Identification of the p34 Protein of African Swine Fever Virus as a Novel Viral Antigen with Protection Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Sequence Analysis
2.3. Construction of the Prokaryotic and Eukaryotic Plasmids Expressing the ASFV p34 Protein
2.4. Expression and Purification of the Recombinant p34 Protein
2.5. Antigenic Analysis of p34
2.6. The Cell-Mediated Immune Response Induced by p34
2.7. Preparation of Anti-p34 Antibodies in Mice
2.8. Generation of a Recombinant HCLV Expressing p34
2.9. Animal Immunization Experiment
2.10. Detection of the Serum Anti-p34 Antibodies in Rabbits
2.11. ELIspot Assay
2.12. Serum Neutralization Test
2.13. Screen of the T-Cell Epitopes on p34
2.14. Multiple Sequence Analysis of the Identified Epitope
2.15. In Silico Validation of the Candidate Epitopes
2.16. Statistical Analysis
3. Results
3.1. The p34 Protein Is a Novel Antigenic Protein of ASFV
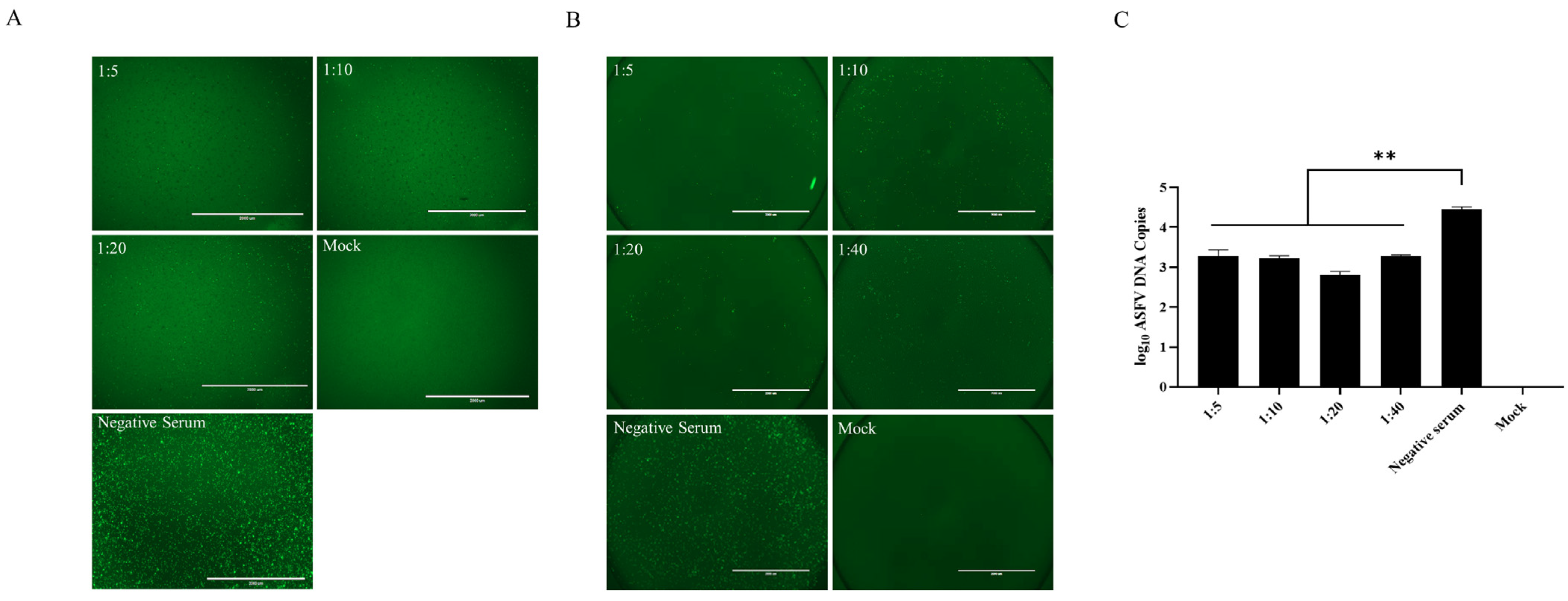
3.2. Anti-p34 Antibodies can Significantly Inhibit ASFV Replication
3.3. p34 Is Able to Activate a Cell-Mediated Immune Response
3.4. A T-Cell Epitope Is Present on the p34 Protein
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez, V.J.; Pfeiffer, D. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.L.; Vosloo, W.; Jori, F.; Bastos, A.D. African swine fever virus eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef] [PubMed]
- Zakaryan, H.; Karalova, E.; Voskanyan, H.; Terpogossyan, Z.; Nersisyan, N.; Hakobyan, A.; Saroyan, D.; Karalyan, Z. Evaluation of hemostaseological status of pigs experimentally infected with African swine fever virus. Vet. Microbiol. 2014, 174, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Rodriguez, A.; Feliziani, F.; Rolesu, S.; Dela, T.A. Spatio-temporal analysis of African swine fever in Sardinia (2012–2014): Trends in domestic pigs and wild boar. Transbound. Emerg. Dis. 2017, 64, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Waterson, A.; Plowright, W. The morphological characteristics of African swine fever virus and its resemblance to tipula iridescent virus. Arch. Gesamte Virusforsch. 1967, 20, 392–396. [Google Scholar] [CrossRef]
- Kuno, G.; Mackenzie, J.; Junglen, S.; Hubálek, Z.; Plyusnin, A.; Gubler, D. Vertebrate reservoirs of Arboviruses: Myth, synonym of amplifier, or reality? Viruses 2017, 9, 185. [Google Scholar] [CrossRef]
- Chapman, D.A.; Tcherepanov, V.; Upton, C.; Dixon, L.K. Comparison of the genome sequences of non-pathogenic and pathogenic African swine fever virus isolates. J. Gen. Virol. 2008, 89, 397–408. [Google Scholar] [CrossRef]
- Portugal, R.; Coelho, J.; Hoper, D.; Little, N.S.; Smithson, C.; Upton, C.; Martins, C.; Leitao, A.; Keil, G.M. Related strains of African swine fever virus with different virulence: Genome comparison and analysis. J. Gen. Virol. 2015, 96, 408–419. [Google Scholar] [CrossRef]
- Hernáez, B.; Guerra, M.; Salas, M.L.; Andrés, G. African swine fever virus undergoes outer envelope disruption, capsid disassembly and inner envelope fusion before core release from multivesicular endosomes. PLoS Pathog. 2016, 12, e1005595. [Google Scholar] [CrossRef]
