Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review
Abstract
:1. Introduction
2. Technical Notes
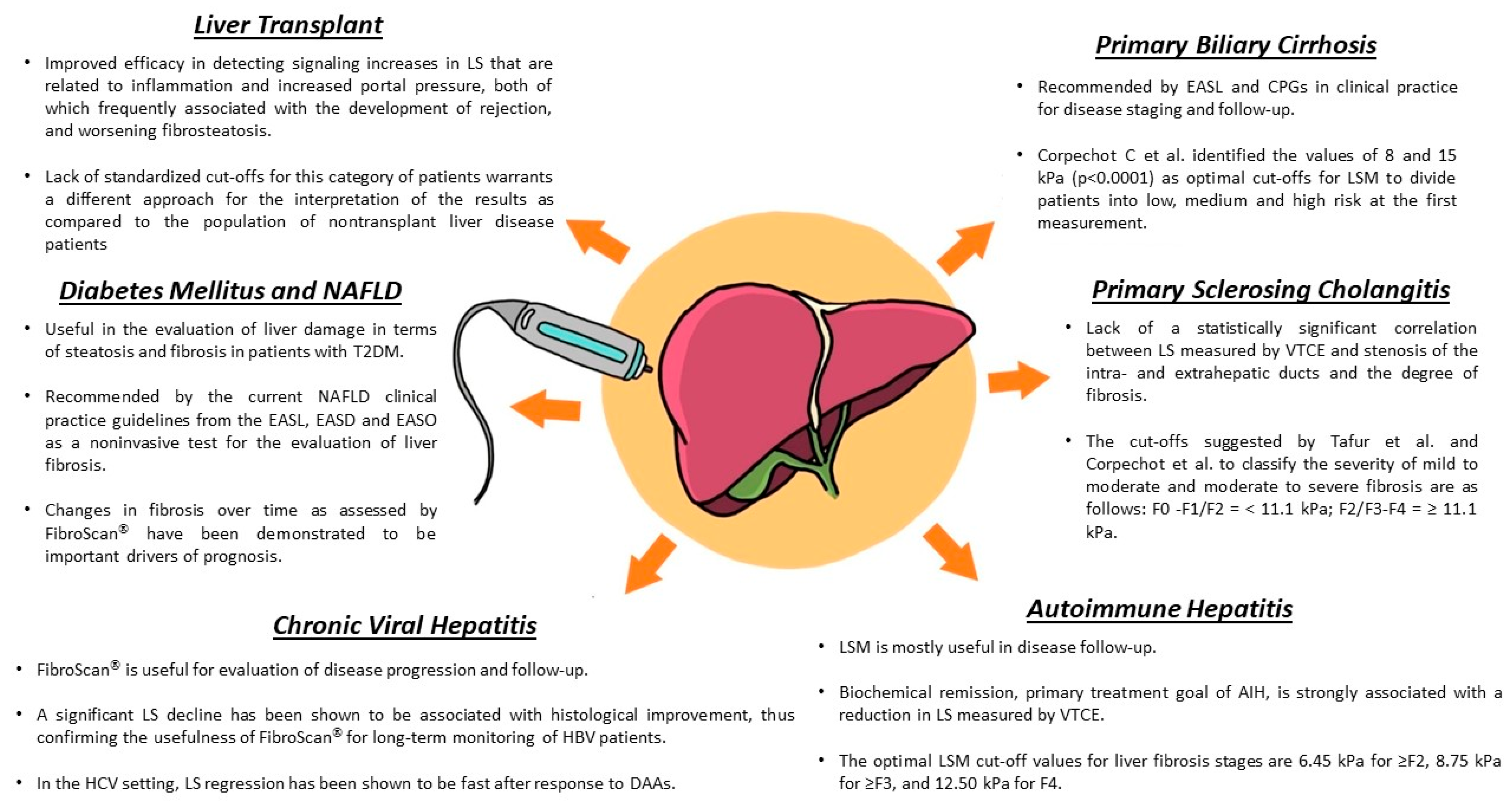
3. Transient Elastography in Chronic Viral Hepatitis
4. Changes in LS in Patients Undergoing Antiviral Therapy
5. LS in Portal Hypertension
6. LS in NAFLD
7. LS in Type 2 Diabetes Mellitus
8. LS in Autoimmune Liver Diseases
8.1. PSC and LS
8.2. AIH and LS
8.3. PBC and LS
9. TE in Liver Transplant Recipients
10. TE and Future Prospects
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, A.C.; Carvalho-Filho, R.J.; Marcellin, P. Transient elastography in chronic viral hepatitis: A critical appraisal. Gut 2011, 60, 759–764. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Dal Bello, B.; Zicchetti, M.; Lissandrin, R.; Filice, G.; Filice, C.; Above, E.; Barbarini, G.; Brunetti, E.; et al. Performance of liver stiffness measurements by transient elastography in chronic hepatitis. World J. Gastroenterol. 2013, 19, 49–56. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Ong, M.F.; Martens, S.; Sarrazin, C.; Bojunga, J.; Zeuzem, S.; Herrmann, E. Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology 2008, 134, 960–974. [Google Scholar] [CrossRef] [Green Version]
- Cristoferi, L.; Nardi, A.; Carbone, M. Transient elastography in chronic liver disease: Beware of the cut-offs! J. Hepatol. 2021, 75, 1245–1246. [Google Scholar] [CrossRef]
- Fang, J.M.; Cheng, J.; Chang, M.F.; Ahn, J.; Westerhoff, M. Transient elastography versus liver biopsy: Discordance in evaluations for fibrosis and steatosis from a pathology standpoint. Mod. Pathol. 2021, 34, 1955–1962. [Google Scholar] [CrossRef]
- Bonder, A.; Afdhal, N. Utilization of FibroScan in clinical practice. Curr. Gastroenterol. Rep. 2014, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Goh, M.J.; Park, Y.; Kim, J.; Kang, W.; Sinn, D.H.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Different Performance of Liver Stiffness Measurement According to Etiology and Outcome for the Prediction of Liver-Related Events. Dig. Dis. Sci. 2021, 66, 2816–2825. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J. Hepatol. 2017, 66, 153–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Non-invasive Assessment of Liver Disease in Patients With Non-alcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.J.; Matic, V.; Mare, R.; Maiocchi, L.; Chromy, D.; Müllner-Bucsics, T.; Mandorfer, M.; Mustapic, S.; Sporea, I.; Ferraioli, G.; et al. Point Shear Wave Elastography by ElastPQ for Fibrosis Screening in Patients with NAFLD: A Prospective, Multicentre Comparison to Vibration-Controlled Elastography. Ultraschall Med. 2023, 44, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.N.; Han, K.H.; Kim, S.U. Clinical application of transient elastography in patients with chronic viral hepatitis receiving antiviral treatment. Liver Int. 2015, 35, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Jayaswal, A.N.A.; Levick, C.; Selvaraj, E.A.; Dennis, A.; Booth, J.C.; Collier, J.; Cobbold, J.; Tunnicliffe, E.M.; Kelly, M.; Barnes, E.; et al. Prognostic value of multiparametric magnetic resonance imaging, transient elastography and blood-based fibrosis markers in patients with chronic liver disease. Liver Int. 2020, 40, 3071–3082. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.P.; de Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, R.P.; Crotty, P.; Pomier-Layrargues, G.; Ma, M.; Urbanski, S.J.; Elkashab, M. Prevalence, risk factors and causes of discordance in fibrosis staging by transient elastography and liver biopsy. Liver Int. 2010, 30, 1471–1480. [Google Scholar] [CrossRef]
- Lucidarme, D.; Foucher, J.; Le Bail, B.; Vergniol, J.; Castera, L.; Duburque, C.; Forzy, G.; Filoche, B.; Couzigou, P.; de Lédinghen, V. Factors of accuracy of transient elastography (Fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology 2009, 49, 1083–1089. [Google Scholar] [CrossRef]
- Boursier, J.; Decraecker, M.; Bourlière, M.; Bureau, C.; Ganne-Carrié, N.; de Lédinghen, V. Quality criteria for the measurement of liver stiffness. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101761. [Google Scholar] [CrossRef]
- Kim, S.U.; Kim, J.K.; Park, J.Y.; Ahn, S.H.; Lee, J.M.; Baatarkhuu, O.; Choi, E.H.; Han, K.H.; Chon, C.Y.; Kim, D.Y. Variability in liver stiffness values from different intercostal spaces. Liver Int. 2009, 29, 760–766. [Google Scholar] [CrossRef]
- Lee, J.; Kang, H.J.; Yoon, J.H.; Lee, J.M. Ultrasound-guided transient elastography and two-dimensional shear wave elastography for assessment of liver fibrosis: Emphasis on technical success and reliable measurements. Ultrasonography 2021, 40, 217–227. [Google Scholar] [CrossRef]
- Gatos, I.; Yarmenitis, S.; Theotokas, I.; Koskinas, J.; Manesis, E.; Zoumpoulis, S.P.; Zoumpoulis, P.S. Comparison of Visual Transient Elastography, Vibration Controlled Transient Elastography, Shear Wave Elastography and Sound Touch Elastography in Chronic liver Disease assessment using liver biopsy as ‘Gold Standard’. Eur. J. Radiol. 2022, 157, 110557. [Google Scholar] [CrossRef]
- Mendes, L.C.; Ferreira, P.A.; Miotto, N.; Zanaga, L.; Gonçales, E.S.L.; Pedro, M.N.; Lazarini, M.S.; Júnior, F.L.G.; Stucchi, R.S.B.; Vigani, A.G. Elastogram quality assessment score in vibration-controlled transient elastography: Diagnostic performance compared to digital morphometric analysis of liver biopsy in chronic hepatitis C. J. Viral Hepat. 2018, 25, 335–343. [Google Scholar] [CrossRef]
