Components of the Nucleotide Salvage Pathway Increase Frog Virus 3 (FV3) Replication
Abstract
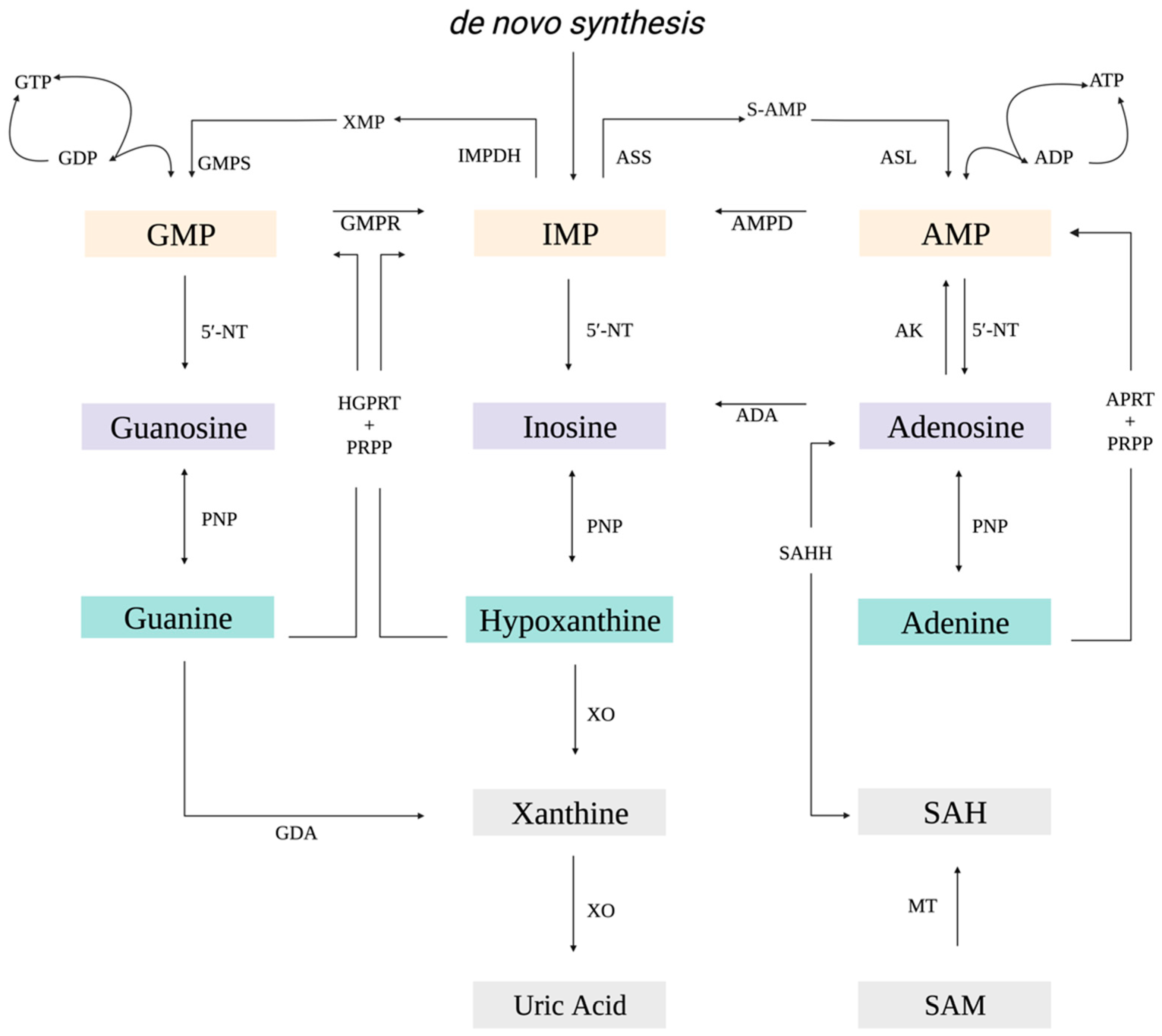
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Reagents, and Virus
2.2. Relative Plaque Formation Assay
2.3. Plaque Size Assay
2.4. Cytotoxicity and Proliferation Assays
3. Results
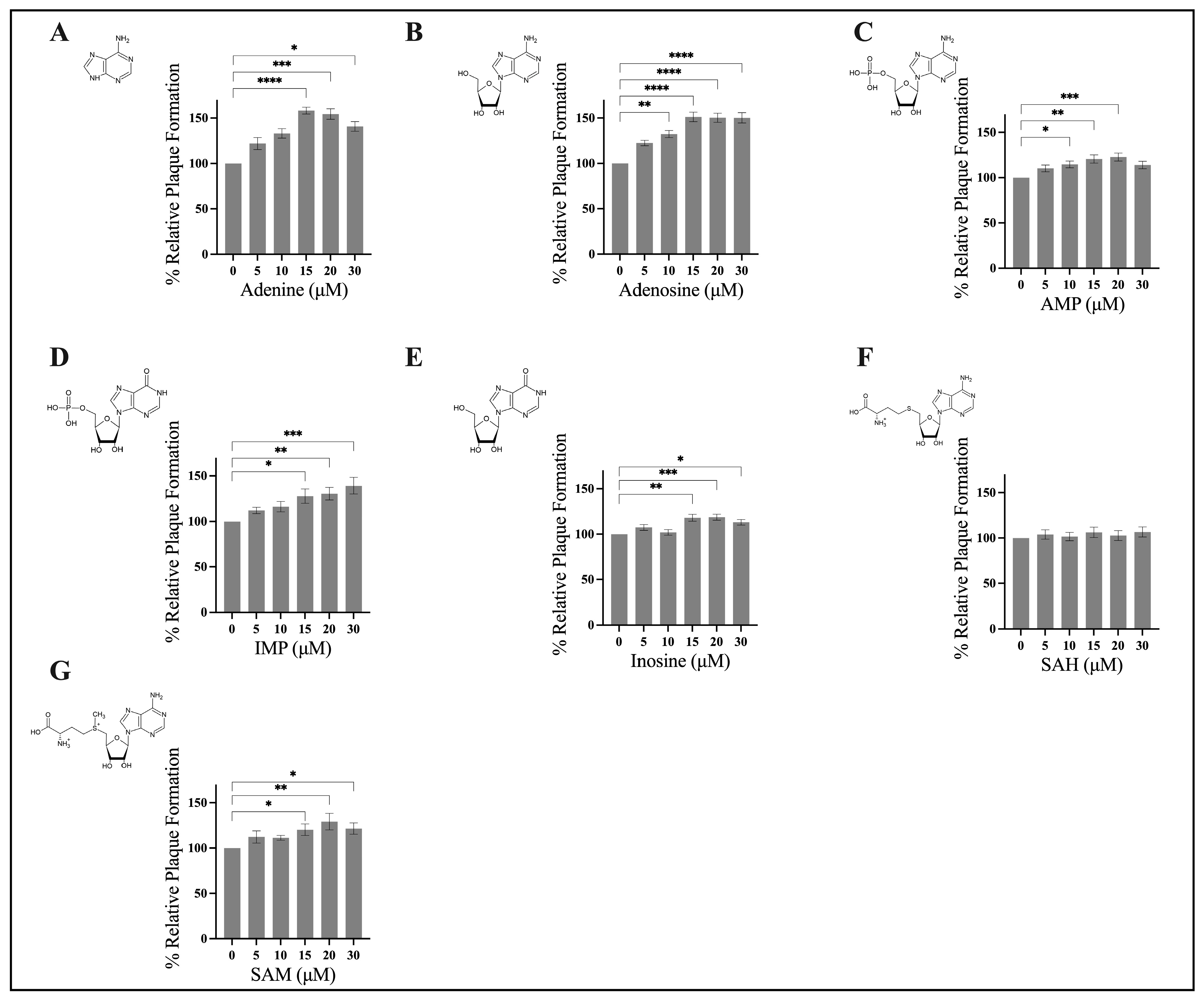
3.1. Exogenous Application of Adenine, Adenosine, AMP, IMP, Inosine, and SAM Stimulate FV3 Replication
3.2. Application of Exogenous Adenine Increases FV3 Plaque Area
3.3. Pre-Treatment Assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, A.N.; Fan, T.W. Regulation of Mammalian Nucleotide Metabolism and Biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Ye, W.; Chen, H.; Kuang, X.; Guo, J.; Xiang, M.; Peng, C.; Chen, X.; Liu, H. Role of Purines in Regulation of Metabolic Reprogramming. Purinergic Signal. 2019, 15, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, B.A.; Ashihara, H. Purine and Pyrimidine Nucleotide Synthesis and Metabolism. Arab. Book 2002, 1, e0018. [Google Scholar] [CrossRef] [Green Version]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de Novo Purine Biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pedley, A.M.; Benkovic, S.J. A New View into the Regulation of Purine Metabolism: The Purinosome. Trends Biochem. Sci. 2017, 42, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Eads, J.; Sacchettini, J.C.; Grubmeyer, C. Kinetic Mechanism of Human Hypoxanthine−Guanine Phosphoribosyltransferase: Rapid Phosphoribosyl Transfer Chemistry. Biochemistry 1997, 36, 3700–3712. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, K.; Mittal, J.; Chan, B.; Yan, D.; Grati, M.; Liu, X.Z. Association of PRPS1 Mutations with Disease Phenotypes. Dis. Markers 2015, 2015, 127013. [Google Scholar] [CrossRef] [Green Version]
- Vizán, P.; Di Croce, L.; Aranda, S. Functional and Pathological Roles of AHCY. Front. Cell Dev. Biol. 2021, 9, 587. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Xu, S.; Munger, J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015, 23, 789–798. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral Activation of Cellular Metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral Hijacking of Cellular Metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Polcicova, K.; Badurova, L.; Tomaskova, J. Metabolic Reprogramming as a Feast for Virus Replication. Acta Virol. 2020, 64, 201–215. [Google Scholar] [CrossRef]
