2023 International African Swine Fever Workshop: Critical Issues That Need to Be Addressed for ASF Control
Abstract
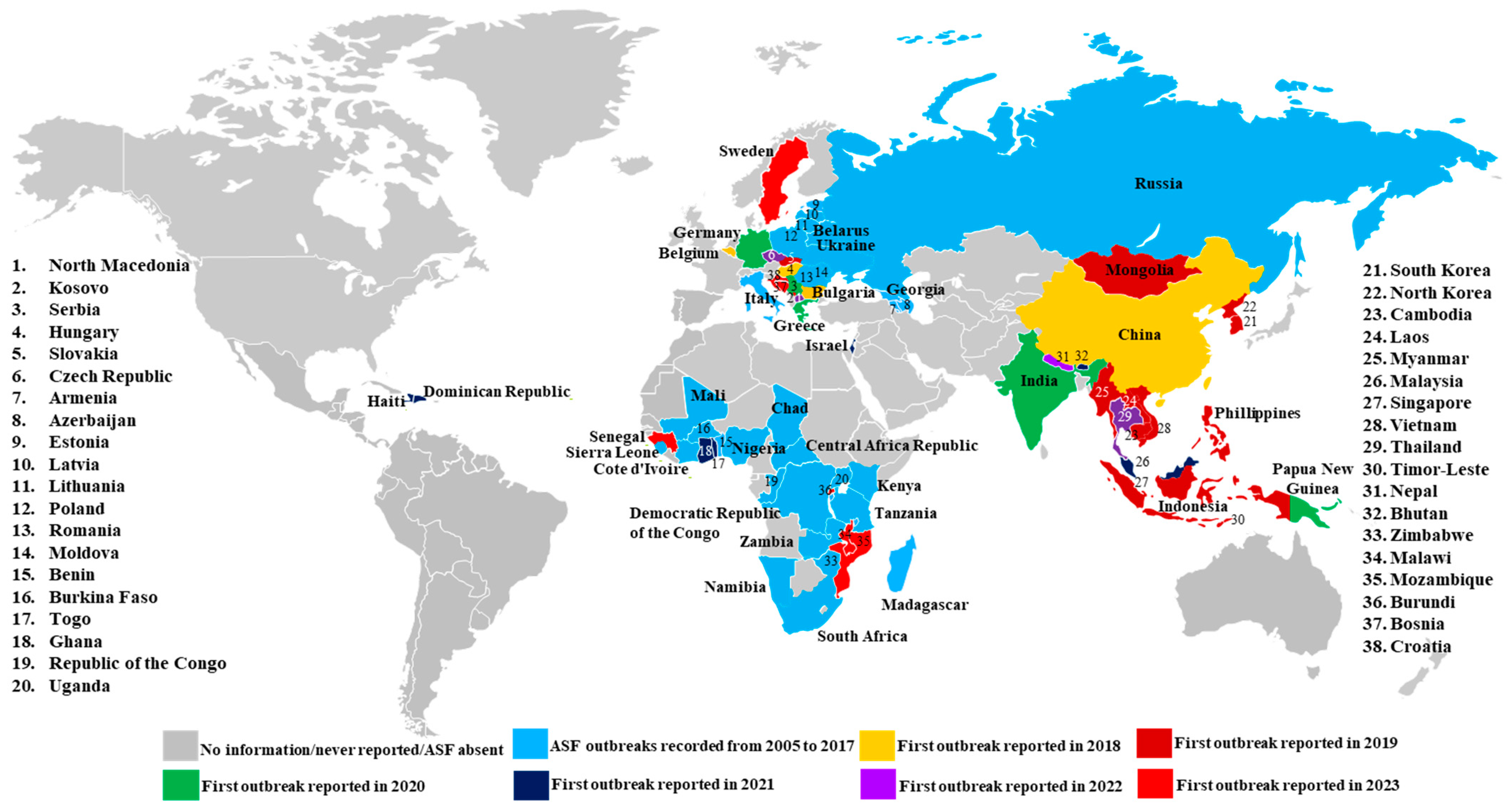
:1. Introduction
2. Workshop Sessions and Highlights
2.1. Keynote Lectures (Moderator: Jishu Shi)
- (i)
- More studies on ASFV proteins/complexes and ASFV–cell interactions are needed to understand ASFV replication and assembly further and find targets for antibodies and antivirals.