- Harris, R.; Card, T.R.; Delahooke, T.; Aithal, G.P.; Guha, I.N. The XL probe: A luxury or a necessity? Risk stratification in an obese community cohort using transient elastography. United Eur. Gastroenterol. J. 2018, 6, 1372–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Wang, F.; Friedrich-Rust, M.; Zhou, F.; Zhu, J.; Yang, H.; Ruan, W.; Zeng, Z. Feasibility and Efficacy of Transient Elastography using the XL probe to diagnose liver fibrosis and cirrhosis: A meta-analysis. Medicine 2018, 97, e11816. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Bortolotti, F.; Donato, F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008, 48, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.C.; Carvalho-Filho, R.J.; Stern, C.; Dipumpo, A.; Giuily, N.; Ripault, M.P.; Asselah, T.; Boyer, N.; Lada, O.; Castelnau, C.; et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012, 32, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.P.; Tainturier, M.H.; Ratziu, V.; Piton, A.; Thibault, V.; Imbert-Bismut, F.; Messous, D.; Charlotte, F.; Di Martino, V.; Benhamou, Y.; et al. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J. Hepatol. 2003, 39, 222–230. [Google Scholar] [CrossRef]
- Foucher, J.; Chanteloup, E.; Vergniol, J.; Castéra, L.; Le Bail, B.; Adhoute, X.; Bertet, J.; Couzigou, P.; de Lédinghen, V. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut 2006, 55, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Munteanu, M.; Deckmyn, O.; Ngo, Y.; Drane, F.; Castille, J.M.; Housset, C.; Ratziu, V.; Imbert-Bismut, F. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: Proof of concept and first application in a large population. J. Hepatol. 2012, 57, 541–548. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Q.; Chen, C.; Li, W.; Li, Q.; Chen, L. FibroScan Predicts Liver Fibrosis Progression in Chronic HBV Infection Patients with No Clear Indication for Antiviral Therapy: A Retrospective Cohort Study. Infect Drug Resist. 2023, 16, 1777–1785. [Google Scholar] [CrossRef]
- Lai, C.L.; Yuen, M.F. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology 2013, 57, 399–408. [Google Scholar] [CrossRef]
- Poynard, T.; Vergniol, J.; Ngo, Y.; Foucher, J.; Thibault, V.; Munteanu, M.; Merrouche, W.; Lebray, P.; Rudler, M.; Deckmyn, O.; et al. FibroFrance Study Group and the Bordeaux HBV Study Group. Staging chronic hepatitis B into seven categories, defining inactive carriers and assessing treatment impact using a fibrosis biomarker (FibroTest®) and elastography (FibroScan®). J. Hepatol. 2014, 61, 994–1003. [Google Scholar] [CrossRef]
- Liang, X.E.; Chen, Y.P. Clinical Application of Vibration Controlled Transient Elastography in Patients with Chronic Hepatitis B. J. Clin. Transl. Hepatol. 2017, 5, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.E.; Chen, Y.P.; Zhang, Q.; Dai, L.; Zhu, Y.F.; Hou, J.L. Dynamic evaluation of liver stiffness measurement to improve diagnostic accuracy of liver cirrhosis in patients with chronic hepatitis B acute exacerbation. J. Viral Hepat. 2011, 18, 884–891. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castéra, L.; Bernard, P.H.; Le Bail, B.; Foucher, J.; Trimoulet, P.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Transient elastography and biomarkers for liver fibrosis assessment and follow-up of inactive hepatitis B carriers. Aliment. Pharmacol. Ther. 2011, 33, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Papatheodoridis, G.V.; Manolakopoulos, S.; Margariti, A.; Papageorgiou, M.V.; Kranidioti, H.; Katoglou, A.; Kontos, G.; Adamidi, S.; Kafiri, G.; Deutsch, M.; et al. The usefulness of transient elastography in the assessment of patients with HBeAg-negative chronic hepatitis B virus infection. J. Viral Hepat. 2014, 21, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liang, X.E.; Dai, L.; Zhang, Q.; Peng, J.; Zhu, Y.F.; Wen, W.Q.; Chan, H.L.; Hou, J.L. Improving transient elastography performance for detecting hepatitis B cirrhosis. Dig. Liver Dis. 2012, 44, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Wong, C.K.; Leung, C.; Chan, C.Y.; Ho, P.P.; Chung, V.C.; Chan, Z.C.; Tse, Y.K.; Chim, A.M.; et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J. Hepatol. 2014, 60, 339–345. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Vergniol, J.; Barthe, C.; Foucher, J.; Chermak, F.; Le Bail, B.; Merrouche, W.; Bernard, P.H. Non-invasive tests for fibrosis and liver stiffness predict 5-year survival of patients chronically infected with hepatitis B virus. Aliment. Pharmacol. Ther. 2013, 37, 979–988. [Google Scholar] [CrossRef]
- Lee, H.W.; Yoo, E.J.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am. J. Gastroenterol. 2014, 109, 1241–1249. [Google Scholar] [CrossRef]
- Elsharkawy, A.; Alem, S.A.; Fouad, R.; El Raziky, M.; El Akel, W.; Abdo, M.; Tantawi, O.; AbdAllah, M.; Bourliere, M.; Esmat, G. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J. Gastroenterol. Hepatol. 2017, 32, 1624–1630. [Google Scholar] [CrossRef]
- Saleem, N.; Miller, L.S.; Dadabhai, A.S.; Cartwright, E.J. Using vibration controlled transient elastography and FIB-4 to assess liver cirrhosis in a hepatitis C virus infected population. Medicine 2021, 100, e26200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.N.; Chiu, H.C.; Chiu, Y.C.; Chen, S.C.; Chen, Y. Comparison of FIB-4 and transient elastography in evaluating liver fibrosis of chronic hepatitis C subjects in community. PLoS ONE 2018, 13, e0206947. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, H.; Fernandes, F.F.; Gomes, A.; Terra, C.; Perez, R.M.; Figueiredo, F.A. Interobserver variability in transient elastography analysis of patients with chronic hepatitis C. Liver Int. 2015, 35, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Arena, U.; Lupsor Platon, M.; Stasi, C.; Moscarella, S.; Assarat, A.; Bedogni, G.; Piazzolla, V.; Badea, R.; Laffi, G.; Marra, F.; et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 2013, 58, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sabbá, C.; Ferraioli, G.; Genecin, P.; Colombato, L.; Buonamico, P.; Lerner, E.; Taylor, K.J.; Groszmann, R.J. Evaluation of postprandial hyperemia in superior mesenteric artery and portal vein in healthy and cirrhotic humans: An operator-blind echo-Doppler study. Hepatology 1991, 13, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Groszmann, R.J. Vascular endothelial dysfunction in cirrhosis. J. Hepatol. 2007, 46, 927–934. [Google Scholar] [CrossRef]
- Fernández, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in liver disease. J. Hepatol. 2009, 50, 604–620. [Google Scholar] [CrossRef]