- Sabariegos, R.; Ortega-Prieto, A.M.; Díaz-Martínez, L.; Grande-Pérez, A.; García Crespo, C.; Gallego, I.; de Ávila, A.I.; Albentosa-González, L.; Soria, M.E.; Gastaminza, P.; et al. Guanosine Inhibits Hepatitis C Virus Replication and Increases Indel Frequencies, Associated with Altered Intracellular Nucleotide Pools. PLOS Pathog. 2022, 18, e1010210. [Google Scholar] [CrossRef]
- Lozano-Sepulveda, S.A.; Bautista-Osorio, E.; Merino-Mascorro, J.A.; Varela-Rey, M.; Muñoz-Espinosa, L.E.; Cordero-Perez, P.; Martinez-Chantar, M.L.; Rivas-Estilla, A.M. S-Adenosyl-L-Methionine Modifies Antioxidant-Enzymes, Glutathione-Biosynthesis and Methionine Adenosyltransferases-1/2 in Hepatitis C Virus-Expressing Cells. World J. Gastroenterol. 2016, 22, 3746. [Google Scholar] [CrossRef]
- Feld, J.J.; Modi, A.A.; El–Diwany, R.; Rotman, Y.; Thomas, E.; Ahlenstiel, G.; Titerence, R.; Koh, C.; Cherepanov, V.; Heller, T. S-Adenosyl Methionine Improves Early Viral Responses and Interferon-Stimulated Gene Induction in Hepatitis C Nonresponders. Gastroenterology 2011, 140, 830–839.e3. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, W.; Xu, L.; Zhou, X.; Shokrollahi, E.; Felczak, K.; van der Laan, L.J.W.; Pankiewicz, K.W.; Sprengers, D.; Raat, N.J.H.; et al. Cross Talk between Nucleotide Synthesis Pathways with Cellular Immunity in Constraining Hepatitis E Virus Replication. Antimicrob. Agents Chemother. 2016, 60, 2834–2848. [Google Scholar] [CrossRef] [Green Version]
- Leão-Ferreira, L.R.; Paes-de-Carvalho, R.; de Mello, F.G.; Moussatché, N. Inhibition of Vaccinia Virus Replication by Adenosine in BSC-40 Cells: Involvement of A2 Receptor-Mediated PKA Activation. Arch. Virol. 2002, 147, 1407–1423. [Google Scholar] [CrossRef]
- Li, J.-S.; Cheng, Y.-C. Interaction of Epstein-Barr Virus DNA Polymerase with Aphidicolin, Phosphonoformate and 5′-GMP. Virus Genes 1988, 1, 369–374. [Google Scholar] [CrossRef]
- Frank, K.B.; Cheng, Y.C. Inhibition of Herpes Simplex Virus DNA Polymerase by Purine Ribonucleoside Monophosphates. J. Biol. Chem. 1986, 261, 1510–1513. [Google Scholar] [CrossRef]