- Cuesta, M.A.; Galindo, I.; Hernáez, B.; Quetglas, J.I.; Dalmau, I.; Alonso, C. Endosomal maturation, Rab7 GTPase and phosphoinositides in African swine fever virus entry. PLoS ONE 2012, 7, e48853. [Google Scholar]
- Valdeira, M.L.; Bernardes, C.; Cruz, B.; Geraldes, A. Entry of African swine fever virus into Vero cells and uncoating. Vet. Microbiol. 1998, 60, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.L.; Andrés, G. African swine fever virus morphogenesis. Virus Res. 2013, 173, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Spinard, E.; Dinhobl, M.; Tesler, N.; Birtley, H.; Signore, A.V.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. A re-evaluation of African swine fever genotypes based on p72 sequences reveals the existence of only six distinct p72 groups. Viruses 2023, 15, 2246. [Google Scholar] [CrossRef]
- Zhu, J.; Ramanathan, P.; Bishop, E.A.; Gladue, D.P.; Borca, M.V. Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages. PLoS ONE 2019, 14, e0223955. [Google Scholar] [CrossRef]
- Correia, S.; Ventura, S.; Parkhouse, R.M. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res. 2013, 173, 87–100. [Google Scholar] [CrossRef]
- Hubner, A.; Petersen, B.; Keil, G.M.; Niemann, H.; Mettenleiter, T.C.; Fuchs, W. Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci. Rep. 2018, 8, 1449. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Ghochikyan, A.; Petrushina, I.; Davtyan, H.; Hovakimyan, A.; Saing, T.; Davtyan, A.; Cribbs, D.H.; Agadjanyan, M.G. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: Feasibility study. Neurosci. Lett. 2014, 560, 86–91. [Google Scholar] [CrossRef]
- Andrés, G.; Simón, M.C.; Viñuela, E. Assembly of African swine fever virus: Role of polyprotein pp220. J. Virol. 1997, 71, 2331–2341. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Zhou, Y. Advances and challenges in African swine fever virus vaccine development. J. Immunol. 2019, 202, 2253–2260. [Google Scholar]
- Zajac, M.D.; Sangewar, N.; Lokhandwala, S.; Bray, J.; Sang, H.; McCall, J.; Bishop, R.P.; Waghela, S.D.; Kumar, R.; Kim, T.; et al. Adenovirus-vectored African swine fever virus pp220 induces robust antibody, IFN-γ, and CTL responses in pigs. Front. Vet. Sci. 2022, 9, 921481. [Google Scholar] [CrossRef]
- Eulálio, A.; Nunes, C.I.; Carvalho, A.L.; Faro, C.; Citovsky, V.; Simões, S.; Pedroso, M.C. Two African swine fever virus proteins derived from a common precursor exhibit different nucleocytoplasmic transport activities. J. Virol. 2004, 78, 9731–9739. [Google Scholar] [CrossRef] [PubMed]
