, V.; Schupp, J.C.; Asashima, H.; Liu, Y.; Cosme, C.; et al. Single-cell multi-omics reveals dyssynchrony of the innate and adaptive immune system in progressive COVID-19. Nat. Commun. 2022, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Paidas, M.J.; Sampath, N.; Schindler, E.A.; Cosio, D.S.; Ndubizu, C.O.; Shamaladevi, N.; Kwal, J.; Rodriguez, S.; Ahmad, A.; Kenyon, N.S.; et al. Mechanism of Multi-Organ Injury in Experimental COVID-19 and Its Inhibition by a Small Molecule Peptide. Front. Pharmacol. 2022, 13, 864798. [Google Scholar] [CrossRef]
- Queiroz, M.A.F.; das Neves, P.F.M.; Lima, S.S.; Lopes, J.d.C.; Torres, M.K.d.S.; Vallinoto, I.M.V.C.; Bichara, C.D.A.; dos Santos, E.F.; de Brito, M.T.F.M.; da Silva, A.L.S.; et al. Cytokine Profiles Associated with Acute COVID-19 and Long COVID-19 Syndrome. Front. Cell. Infect. Microbiol. 2022, 12, 922422. [Google Scholar] [CrossRef]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Dedkov, V.G.; Gladkikh, A.S.; Sharova, A.A.; Chernykh, E.I.; Kashchenko, V.A.; Ratnikov, V.A.; Gorelov, V.P.; et al. A Comparative Study of the Plasma Chemokine Profile in COVID-19 Patients Infected with Different SARS-CoV-2 Variants. Int. J. Mol. Sci. 2022, 23, 9058. [Google Scholar] [CrossRef] [PubMed]
- Kesmez Can, F.; Ozkurt, Z.; Ozturk, N.; Sezen, S. Effect of IL-6, IL-8/CXCL8, IP-10/CXCL 10 levels on the severity in COVID-19 infection. Int. J. Clin. Pract. 2021, 75, e14970. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Singh, G.; Rumende, C.M.; Sharma, S.K.; Rengganis, I.; Amin, Z.; Loho, T.; Hermiyanti, E.; Harimurti, K.; Wibowo, H. Low BALF CD4 T cells count is associated with extubation failure and mortality in critically ill COVID-19 pneumonia. Ann. Med. 2022, 54, 1894–1905. [Google Scholar] [CrossRef]
- Yang, L.; Gou, J.; Gao, J.; Huang, L.; Zhu, Z.; Ji, S.; Liu, H.; Xing, L.; Yao, M.; Zhang, Y. Immune characteristics of severe and critical COVID-19 patients. Signal Transduct. Target. Ther. 2020, 5, 179. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Tamburello, A.; Castelnovo, L.; Laria, A.; Mumoli, N.; Faggioli, P.M.; Stefani, I.; Mazzone, A. Immunotherapy of COVID-19: Inside and Beyond IL-6 Signalling. Front. Immunol. 2022, 13, 795315. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 20 January 2023).

| Total | Survivors | Nonsurvivors | |
|---|---|---|---|
| n = 34 | n = 16 | n = 18 | |
| Baseline | |||
| Age—median (Q1–Q3) | 58 (41–70) | 47 * (40–61) | 64 * (51–74) |
| Gender (M)—no. (%) | 21 (62) | 11 (69) | 10 (55) |
| Symptom days—median (Q1–Q3) | 8 (7–11.3) | 8 (7–13) | 7 (5–11) |
| SOFA (points)—median (Q1–Q3) | 7.5 (5–9) | 7 (4–10) | 8 (6–9) |
| APACHE II (points)—median (Q1–Q3) | 16.5 (13.5–22) | 16 (10.5–21.5) | 17.5 (15–23.8) |
| Chest CT changes ≥ 50% GGO—no. (%) | 12/24 (50) | 6/12 (50) | 6/12 (50) |
| PaO2/FiO2—median (Q1–Q3) | 168 (113–201) | 164.1 (106–225) | 168.7 (113–223) |
| Continuous use of immunosuppressants drugs—no. (%) | 3 (9) | 2 (12.5) | 1 (5.6) |
| Comorbidities—no. (%) | 27 (79.4) | 10 * (62.5) | 17 * (94.4) |
| Systemic arterial hypertension—no. (%) | 16 (47) | 6 (37.5) | 10 (55.5) |