- Blue, W.T.; Macias, E.A.; Sklar, S.H. Activity of AMP against Experimental Herpes Simplex Virus Type 1 Infections in Mice. Antimicrob. Agents Chemother. 1983, 24, 807–809. [Google Scholar] [CrossRef] [Green Version]
- Goorha, R.; Murti, K.G. The Genome of Frog Virus 3, an Animal DNA Virus, Is Circularly Permuted and Terminally Redundant. Proc. Natl. Acad. Sci. USA 1982, 79, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Waltzek, T.B.; Subramaniam, K. Ranaviruses and Other Members of the Family Iridoviridae: Their Place in the Virosphere. Virology 2017, 511, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Steckler, N.K.; Waltzek, T.B. Ranavirus Taxonomy and Phylogeny. In Ranaviruses; Springer International Publishing: Cham, Switzerland, 2015; pp. 59–70. [Google Scholar]
- Braunwald, J.; Nonnenmacher, H.; Tripier-Darcy, F. Ultrastructural and Biochemical Study of Frog Virus 3 Uptake by BHK-21 Cells. J. Gen. Virol. 1985, 66, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Qin, Q.; Zhang, Q.-Y.; Chinchar, V.G. Ranavirus Replication: Molecular, Cellular, and Immunological Events. In Ranaviruses; Springer International Publishing: Cham, Switzerland, 2015; pp. 105–139. [Google Scholar]
- Gendrault, J.-L.; Steffan, A.-M.; Bingen, A.; Kirn, A. Penetration and Uncoating of Frog Virus 3 (FV3) in Cultured Rat Kupffer Cells. Virology 1981, 112, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Majji, S.; Thodima, V.; Sample, R.; Whitley, D.; Deng, Y.; Mao, J.; Chinchar, V.G. Transcriptome Analysis of Frog Virus 3, the Type Species of the Genus Ranavirus, Family Iridoviridae. Virology 2009, 391, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Willis, D.B.; Granoff, A. Frog Virus 3 DNA Is Heavily Methylated at CpG Sequences. Virology 1980, 107, 250–257. [Google Scholar] [CrossRef]
- Willis, D.B.; Goorha, R.; Granoff, A. DNA Methyltransferase Induced by Frog Virus 3. J. Virol. 1984, 49, 86–91. [Google Scholar] [CrossRef]