- Murawaki, Y.; Ikuta, Y.; Koda, M.; Kawasaki, H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: Relationship to liver histology. Hepatology 1994, 20, 780–787. [Google Scholar] [CrossRef]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. Serum hyaluronan as a disease marker. Ann. Med. 1996, 28, 241–253. [Google Scholar] [CrossRef]
- Atsukawa, M.; Tsubota, A.; Kondo, C.; Uchida-Kobayashi, S.; Takaguchi, K.; Tsutsui, A.; Nozaki, A.; Chuma, M.; Hidaka, I.; Ishikawa, T.; et al. A novel non-invasive formula for predicting cirrhosis in patients with chronic hepatitis C. PLoS ONE 2021, 16, e0257166. [Google Scholar] [CrossRef]
- Erman, A.; Sathya, A.; Nam, A.; Bielecki, J.M.; Feld, J.J.; Thein, H.H.; Wong, W.W.L.; Grootendorst, P.; Krahn, M.D. Estimating chronic hepatitis C prognosis using transient elastography-based liver stiffness: A systematic review and meta-analysis. J. Viral Hepat. 2018, 25, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Falade-Nwulia, O.; Suarez-Cuervo, C.; Nelson, D.R.; Fried, M.W.; Segal, J.B.; Sulkowski, M.S. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann. Intern. Med. 2017, 166, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lok, A.S.; Zoulim, F.; Dusheiko, G.; Ghany, M.G. Hepatitis B cure: From discovery to regulatory approval. Hepatology 2017, 66, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Feld, J.J.; Zeuzem, S.; Hoofnagle, J.H. From non-A, non-B hepatitis to hepatitis C virus cure. J. Hepatol. 2015, 62, S87–S99. [Google Scholar] [CrossRef] [Green Version]
- Chien, R.N.; Liaw, Y.F. Current Trend in Antiviral Therapy for Chronic Hepatitis B. Viruses 2022, 14, 434. [Google Scholar] [CrossRef]
- Daniel, K.E.; Saeian, K.; Rizvi, S. Real-world experiences with direct acting antiviral agents for chronic hepatitis C treatment. J. Viral Hepat. 2020, 27, 195–204. [Google Scholar] [CrossRef]
- Schiff, E.R.; Lee, S.S.; Chao, Y.C.; Kew Yoon, S.; Bessone, F.; Wu, S.S.; Kryczka, W.; Lurie, Y.; Gadano, A.; Kitis, G.; et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin. Gastroenterol. Hepatol. 2011, 9, 274–276. [Google Scholar] [CrossRef]
- Chang, T.T.; Liaw, Y.F.; Wu, S.S.; Schiff, E.; Han, K.H.; Lai, C.L.; Safadi, R.; Lee, S.S.; Halota, W.; Goodman, Z.; et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010, 52, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F. Re-appraisal of old and new diagnostic tools in the current management of chronic hepatitis B. Liver Int. 2014, 34, 991–1000. [Google Scholar] [CrossRef]
- Martínez-Campreciós, J.; Bonis Puig, S.; Pons Delgado, M.; Salcedo Allende, M.T.; Mínguez Rosique, B.; Genescà Ferrer, J. Transient elastography in DAA era. Relation between post-SVR LSM and histology. J. Viral Hepat. 2020, 27, 453–455. [Google Scholar] [CrossRef]
- Ramji, A.; Doucette, K.; Cooper, C.; Minuk, G.Y.; Ma, M.; Wong, A.; Wong, D.; Tam, E.; Conway, B.; Truong, D.; et al. Nationwide retrospective study of hepatitis B virological response and liver stiffness improvement in 465 patients on nucleos(t)ide analogue. World J. Gastroenterol. 2022, 28, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.; Sporea, I.; Popa, A.; Lupusoru, R.; Gherhardt, D.; Mare, R.; Apostu, A.; Hnatiuc, M. Dynamic Changes in Liver Stiffness in Patients with Chronic Hepatitis B Undergoing Antiviral Ther apy. Diagnostics 2022, 12, 2646. [Google Scholar] [CrossRef]
- Rinaldi, L.; Ascione, A.; Messina, V.; Rosato, V.; Valente, G.; Sangiovanni, V.; Zampino, R.; Marrone, A.; Fontanella, L.; de Rosa, N.; et al. Influence of antiviral therapy on the liver stiffness in chronic HBV hepatitis. Infection 2018, 46, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.K.; Fung, J.; Cheung, K.S.; Mak, L.Y.; Hui, R.W.; Liu, K.S.; Lai, C.L.; Yuen, M.F. Body-mass index is associated with fibrosis regression during long-term nucleoside analogue therapy in chronic hepatitis B. Aliment. Pharmacol. Ther. 2016, 44, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.H.; Lu, S.N.; Chen, C.H.; Chang, K.C.; Hung, C.H.; Tai, W.C.; Tsai, M.C.; Tseng, P.L.; Hu, T.H.; Wang, J.H. The changes of liver stiffness and its associated factors for chronic hepatitis B patients with entecavir therapy. PLoS ONE 2014, 9, e93160. [Google Scholar] [CrossRef]
- Kim, M.N.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Long-term changes of liver stiffness values assessed using transient elastography in patients with chronic hepatitis B receiving entecavir. Liver Int. 2014, 34, 1216–1223. [Google Scholar] [CrossRef]
- Chon, Y.E.; Park, J.Y.; Myoung, S.M.; Jung, K.S.; Kim, B.K.; Kim, S.U.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Improvement of Liver Fibrosis after Long-Term Antiviral Therapy Assessed by Fibroscan in Chronic Hepatitis B Patients With Advanced Fibrosis. Am. J. Gastroenterol. 2017, 112, 882–891. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Aguilar Schall, R.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label followup study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Sun, X.; Yu, H.; Liu, Y. Dynamic Changes of the Aspartate Aminotransferase-to-Platelet Ratio and Transient Elastography in Predicting a Histologic Response in Patients With Chronic Hepatitis B After Entecavir Treatment. J. Ultrasound Med. 2019, 38, 1441–1448. [Google Scholar] [CrossRef]
- Chan, J.; Gogela, N.; Zheng, H.; Lammert, S.; Ajayi, T.; Fricker, Z.; Kim, A.Y.; Robbins, G.K.; Chung, R.T. Direct-acting antivirals Therapy for Chronic HCV Infection Results in Liver Stiffness Regression Over 12 Months Post-treatment. Dig. Dis. Sci. 2018, 63, 486–492. [Google Scholar] [CrossRef]
- Bachofner, J.A.; Valli, P.V.; Kröger, A.; Bergamin, I.; Künzler, P.; Baserga, A.; Braun, D.; Seifert, B.; Moncsek, A.; Fehr, J.; et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017, 37, 369–376. [Google Scholar] [CrossRef]