- Eulálio, A.; Nunes, C.I.; Salas, J.; Salas, M.L.; Simões, S.; Pedroso, M.C. African swine fever virus p37 structural protein is localized in nuclear foci containing the viral DNA at early post-infection times. Virus Res. 2007, 130, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Aicher, S.M.; Monaghan, P.; Netherton, C.L.; Hawes, P.C. Unpicking the secrets of African swine fever viral replication sites. Viruses 2021, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Shimmon, G.L.; Hui, Y.K.; Wileman, T.E.; Netherton, C.L. Autophagy impairment by African swine fever virus. J. Gen. Virol. 2021, 102, 1637. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, E.V.; Villinger, F.; Gerstner, D.J.; Whyard, T.C.; Knudsen, R.C. Effect of macrophage-specific colony-stimulating factor (CSF-1) on swine monocyte/ macrophage susceptibility to in vitro infection by African swine fever virus. Vet. Microbiol. 1990, 25, 153–176. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef]
- Arias, M.; Dela, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.; et al. Approaches and perspectives for development of African swine fever virus vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef]
- Revilla, Y.; Pérez, N.D.; Richt, J.A. African swine fever virus biology and vaccine approaches. Adv. Virus Res. 2018, 100, 41–74. [Google Scholar] [PubMed]
- Lacasta, A.; Ballester, M.; Monteagudo, P.L.; Rodríguez, J.M.; Salas, M.L.; Accensi, F.; Pina, P.S.; Bensaid, A.; Argilaguet, J.; López, S.S.; et al. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J. Virol. 2014, 88, 13322–13332. [Google Scholar] [CrossRef] [PubMed]
- Zakaryan, H.; Revilla, Y. African swine fever virus: Current state and future perspectives in vaccine and antiviral research. Vet. Microbiol. 2016, 185, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Onisk, D.V.; Borca, M.V.; Kutish, G.; Kramer, E.; Irusta, P.; Rock, D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 1994, 198, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.M.; Galindo, I.; Alonso, C. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013, 173, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Richt, J.A. Subunit vaccine approaches for African swine fever virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Han, Y.; Pan, L.; Yang, J.; Sun, M.; Zhou, P.; Sun, Y.; Bi, Y.; Qiu, H.J. Adaptation of African swine fever virus to HEK293T cells. Transbound. Emerg. Dis. 2021, 68, 2853–2866. [Google Scholar] [CrossRef]
- Walczak, M.; Juszkiewicz, M.; Szymankiewicz, K.; Szczotka, A.; Woźniakowski, G. ASF-survivors’ sera do not inhibit African swine fever virus replication in vitro. J. Vet. Res. 2022, 25, 21–27. [Google Scholar] [CrossRef]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C.; et al. Identification and immunogenicity of African swine fever virus antigens. Front. Immunol. 2019, 10, 1318. [Google Scholar] [CrossRef]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Xu, W.; Delhon, G.; Kolbasov, D.; Rock, D.L. Identification of T-cell epitopes in African swine fever virus CD2v and C-type lectin proteins. J. Gen. Virol. 2019, 100, 259–265. [Google Scholar] [CrossRef]