- Goorha, R. Frog Virus 3 DNA Replication Occurs in Two Stages. J. Virol. 1982, 43, 519–528. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, Y.; Wang, L.; Zhang, Y.; Guo, X.; Huang, X.; Qin, Q. SGIV Induced and Exploited Cellular De Novo Fatty Acid Synthesis for Virus Entry and Replication. Viruses 2022, 14, 180. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, Q.; Pan, Z.; Huang, Y.; Huang, X.; Qin, Q. Singapore Grouper Iridovirus Induces Glucose Metabolism in Infected Cells by Activation of Mammalian Target of Rapamycin Signaling. Front. Microbiol. 2022, 13, 827818. [Google Scholar] [CrossRef]
- Guo, X.; Wu, S.; Li, N.; Lin, Q.; Liu, L.; Liang, H.; Niu, Y.; Huang, Z.; Fu, X. Accelerated Metabolite Levels of Aerobic Glycolysis and the Pentose Phosphate Pathway Are Required for Efficient Replication of Infectious Spleen and Kidney Necrosis Virus in Chinese Perch Brain Cells. Biomolecules 2019, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Hu, X.; Li, N.; Zheng, F.; Dong, X.; Duan, J.; Lin, Q.; Tu, J.; Zhao, L.; Huang, Z.; et al. Glutamine and Glutaminolysis Are Required for Efficient Replication of Infectious Spleen and Kidney Necrosis Virus in Chinese Perch Brain Cells. Oncotarget 2017, 8, 2400–2412. [Google Scholar] [CrossRef] [Green Version]
- Ariel, E.; Nicolajsen, N.; Christophersen, M.-B.; Holopainen, R.; Tapiovaara, H.; Bang Jensen, B. Propagation and Isolation of Ranaviruses in Cell Culture. Aquaculture 2009, 294, 159–164. [Google Scholar] [CrossRef]
- Holopainen, R.; Tapiovaara, H.; Honkanen, J. Expression analysis of immune response genes in fish epithelial cells following ranavirus infection. Fish Shellfish. Immunol. 2012, 32, 1095–1105. [Google Scholar] [CrossRef]
- Abràmoff, M.; Magalhães, P.; Ram, S. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 249–258. [Google Scholar]
- Cheng, K.; Escalon, B.L.; Robert, J.; Chinchar, V.G.; Garcia-Reyero, N. Differential Transcription of Fathead Minnow Immune-Related Genes Following Infection with Frog Virus 3, an Emerging Pathogen of Ectothermic Vertebrates. Virology 2014, 456–457, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Grayfer, L.; Edholm, E.-S.; De Jesús Andino, F.; Chinchar, V.G.; Robert, J. Ranavirus Host Immunity and Immune Evasion. In Ranaviruses; Springer International Publishing: Cham, Switzerland, 2015; pp. 141–170. [Google Scholar]