- Sporea, I.; Lupușoru, R.; Mare, R.; Popescu, A.; Gheorghe, L.; Iacob, S.; Șirli, R. Dynamics of liver stiffness values by means of transient elastography in patients with HCV liver cirrhosis undergoing interferon free treatment. J. Gastrointestin. Liver Dis. 2017, 26, 145–150. [Google Scholar] [CrossRef]
- Knop, V.; Mauss, S.; Goeser, T.; Geier, A.; Zimmermann, T.; Herzer, K.; Postel, N.; Friedrich-Rust, M.; Hofmann, W.P. German Hepatitis C-Registry. Dynamics of liver stiffness by transient elastography in patients with chronic hepatitis C virus infection receiving direct-acting antiviral therapy-Results from the German Hepatitis C-Registry. J. Viral. Hepat. 2020, 27, 690–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezina, A.; Krishnan, A.; Woreta, T.A.; Rubenstein, K.B.; Watson, E.; Chen, P.H.; Rodriguez-Watson, C. Longitudinal assessment of liver stiffness by transient elastography for chronic hepatitis C patients. World J. Clin. Cases 2022, 10, 5566–5576. [Google Scholar] [CrossRef] [PubMed]
- Piedade, J.; Pereira, G.; Guimarães, L.; Duarte, J.; Victor, L.; Baldin, C.; Inacio, C.; Santos, R.; Chaves, Ú.; Nunes, E.P.; et al. Liver stiffness egression after sustained virological response by diret acting antivirals reduces the risk of outcomes. Sci. Rep. 2021, 11, 11681. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Ascione, A.; Nevola, R.; Fracanzani, A.L.; Piai, G.; Messina, V.; Claar, E.; Coppola, C.; Fontanella, L.; Lombardi, R.; et al. Factors affecting long-term changes of liver stiffness in direct-acting anti-hepatitis C virus therapy: A multicentre prospective study. J. Viral Hepat. 2022, 29, 26–34. [Google Scholar] [CrossRef]
- Rout, G.; Nayak, B.; Patel, A.H.; Gunjan, D.; Singh, V.; Kedia, S. Therapy with Oral Directly Acting Agents in Hepatitis C Infection Is Associated with Reduction in Fibrosis and Increase in Hepatic Steatosis on Transient Elastography. J. Clin. Exp Hepatol. 2019, 9, 207–214. [Google Scholar] [CrossRef]
- Ogasawara, N.; Kobayashi, M.; Akuta, N.; Kominami, Y.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Suzuki, F.; Saitoh, S.; et al. Serial changes in liver stiffness and controlled attenuation parameter following direct-acting antiviral therapy against hepatitis C virus genotype 1b. J. Med. Virol. 2018, 90, 313–319. [Google Scholar] [CrossRef]
- Singh, S.; Facciorusso, A.; Loomba, R.; Falck-Ytter, Y.T. Magnitude and kinetics of decrease in liver stiffness after antiviral therapy in patients with chronic hepatitis C: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.J.; Bao, F.; Du, E.; Skillin, C.; Frenette, C.T.; Waalen, J.; Alaparthi, L.; Goodman, Z.D.; Pockros, P. Morphometry confirms fibrosis regression from sustained virologic response to direct-acting antivirals for hepatitis C. Hepatol. Commun. 2018, 2, 1320–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Berzigotti, A.; Cardenas, A.; Sarin, S.K.l. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol. Hepatol. 2018, 3, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, L.S.; Rajapaksha, H.; Shackel, N.; Angus, P.W.; Herath, C.B. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J. Gastroenterol. 2020, 26, 6111–6140. [Google Scholar] [CrossRef] [PubMed]
- Vuille-Lessard, É.; Rodrigues, S.G.; Berzigotti, A. Non-invasive Detection of Clinically Significant Portal Hypertension in Compensated Advanced Chronic Liver Disease. Clin. Liver Dis. 2021, 25, 253–289. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Maruyama, H.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Kumar, M.; Ranjan, P.; Sachdeva, M.; Khare, S. Diagnostic accuracy of transient elastography in diagnosing clinically signifcant portal hypertension in patients with chronic liver disease: A systematic review and meta-analysis. J. Med. Ultrason. 2022, 49, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Shi, J.H.; Wang, X.; Tang, Q.; Wang, X.J.; Tang, K.L.; Long, Z.Q.; Hu, X.S. Diagnostic accuracy of transient elastography (Fibroscan) in detection of esophageal varices in patients with cirrhosis; A meta analysis. World J. Gastroenterol. 2017, 23, 345–356. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII Faculty. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Wong, Y.J.; Zhaojin, C.; Tosetti, G.; Degasperi, E.; Sharma, S.; Agarwal, S.; Chuan, L.; Huak, C.Y.; Jia, L.; Xiaolong, Q.; et al. Baveno-VII criteria to predict decompensation and initiate non-selective beta-blocker in compensated advanced chronic liver disease patients. Clin. Mol. Hepatol. 2023, 29, 135–145. [Google Scholar] [CrossRef]
- Jachs, M.; Hartl, L.; Simbrunner, B.; Bauer, D.; Paternostro, R.; Scheiner, B.; Balcar, L.; Semmler, G.; Stättermayer, A.F.; Pinter, M.; et al. The Sequential Application of Baveno VII Criteria and VITRO Score Improves Diagnosis of Clinically Significant Portal Hypertension. Clin. Gastroenterol. Hepatol. 2023, 21, 1854–1863. [Google Scholar] [CrossRef]
- Dajti, E.; Ravaioli, F.; Marasco, G.; Alemanni, L.V.; Colecchia, L.; Ferrarese, A.; Cusumano, C.; Gemini, S.; Vestito, A.; Renzulli, M.; et al. A combined Baveno VII and spleen stiffness algorithm to improve the non-invasive diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease. Am. J. Gastroenterol. 2022, 117, 1825–1833. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Hiriart, J.B.; Lupsor-Platon, M.; Bronte, F.; Boursier, J.; Elshaarawy, O.; Marra, F.; Thiele, M.; Markakis, G.; Payance, A.; et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021, 74, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of non-alcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Watanabe, S.; Hashimoto, E.; Ikejima, K.; Uto, H.; Ono, M.; Sumida, Y.; Seike, M.; Takei, Y.; Takehara, T.; Tokushige, K.; et al. Japanese Society of Gastroenterology; Japan Society of Hepatology. Evidence-based clinical practice guidelines for non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. J. Gastroenterol. 2015, 50, 364–377. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, A.J. American Gastroenterological Association. AGA technical review on non-alcoholic fatty liver disease. Gastroenterology 2002, 123, 1705–1725. [Google Scholar] [CrossRef]