- Goatley, L.C.; Reis, A.L.; Portugal, R.; Goldswain, H.; Shimmon, G.L.; Hargreaves, Z.; Ho, C.S.; Montoya, M.; Sánchez, P.J.; Taylor, G.; et al. A pool of eight virally vectored African swine fever antigens protect pigs against fatal disease. Vaccines 2020, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Argilaguet, J.M.; Pérez, M.E.; López, S.; Goethe, M.; Escribano, J.M.; Giesow, K.; Keil, G.M.; Rodríguez, F. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antiviral Res. 2013, 98, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, F.E.; Sánchez, J.M.; Kosowska, A.; Rivera, B.; Mayoral-Alegre, F.; Rodríguez, B.A.; Yao, J.; Bray, J.; Lokhandwala, S.; Mwangi, W.; et al. Adenovirus-vectored African swine fever virus antigens cocktail is not protective against virulent Arm07 isolate in Eurasian wild boar. Pathogens 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequences (5′-3′) |
|---|---|
| pET-p34-F | GGTGGACAGCAAATGGGTCGCGGATCCATGGACAAAAATCCTGTACAACAC |
| pET-p34-R | GCAAGCTTGTCGACGGAGCTCGAATTCTTAATCGCCCCCCTTTTTGGCACAAC |
| pShuttle-p34-F | ACCGGCGTGCACTCCGTCGACATGGACAAAAATCCTGTACAAC |
| pShuttle-p34-R | GGATATCTTATCTAGAAGCTTTTAATCGCCCCCCTTTTTGGCAC |
| pHCLV-p34-1F | GGAACCGGTGTACGATGCCACGGGGAG |
| pHCLV-p34-1R | GTGTTGTACAGGATTTTTGTCCATCCCACTTGCGCCATCATCGGA |
| pHCLV-p34-2F | TCCGATGATGGCGCAAGTGGGATGGACAAAAATCCTGTACAACAC |
| pHCLV-p34-2R | CAGCAGCGAAAAGTTTGTGGCATCGCCCCCCTTTTTGGCACA |
| pHCLV-p34-3F | TGTGCCAAAAAGGGGGGCGATGCCACAAACTTTTCGCTGCTG |
| pHCLV-p34-3R | CTGATGCATGCACCTTGACAGTCGTG |
| Groups | Viruses | Dose (Boost at Day 14 and 28) | Route | Number of Pigs | Fever Response | Anti-p34 Antibodies | Anti-E2 Antibodies |
|---|---|---|---|---|---|---|---|
| A | HCLV | 104 TCID50 | i.v. | 6 | + | - | 7 dpi |
| B | rHCLV-p34 | 104 TCID50 | i.v. | 6 | + | 7 dpi | 7 dpi |
| C | DMEM | 1 mL | i.v. | 4 | - | - | - |
| Epitopes Docked to SLA-1*0401 | ΔGbind (kJ/mol) | Kd (mol/L) | Antigen Source |
|---|---|---|---|
| IADAINQEF | −9.8 | 1.3 × 10−7 | ASFV p34 |
| NSDTVGWSW | −9.8 | 1.4 × 10−7 | Positive epitope |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Guan, X.; Wang, Q.; Wang, X.; Yang, X.; Li, S.; Zhao, X.-T.; Yuan, M.; Liu, X.; Qiu, H.-J.; et al. Identification of the p34 Protein of African Swine Fever Virus as a Novel Viral Antigen with Protection Potential. Viruses 2024, 16, 38. https://doi.org/10.3390/v16010038
Zhang X, Guan X, Wang Q, Wang X, Yang X, Li S, Zhao X-T, Yuan M, Liu X, Qiu H-J, et al. Identification of the p34 Protein of African Swine Fever Virus as a Novel Viral Antigen with Protection Potential. Viruses. 2024; 16(1):38. https://doi.org/10.3390/v16010038
Chicago/Turabian StyleZhang, Xin, Xiangyu Guan, Qiuxia Wang, Xiao Wang, Xiaoke Yang, Shuwen Li, Xiao-Tian Zhao, Mengqi Yuan, Xingyou Liu, Hua-Ji Qiu, and et al. 2024. "Identification of the p34 Protein of African Swine Fever Virus as a Novel Viral Antigen with Protection Potential" Viruses 16, no. 1: 38. https://doi.org/10.3390/v16010038
APA StyleZhang, X., Guan, X., Wang, Q., Wang, X., Yang, X., Li, S., Zhao, X.-T., Yuan, M., Liu, X., Qiu, H.-J., & Li, Y. (2024). Identification of the p34 Protein of African Swine Fever Virus as a Novel Viral Antigen with Protection Potential. Viruses, 16(1), 38. https://doi.org/10.3390/v16010038