- Yu, Y.; Huang, Y.; Wei, S.; Li, P.; Zhou, L.; Ni, S.; Huang, X.; Qin, Q. A Tumour Necrosis Factor Receptor-like Protein Encoded by Singapore Grouper Iridovirus Modulates Cell Proliferation, Apoptosis and Viral Replication. J. Gen. Virol. 2016, 97, 756. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Aggarwal, B.B. Adenosine Suppresses Activation of Nuclear Factor-[Kappa]B Selectively Induced by Tumor Necrosis Factor in Different Cell Types. Oncogene 2003, 22, 1206–1218. [Google Scholar] [CrossRef]
- Ren, T.; Qiu, Y.; Wu, W.; Feng, X.; Ye, S.; Wang, Z.; Tian, T.; He, Y.; Yu, C.; Zhou, Y. Activation of Adenosine A3 Receptor Alleviates TNF- α -Induced Inflammation through Inhibition of the NF- κ B Signaling Pathway in Human Colonic Epithelial Cells. Mediat. Inflamm. 2014, 2014, 818251. [Google Scholar] [CrossRef]
- Jijon, H.B.; Walker, J.; Hoentjen, F.; Diaz, H.; Ewaschuk, J.; Jobin, C.; Madsen, K.L. Adenosine Is a Negative Regulator of NF-ΚB and MAPK Signaling in Human Intestinal Epithelial Cells. Cell. Immunol. 2005, 237, 86–95. [Google Scholar] [CrossRef]
- Bouma, M.G.; van den Wildenberg, F.A.; Buurman, W.A. Adenosine Inhibits Cytokine Release and Expression of Adhesion Molecules by Activated Human Endothelial Cells. Am. J. Physiol. Physiol. 1996, 270, C522–C529. [Google Scholar] [CrossRef]
- Haskó, G.; Kuhel, D.G.; Németh, Z.H.; Mabley, J.G.; Stachlewitz, R.F.; Virág, L.; Lohinai, Z.; Southan, G.J.; Salzman, A.L.; Szabó, C. Inosine Inhibits Inflammatory Cytokine Production by a Posttranscriptional Mechanism and Protects Against Endotoxin-Induced Shock 1. J. Immunol. 2000, 164, 1013–1019. [Google Scholar] [PubMed] [Green Version]
- Marton, A.; Pacher, P.; Murthy, K.; Nemeth, Z.; Hasko, G.; Szabo, C. Anti-Inflammatory Effects of Inosine in Human Monocytes, Neutrophils and Epithelial Cells in Vitro. Int. J. Mol. Med. 2001, 8, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Ariav, Y.; Ch’ng, J.H.; Christofk, H.R.; Ron-Harel, N.; Erez, A. Targeting Nucleotide Metabolism as the Nexus of Viral Infections, Cancer, and the Immune Response. Sci. Adv. 2021, 7, 6165. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W. Physiological Concentrations of Purines and Pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Haskó, G.; Cronstein, B.N. Adenosine: An Endogenous Regulator of Innate Immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef]