- Pu, K.; Wang, Y.; Bai, S.; Wei, H.; Zhou, Y.; Fan, J.; Qiao, L. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: A systematic review and meta-analysis. BMC Gastroenterol. 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzetti, E.; Lombardi, R.; De Luca, L.; Tsochatzis, E.A. Non-invasive Assessment of Fibrosis in Patients with Non-alcoholic Fatty Liver Disease. Int. J. Endocrinol. 2015, 2015, 343828. [Google Scholar] [CrossRef] [Green Version]
- EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [CrossRef] [PubMed] [Green Version]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in non-alcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Lin, Y.S. Ultrasound Evaluation of Liver Fibrosis. J. Med Ultrasound. 2017, 25, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Ozercan, A.M.; Ozkan, H. Vibration-controlled Transient Elastography in NAFLD: Review Study. Euroasian J. Hepatogastroenterol. 2022, 12, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Challies, T.; Nasser, I.; Afdhal, N.H.; Lai, M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients With Non-alcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2016, 111, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Non-alcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Oeda, S.; Takahashi, H.; Imajo, K.; Seko, Y.; Ogawa, Y.; Moriguchi, M.; Yoneda, M.; Anzai, K.; Aishima, S.; Kage, M.; et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with non-alcoholic fatty liver disease: A multicentre prospective study. J. Gastroenterol. 2020, 55, 428–440. [Google Scholar] [CrossRef]
- Mederacke, I.; Wursthorn, K.; Kirschner, J.; Rifai, K.; Manns, M.P.; Wedemeyer, H.; Bahr, M.J. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009, 29, 1500–1506. [Google Scholar] [CrossRef]
- Berzigotti, A.; De Gottardi, A.; Vukotic, R.; Siramolpiwat, S.; Abraldes, J.G.; García-Pagan, J.C.; Bosch, J. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS ONE 2013, 8, e58742. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Costa Moreira, P.; Peixoto, A.; Santos, A.L.; Lopes, S.; Gonçalves, R.; Pereira, P.; Cardoso, H.; Macedo, G. Effect of Meal Ingestion on Liver Stiffness and Controlled Attenuation Parameter. GE Port. J. Gastroenterol. 2019, 26, 99–104. [Google Scholar] [CrossRef]
- Mueller, S.; Sandrin, L. Liver stiffness: A novel parameter for the diagnosis of liver disease. Hepat. Med. 2010, 2, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, Y.P.; Rodrigues, S.G.; Delgado, M.G.; Murgia, G.; Lange, N.F.; Schropp, J.; Montani, M.; Dufour, J.F.; Berzigotti, A. Inflammatory activity affects the accuracy of liver stiffness measurement by transient elastography but not by two-dimensional shear wave elastography in non-alcoholic fatty liver disease. Liver Int. 2022, 42, 102–111. [Google Scholar] [CrossRef]
- Zhang, X.; Heredia, N.I.; Balakrishnan, M.; Thrift, A.P. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017-2018. PLoS ONE 2021, 16, e0252164. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, S.; Pipitone, R.M.; Pennisi, G.; Celsa, C.; Cammà, C.; Di Marco, V.; Barcellona, M.R.; Boemi, R.; Enea, M.; Giannetti, A.; et al. Association Between PNPLA3 rs738409 C>G Variant and Liver-Related Outcomes in Patients With Non-alcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 935–944.e3. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Non-alcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Petta, S.; Sebastiani, G.; Viganò, M.; Ampuero, J.; Wai-Sun Wong, V.; Boursier, J.; Berzigotti, A.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Non-alcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 806–815.e5. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. NASH Clinical Research Network. Performance characteristics of vibration-controlled transient elastography for evaluation of non-alcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Targher, G.; Lonardo, A.; Byrne, C.D. Non-alcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020, 40, 347–354. [Google Scholar] [CrossRef]
- Rinaldi, L.; Guarino, M.; Perrella, A.; Pafundi, P.C.; Valente, G.; Fontanella, L.; Nevola, R.; Guerrera, B.; Iuliano, N.; Imparato, M.; et al. Role of Liver Stiffness Measurement in Predicting HCC Occurrence in Direct-Acting Antivirals Setting: A Real-Life Experience. Dig. Dis. Sci. 2019, 64, 3013–3019. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Domislovic, V.; Klapan, M.; Juric, T.; Lukic, A.; Krznaric-Zrnic, I.; Fuckar-Cupic, D.; Stimac, D.; Filipec Kanizaj, T.; Krznaric, Z.; et al. Accuracy of Controlled Attenuation Parameter and Liver Stiffness Measurement in Patients with Non-alcoholic Fatty Liver Disease. Ultrasound Med. Biol. 2021, 47, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Racki, S.; Bubic, I.; Jelic, I.; Stimac, D.; Orlic, L. Chronic kidney disease and non-alcoholic Fatty liver disease proven by transient elastography. Kidney Blood Press. Res. 2013, 37, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Orlic, L.; Milic, S.; Lukenda, V.; Racki, S.; Stimac, D.; Avdovic, E.; Zaputovic, L. Non-alcoholic fatty liver disease (NAFLD) proven by transient elastography in patients with coronary heart disease. Wien Klin Wochenschr. 2014, 126, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Rahelic, D.; Turk-Wensween, T.; Ruzic, A.; Domislovic, V.; Hauser, G.; Matic, T.; Radic-Kristo, D.; Krznaric, Z.; Radic, M.; et al. Significant liver fibrosis, as assessed by fibroscan, is independently associated with chronic vascular complications of type 2 diabetes: A multicentre study. Diabetes Res. Clin. Pract. 2021, 177, 108884. [Google Scholar] [CrossRef]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107684. [Google Scholar] [CrossRef]