| Diabetes mellitus—no. (%) | 12 (35.3) | 4 (25) | 8 (44.4) |
| COPD—no. (%) | 2 (6) | 2 (12.5) | 2 (11.1) |
| Asthma—no. (%) | 2 (6) | 1 (6.3) | 1 (5.6) |
| Cardiovascular diseases—no. (%) | 9 (26.5) | 2 (12.5) | 7 (38.9) |
| CKD dialytic—no. (%) | 2 (6) | 0 | 2 (11.1) |
| Active neoplasm—no. (%) | 4 (11.8) | 1 (6.3) | 3 (16.7) |
| Transplanted—no. (%) | 1 (2.9) | 1 (6.3) | 0 |
| Obesity: BMI > 30 (weight (Kg)/height (m2))—no. (%) | 7 (20.6) | 4(25) | 3 (16.7) |
| Other comorbidities—no. (%) | 8 (23.5) | 2 (12.5) | 6 (33.3) |
| Laboratory characteristics at baseline | |||
| Leukocyte × 10³/µL—median (Q1–Q3) | 10.5 (7.1–14) | 11.1 (6.7–13.6) | 10.4 (6.6–15.1) |
| Neutrophil × 10³/µL—median (Q1–Q3) | 8.7 (6.6–13) | 8.6 (5.6–12.2) | 8.9 (6.3–14) |
| Lymphocyte × 10³/µL—median (Q1–Q3) | 0.6 (0.4–0.8) | 0.7 (0.6–0.8) | 0.5 (0.2–0.9) |
| Platelet × 10³/µL—median (Q1–Q3) | 189 (145–450) | 199 (155–285) | 183 (123–219) |
| CRP mg/l—median (Q1–Q3) | 180 (70–229) | 160.2 (57.6–254.4) | 190.4 (141.5–228) |
| Lactate mmol/L—median (Q1–Q3) | 1.6 (1.4–4.8) | 1.5 (1.2–2.1) | 1.7 (1.5–2.2) |
| Creatinine mg/dL—median (Q1–Q3) | 1.6 (0.7–2.2) | 1.3 (0.5–6.3) | 1.2 (0.6–6.4) |
| Bilirubin mg/dL—median (Q1–Q3) | 0.6 (0.4–5.7) | 0.5 (0.3–1.8) | 0.6 (0.5–5.7) |
| Followup | |||
| Antibiotic use during the ICU stay—no. (%) | 33 (97) | 16 (100) | 17 (94.4) |
| Use of dexamethasone during hospitalization—no. (%) | 28 (82.4) | 14 (87.5) | 14 (78) |
| Vasopressor or inotropic during hospitalization—no. (%) | 32 (94.1) | 14 (87.5) | 18 (100) |
| AKI during hospitalization—no. (%) | 25 (73.5) | 10 (62.5) | 15 (83.3) |
| Length of ICU stay in days—median (Q1–Q3) | 17 (10.5–33) | 21.5 (11–33) | 16.5 (9–30) |
| Length of hospitalization stay in days—median (Q1–Q3) | 31 (18.8–44.8) | 39 ** (30.5–52.5) | 22 ** (13–34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, L.; Gonçalves-Pereira, M.H.; Vieira, M.S.; Gómez Ravetti, C.; Vassallo, P.F.; Silva e Castro, R.; Costa Pimenta, P.P.; Andrade, M.V.M.d.; Santiago, H.d.C.; Nobre, V. Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients. Viruses 2023, 15, 1704. https://doi.org/10.3390/v15081704
Santiago L, Gonçalves-Pereira MH, Vieira MS, Gómez Ravetti C, Vassallo PF, Silva e Castro R, Costa Pimenta PP, Andrade MVMd, Santiago HdC, Nobre V. Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients. Viruses. 2023; 15(8):1704. https://doi.org/10.3390/v15081704
Chicago/Turabian StyleSantiago, Luciana, Marcela Helena Gonçalves-Pereira, Mariana Sousa Vieira, Cecilia Gómez Ravetti, Paula Frizera Vassallo, Rafael Silva e Castro, Pedro Pires Costa Pimenta, Marcus Vinícius Melo de Andrade, Helton da Costa Santiago, and Vandack Nobre. 2023. "Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients" Viruses 15, no. 8: 1704. https://doi.org/10.3390/v15081704
APA StyleSantiago, L., Gonçalves-Pereira, M. H., Vieira, M. S., Gómez Ravetti, C., Vassallo, P. F., Silva e Castro, R., Costa Pimenta, P. P., Andrade, M. V. M. d., Santiago, H. d. C., & Nobre, V. (2023). Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients. Viruses, 15(8), 1704. https://doi.org/10.3390/v15081704