- Zhao, H.; Chiaro, C.R.; Zhang, L.; Smith, P.B.; Chan, C.Y.; Pedley, A.M.; Pugh, R.J.; French, J.B.; Patterson, A.D.; Benkovic, S.J. Quantitative Analysis of Purine Nucleotides Indicates That Purinosomes Increase de Novo Purine Biosynthesis. J. Biol. Chem. 2015, 290, 6705–6713. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, D.C.J.; Van Loggerenberg, A.; Dieckmann, N.M.G.; Smith, G.L. Vaccinia Virus Egress Mediated by Virus Protein A36 Is Reliant on the F12 Protein. J. Gen. Virol. 2017, 98, 1500–1514. [Google Scholar] [CrossRef]
- Goh, K.C.M.; Tang, C.K.; Norton, D.C.; Gan, E.S.; Tan, H.C.; Sun, B.; Syenina, A.; Yousuf, A.; Ong, X.M.; Kamaraj, U.S.; et al. Molecular Determinants of Plaque Size as an Indicator of Dengue Virus Attenuation. Sci. Rep. 2016, 6, 26100. [Google Scholar] [CrossRef] [Green Version]
- Dobson, B.M.; Procter, D.J.; Hollett, N.A.; Flesch, I.E.A.; Newsome, T.P.; Tscharke, D.C. Vaccinia Virus F5 Is Required for Normal Plaque Morphology in Multiple Cell Lines but Not Replication in Culture or Virulence in Mice. Virology 2014, 456–457, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.N.; Zhang, Y.; Allan, P.W.; Parker, W.B.; Ting, J.W.; Chang, C.Y.; Ealick, S.E. Structure of Grouper Iridovirus Purine Nucleoside Phosphorylase. Acta Crystallogr. Sect. D 2010, 66, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Ting, J.-W.; Wu, M.-F.; Tsai, C.-T.; Lin, C.-C.; Guo, I.-C.; Chang, C.-Y. Identification and Characterization of a Novel Gene of Grouper Iridovirus Encoding a Purine Nucleoside Phosphorylase. J. Gen. Virol. 2004, 85, 2883–2892. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logan, S.R.; Seegobin, M.; Emery, R.J.N.; Brunetti, C.R. Components of the Nucleotide Salvage Pathway Increase Frog Virus 3 (FV3) Replication. Viruses 2023, 15, 1716. https://doi.org/10.3390/v15081716
Logan SR, Seegobin M, Emery RJN, Brunetti CR. Components of the Nucleotide Salvage Pathway Increase Frog Virus 3 (FV3) Replication. Viruses. 2023; 15(8):1716. https://doi.org/10.3390/v15081716
Chicago/Turabian StyleLogan, Samantha R., Mark Seegobin, R. J. Neil Emery, and Craig R. Brunetti. 2023. "Components of the Nucleotide Salvage Pathway Increase Frog Virus 3 (FV3) Replication" Viruses 15, no. 8: 1716. https://doi.org/10.3390/v15081716