- Lombardi, R.; Petta, S.; Pisano, G.; Dongiovanni, P.; Rinaldi, L.; Adinolfi, L.E.; Acierno, C.; Valenti, L.; Boemi, R.; Spatola, F.; et al. FibroScan Identifies Patients With Non-alcoholic Fatty Liver Disease and Cardiovascular Damage. Clin. Gastroenterol. Hepatol. 2020, 18, 517–519. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.R.L.; Villela-Nogueira, C.A.; Leite, N.C.; Salles, G.F. Prognostic impact of liver fibrosis and steatosis by transient elastography for cardiovascular and mortality outcomes in individuals with non-alcoholic fatty liver disease and type 2 diabetes: The Rio de Janeiro Cohort Study. Cardiovasc Diabetol. 2021, 20, 193. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.L.; et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort ☆. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef]
- Kahali, B.; Liu, Y.L.; Daly, A.K.; Day, C.P.; Anstee, Q.M.; Speliotes, E.K. TM6SF2, catch-22 in the fight against non-alcoholic fatty liver disease and cardiovascular disease? Gastroenterology 2015, 148, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, B.K.; Stender, S.; Kristensen, T.S.; Kofoed, K.F.; Køber, L.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur. Heart J. 2018, 39, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Wong, G.L.; Kwok, R.; Choi, K.C.; Chan, C.K.; Shu, S.S.; Leung, J.K.; Chim, A.M.; Luk, A.O.; Ma, R.C.; et al. Serial Transient Elastography Examinations to Monitor Patients With Type 2 Diabetes: A Prospective Cohort Study. Hepatology 2020, 72, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Wong, G.L.; Choi, P.C.; Chan, A.W.; Li, M.K.; Chan, H.Y.; Chim, A.M.; Yu, J.; Sung, J.J.; Chan, H.L. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut 2010, 59, 969–974. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Blond, E.; Disse, E.; Cuerq, C.; Drai, J.; Valette, P.J.; Laville, M.; Thivolet, C.; Simon, C.; Caussy, C. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: Do they lead to over-referral? Diabetologia 2017, 60, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Seto, W.K.; Ieong, K.; Lui, D.T.W.; Fong, C.H.Y.; Wan, H.Y.; Chow, W.S.; Woo, Y.C.; Yuen, M.F.; Lam, K.S.L. Development of a Non-Invasive Liver Fibrosis Score Based on Transient Elastography for Risk Stratification in Patients with Type 2 Diabetes. Endocrinol. Metab. 2021, 36, 134–145. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Non-alcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Kwok, R.; Choi, K.C.; Wong, G.L.; Zhang, Y.; Chan, H.L.; Luk, A.O.; Shu, S.S.; Chan, A.W.; Yeung, M.W.; Chan, J.C.; et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut 2016, 65, 1359–1368. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Bertolini, L.; Rodella, S.; Zenari, L.; Lippi, G.; Franchini, M.; Zoppini, G.; Muggeo, M. Increased risk of CKD among type 2 diabetics with non-alcoholic fatty liver disease. J. Am. Soc. Nephrol. 2008, 19, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Non-alcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K. LEAN trial team; Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, L.L.; Vethakkan, S.R.; Nik Mustapha, N.R.; Mahadeva, S.; Chan, W.K. Empagliflozin for the Treatment of Non-alcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig. Dis. Sci. 2020, 65, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Non-alcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, J.; Perdereau, D.; Foufelle, F.; Prip-Buus, C.; Ferré, P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994, 8, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jojima, T.; Tomotsune, T.; Iijima, T.; Akimoto, K.; Suzuki, K.; Aso, Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol. Metab. Syndr. 2016, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.M. Primary biliary cirrhosis. N. Engl. J. Med. 1996, 335, 1570–1580. [Google Scholar] [CrossRef]
- Krawitt, E.L. Autoimmune hepatitis. N. Engl. J. Med. 1996, 334, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kaplan, M. Primary sclerosing cholangitis. N. Engl. J. Med. 1995, 332, 924–933. [Google Scholar] [CrossRef]
- Czaja, A. Frequency and nature of the variant forms of autoimmune liver disease. Hepatology 1998, 28, 360–365. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, H.; Wu, S.-D.; Jiang, W. Accuracy of transient elastography in assessing fibrosis at diagnosis in individuals with autoimmune liver disease. J. Hepatol. 2023, 78, e16–e39. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary sclerosing cholangitis. Lancet 2013, 382, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J. Surgical pathology of the syndrome of primary sclerosing cholangitis. Am. J. Surg. Pathol. 1989, 13, 43–49. [Google Scholar] [PubMed]
- Aabakken, L.; Karlsen, T.H.; Albert, J.; Arvanitakis, M.; Chazouilleres, O.; Dumonceau, J.M.; Färkkilä, M.; Fickert, P.; Hirschfield, G.M.; Laghi, A.; et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy 2017, 49, 588–608. [Google Scholar] [CrossRef]
- Dave, M.; Elmunzer, B.J.; Dwamena, B.A.; Higgins, P.D. Primary sclerosing cholangitis: Meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology 2010, 256, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Tafur, M.; Cheung, A.; Menezes, R.J.; Feld, J.; Janssen, H.; Hirschfield, G.M.; Jhaveri, K.S. Risk stratification in primary sclerosing cholangitis: Comparison of biliary stricture severity on MRCP versus liver stiffness by MR elastography and vibration-controlled transient elastography. Eur. Radiol. 2020, 30, 3735–3747. [Google Scholar] [CrossRef]
- Corpechot, C.; Gaouar, F.; El Naggar, A.; Kemgang, A.; Wendum, D.; Poupon, R.; Carrat, F.; Chazouillères, O. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 2014, 146, 970–979. [Google Scholar] [CrossRef]
- Manns, M.P.; Lohse, A.W.; Vergani, D. Autoimmune hepatitis—Update 2015. J. Hepatol. 2015, 62, S100–S111. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yang, F.; Miao, Q.; Krawitt, E.L.; Gershwin, M.E.; Ma, X. The clinical phenotypes of autoimmune hepatitis: A comprehensive review. J. Autoimmun. 2016, 66, 98–107. [Google Scholar] [CrossRef]
- Lohse, A.W.; Mieli-Vergani, G. Autoimmune hepatitis. J. Hepatol. 2011, 55, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Sheng, L.; Bao, H.; Chen, X.; Guo, C.; Li, H.; Ma, X.; Qiu, D.; Hua, J. Evaluation of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis. J. Gastroenterol. Hepatol. 2017, 32, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Ehlken, H.; Sebode, M.; Peiseler, M.; Krech, T.; Zenouzi, R.; von Felden, J.; Weiler-Normann, C.; Schramm, C.; Lohse, A.W. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J. Hepatol. 2018, 68, 754–763. [Google Scholar] [CrossRef]
- Blanc, J.F.; Bioulac-Sage, P.; Balabaud, C.; Desmoulière, A. Investigation of liver fibrosis in clinical practice. Hepatol. Res. 2005, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Joshita, S.; Yamashita, Y.; Sugiura, A.; Uehara, T.; Usami, Y.; Yamazaki, T.; Fujimori, N.; Matsumoto, A.; Tanaka, E.; Umemura, T. Clinical utility of FibroScan as a non-invasive diagnostic test for primary biliary cholangitis. J. Gastroenterol. Hepatol. 2020, 35, 1208–1214. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Osman, K.T.; Maselli, D.B.; Idilman, I.S.; Rowan, D.J.; Viehman, J.K.; Harmsen, W.S.; Harnois, D.M.; Carey, E.J.; Gossard, A.A.; LaRusso, N.F.; et al. Liver Stiffness Measured by Either Magnetic Resonance or Transient Elastography Is Associated With Liver Fibrosis and Is an Independent Predictor of Outcomes Among Patients With Primary Biliary Cholangitis. J. Clin. Gastroenterol. 2021, 55, 449–457. [Google Scholar] [CrossRef]
- Corpechot, C.; Carrat, F.; Gaouar, F.; Chau, F.; Hirschfield, G.; Gulamhusein, A.; Montano-Loza, A.J.; Lytvyak, E.; Schramm, C.; Pares, A.; et al. Global & ERN Rare-Liver PBC Study Groups. Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis. J. Hepatol. 2022, 77, 1545–1553. [Google Scholar] [CrossRef]
- Corpechot, C.; Carrat, F.; Poujol-Robert, A.; Gaouar, F.; Wendum, D.; Chazouillères, O.; Poupon, R. Non-invasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012, 56, 198–208. [Google Scholar] [CrossRef]
- Cristoferi, L.; Calvaruso, V.; Overi, D.; Viganò, M.; Rigamonti, C.; Degasperi, E.; Cardinale, V.; Labanca, S.; Zucchini, N.; Fichera, A.; et al. Accuracy of Transient Elastography in Assessing Fibrosis at Diagnosis in Naïve Patients With Primary Biliary Cholangitis: A Dual Cut-Off Approach. Hepatology 2021, 74, 1496–1508. [Google Scholar] [CrossRef]
- Åberg, F. Quality of life after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101684. [Google Scholar] [CrossRef]
- Valente, G.; Rinaldi, L.; Sgambato, M.; Piai, G. Conversion from twice-daily to once-daily tacrolimus in stable liver transplant patients: Effectiveness in a real-world setting. Transpl. Proc. 2013, 45, 1273–1275. [Google Scholar] [CrossRef]
- Rompianesi, G.; Ravikumar, R.; Jose, S.; Allison, M.; Athale, A.; Creamer, F.; Gunson, B.; Manas, D.; Monaco, A.; Mirza, D.; et al. Incidence and outcome of colorectal cancer in liver transplant recipients: A national, multicentre analysis on 8115 patients. Liver Int. 2019, 39, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Akdur, A.; Kırnap, M.; Yıldırım, S.; Altundağ, Ö.; Moray, G.; Haberal, M. Posttransplant malignancies in liver transplant recipients. Exp. Clin. Transpl. 2014, 12, 162–165. [Google Scholar]
- Lee, K.F.; Tsai, Y.T.; Lin, C.Y.; Hsieh, C.B.; Wu, S.T.; Ke, H.Y.; Lin, Y.C.; Lin, F.Y.; Lee, W.H.; Tsai, C.S. Cancer Incidence among Heart, Kidney, and Liver Transplant Recipients in Taiwan. PLoS ONE 2016, 11, e0155602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Non-alcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Welker, M.W.; Zeuzem, S. Pre- and Post-Transplant Antiviral Therapy (HBV, HCV). Visc. Med. 2016, 32, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonardo, A.; Mantovani, A.; Petta, S.; Carraro, A.; Byrne, C.D.; Targher, G. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation. Nat. Rev. Endocrinol. 2022, 18, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Sensi, B.; Manzia, T.M.; Tisone, G.; Grassi, G.; Signorello, A.; Milana, M.; Lenci, I.; Baiocchi, L. Chronic rejection after liver transplantation: Opening the Pandora’s box. World J. Gastroenterol. 2021, 27, 7771–7783. [Google Scholar] [CrossRef]
- Brookmeyer, C.E.; Bhatt, S.; Fishman, E.K.; Sheth, S. Multimodality Imaging after Liver Transplant: Top 10 Important Complications. Radiographics 2022, 42, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.C.; Mittal, R.; Schiano, T.D. A review of the use of transient elastography in the assessment of fibrosis and steatosis in the post-liver transplant patient. Clin. Transpl. 2019, 33, e13700. [Google Scholar] [CrossRef]
- Mancia, C.; Loustaud-Ratti, V.; Carrier, P.; Naudet, F.; Bellissant, E.; Labrousse, F.; Pichon, N. Controlled attenuation parameter and liver stiffness measurements for steatosis assessment in the liver transplant of brain dead donors. Transplation 2015, 99, 1619–1624. [Google Scholar] [CrossRef]
- Kim, J.M.; Ha, S.Y.; Joh, J.W.; Sinn, D.H.; Jeong, W.K.; Choi, G.S.; Gwak, G.Y.; Kwon, C.H.D.; Kim, Y.K.; Paik, Y.H.; et al. Predicting hepatic steatosis in living liver donors via non-invasive methods. Medicine 2016, 95, e2718. [Google Scholar] [CrossRef]
- Hong, Y.M.; Yoon, K.T.; Cho, M.; Chu, C.W.; Rhu, J.H.; Yang, K.H.; Lee, J.W. Clinical usefulness of controlled attenuation parameter to screen hepatic steatosis for potential donor of living donor liver transplant. Eur. J. Gastroenterol. Hepatol. 2017, 29, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Kuo, F.Y.; Lin, C.C.; Chen, C.L.; Chang, K.C.; Tsai, M.C.; Hu, T.H. Predicting hepatic steatosis in living liver donors via controlled attenuation parameter. Transpl. Proc. 2018, 50, 3533–3538. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.; Castro-Narro, G.; Garcia-Juarez, I.; Benítez, C.; Ruiz, P.; Sastre, L.; Colmenero, J.; Miquel, R.; Sánchez-Fueyo, A.; Forns, X.; et al. Usefulness of liver stiffness measurement during acute cellular rejection in liver transplantation. Liver Transpl. 2016, 22, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigamonti, C.; Fraquelli, M.; Bastiampillai, A.J.; Caccamo, L.; Reggiani, P.; Rossi, G.; Colombo, M.; Donato, M.F. Transient elastography identifies liver recipients with nonviral graft disease after transplantation: A guide for liver biopsy. Liver Transpl. 2012, 18, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Nacif, L.S.; Gomes, C.D.C.; Mischiatti, M.N.; Kim, V.; Paranaguá-Vezozzo, D.; Reinoso, G.L.; Carrilho, F.J.; D’Albuquerque, L.C. Transient Elastography in Acute Cellular Rejection Following Liver Transplantation: Systematic Review. Transpl. Proc. 2018, 50, 772–775. [Google Scholar] [CrossRef]
- Bhat, M.; Tazari, M.; Sebastiani, G. Performance of transient elastog- raphy and serum fibrosis biomarkers for non-invasive evaluation of recurrent fibrosis after liver transplantation: A meta-analysis. PLoS ONE 2017, 12, e0185192. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, L.; Valente, G.; Piai, G. Serial Liver Stiffness Measurements and Monitoring of Liver-Transplanted Patients in a Real-Life Clinical Practice. Hepat. Mon. 2016, 16, e41162. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.S.; Idowu, M.O.; Stromberg, K.; Sima, A.; Lee, E.; Patel, S.; Ghaus, S.; Driscoll, C.; Sterling, R.K.; John, B.; et al. Diagnostic Performance of Vibration-Controlled Transient Elastography in Liver Transplant Recipients. Clin. Gastroenterol. Hepatol. 2021, 19, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, C.; Donato, M.F.; Fraquelli, M.; Agnelli, F.; Ronchi, G.; Casazza, G.; Rossi, G.; Colombo, M. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut 2008, 57, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.; Lens, S.; Gambato, M.; Carrión, J.A.; Mariño, Z.; Londoño, M.C.; Miquel, R.; Bosch, J.; Navasa, M.; Forns, X. Liver stiffness 1 years after transplantation predicts clinical outcomes in patients with recurrence hepatitis C. Am. J. Transpl. 2014, 14, 375–383. [Google Scholar] [CrossRef]
- Mauro, E.; Crespo, G.; Montironi, C.; Londoño, M.C.; Hernández-Gea, V.; Ruiz, P.; Sastre, L.; Lombardo, J.; Mariño, Z.; Díaz, A.; et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology 2018, 67, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Castellaneta, A.; Rendina, M.; Carparelli, S.; Leandro, G.; Di Leo, A. Systematic review with meta-analysis: De novo non-alcoholic fatty liver disease in liver-transplanted patients. Aliment. Pharmacol. Ther. 2018, 47, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.M.; Devera, M.E.; Fontes, P.; Shaikh, O.; Sasatomi, E.; Ahmad, J. Recurrent disease following liver transplantation for non-alcoholic steatohepatitis cirrhosis. Liver Transpl. 2009, 15, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, V.; Mindikoglu, A.L.; Nudo, C.G.; Schiff, E.R.; Tzakis, A.; Regev, A. Outcomes of liver transplantation in patients with cirrhosis due to non-alcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009, 15, 1814–1820. [Google Scholar] [CrossRef]
- Chayanupatkul, M.; Dasani, D.B.; Sogaard, K.; Schiano, T.D. The Utility of Assessing Liver Allograft Fibrosis and Steatosis Post-Liver Transplantation Using Transient Elastography With Controlled Attenuation Parameter. Transpl. Proc. 2021, 53, 159–165. [Google Scholar] [CrossRef]
- Cholongitas, E.; Tsochatzis, E.; Goulis, J.; Burroughs, A.K. Non-invasive tests for evaluation of fibrosis in HCV recurrence after liver trans- plantation: A systematic review. Transpl. Int. 2010, 23, 861–870. [Google Scholar] [CrossRef]
- Rozas, J.S.; González, K.; Estay, C.; Cattaneo, M.; Urzúa, A.; Roblero, J.P.; Sandoval, A.; Poniachik, J. Concordance between ecography and the continuous attenuation parameter (cap) by transient elastography for the diagnosis of liver steatosis. Ann. Hepatol. 2021, 24 (Suppl. S1), 100422. [Google Scholar] [CrossRef]
- Yoshizawa, E.; Yamada, A. MRI-derived proton density fat fraction. J. Med. Ultrason. 2021, 48, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with non-alcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
| Authors | Country | Type of Study | All Patients | HCV Patients | HBV Patients | Significant Fibrosis (≥f2) | Significant Fibrosis in HCV Patients | Significant Fibrosis in HBV Patients | p Value | Liver Stiffness | % Sensitivity | % Specificity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferraioli et al. [2] | Italy | Cross-sectional | 246 | 195 | 41 | 117 | 97 | 11 | 0.004 | >9 kPa | 80 | 85 |
| Friedrich-Rust et al. [3] | Germany | Meta-analysis | 518 | 380 | 51 | 312 | 222 | 27 | <0.0001 | >7.65 kPa | 79 | 85 |
| Cardoso et al. [24] | France | Cross-sectional | 565 | 363 | 202 | 282 | 197 | 85 | <0.001 | >7.2 kPa | 74 | 88 |
| Foucher et al. [26] | France | Prospective | 711 | 398 | 43 | 243 | - | - | <0.0001 | >7.2 kPa | 64 | 85 |
| Chen et al. [36] | China | Prospective | 375 | - | 291 | 231 | - | 231 | <0.05 | >9.8 kPa | 82 | 94.5 |
| Poynard et al. [30] | France | Prospective | 1547 | 28 | 1433 | - | - | 206 | <0.0001 | >9.5 kPa | 84 | 90 |
| Castéra et al. [34] | France | Longitudinal | 412 | 7 | 201 | - | - | 60 | <0.0001 | >7.2 kPa | 87 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, L.; Giorgione, C.; Mormone, A.; Esposito, F.; Rinaldi, M.; Berretta, M.; Marfella, R.; Romano, C. Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review. Viruses 2023, 15, 1730. https://doi.org/10.3390/v15081730
Rinaldi L, Giorgione C, Mormone A, Esposito F, Rinaldi M, Berretta M, Marfella R, Romano C. Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review. Viruses. 2023; 15(8):1730. https://doi.org/10.3390/v15081730
Chicago/Turabian StyleRinaldi, Luca, Chiara Giorgione, Andrea Mormone, Francesca Esposito, Michele Rinaldi, Massimiliano Berretta, Raffaele Marfella, and Ciro Romano. 2023. "Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review" Viruses 15, no. 8: 1730. https://doi.org/10.3390/v15081730
APA StyleRinaldi, L., Giorgione, C., Mormone, A., Esposito, F., Rinaldi, M., Berretta, M., Marfella, R., & Romano, C. (2023). Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review. Viruses, 15(8), 1730. https://doi.org/10.3390/v15081730