- (ii)
- For modified live attenuated vaccines (LAVs), a better understanding of protective immunity, further safety and efficacy testing, the ability to differentiate infected from vaccinated animals (DIVA), permanent cell lines for LAV production, and mathematical modeling and experimental testing to determine vaccine coverage is needed.
- (iii)
- For subunit vaccines, further research on antigens to refine pools required, mechanisms of protection, and testing additional vectors/delivery methods (mRNAs?) are needed.
- (iv)
- We should consider alternative strategies for ASF prevention and control, such as single replication cycle vaccines using helper cell lines and gene-edited resistant pigs.
- (v)
- Considering the rapid spread of the GI-GII recombinant ASFV in China (with possible spread to neighboring countries), comprehensive surveillance, rapid diagnostic tools, and specific control measures for the GI-GII recombinant ASFV are urgently needed.
2.2. Session #1: ASF Live Attenuated Vaccines (Moderator: Jishu Shi)
- (i)
- Safety, efficacy, and genetic stability of current candidate ASF LAVs require comprehensive clinical evaluations prior to country-wide field application.
- (ii)
- Current multi-gene deleted ASFV GII-based LAVs cannot protect pigs against challenge with the GI-GII recombinant ASFV strains circulating in China.
- (iii)
- Cross-protection from different ASFV genotypes (heterologous immunity) combined with a stable, permanent cell line for manufacturing LAVs, as well as the development of DIVA LAVs, are critical issues that need to be resolved.
- (iv)
- LAVs generated by passaging in unnatural hosts or cell lines have proven to be an effective means for preventing many human/animal viral diseases; the successful generation of ASF LAVs through cell passage by a joint effort of researchers at VNUA and KSU indicates that more attention should be given to ASF LAVs developed by cell passage.
- (v)
- A novel virus neutralization test (VNT) method that does not depend on porcine macrophage cell lines should be developed to evaluate the immune responses after vaccination.
- (vi)
- In addition to IFN-gamma ELISpot (enzyme-linked immunosorbent spot) assays, we should develop more assays to test the cellular immune responses for ASF antigens/vaccines, such as assays to measure the induction and specificity of ASFV-specific T-cells.
2.3. Session #2: ASF Subunit Vaccines and Mechanisms of Protective Immunity (Moderator: Dongming Zhao)
- (i)
- Antibodies are not thought to be 100% efficient in protecting pigs from ASFV challenge. Neutralizing antibodies are inconsistently detected. Mechanisms/correlates of protection are still unknown. A better understanding of the mechanisms of protection of LAVs may guide the development of subunit vaccines.
- (ii)
- Subunit vaccines can induce enhancement of ASF disease, most likely due to antibody-dependent enhancement (ADE) as reported for many macrophage-tropic pathogens.
- (iii)
- Host immune status contributes to ASF disease severity following infection with the attenuated Estonia 2014 strain [23]. Although infection with a virulent ASFV strain is lethal for both SPF (specific pathogen-free) and conventional farm pigs, SPF pigs show higher resilience to disease after inoculation with an attenuated (Estonia 2014) ASFV strain compared to farm pigs. This phenomenon may impact the safety of LAV.
- (iv)
- SPF pigs show complete resistance to ASFV Armenia 2008 challenge after recovery from Estonia 2014 infection. Protective immunity correlated with innate and adaptive immune responses in the blood, as shown by transcriptomic analyses. Studies of SPF pigs may help identify key correlates of protection when evaluating ASF vaccine candidates.
- (v)
- Active functional small molecules showed anti-ASFV activities and could protect pigs against virulent ASFV challenge [21]. However, studies on antiviral mechanisms, the structure of the molecules, antimicrobial resistance, and dose- and time-dependent effectiveness, i.e., a more comprehensive evaluation of these small molecules is needed.
2.4. Session #3: ASF Pathogenesis and Diagnostics (Moderator: Charaf Benarafa)
- (i)
- Loop-mediated isothermal amplification (LAMP) with high sensitivity and specificity to amplify efficiently ASFV DNA under isothermal conditions is a field friendly detection method for ASFV. This assay could be used in remote locations, can be adapted to be performed using minimal equipment and with minimally invasive samples. The use of LAMP and other point-of-care diagnostic tests could prove to be a useful alternative for the early detection of ASFV, lending itself to faster action, having an overall positive impact to support the ASF control. Official regulations and guidelines for the use of point-of-care diagnostic tests in surveillance and control programs for notifiable diseases for the WOAH need to be established.
- (ii)
- The mechanisms of ASFV immune evasion are not fully understood. More basic research studies are still needed. These include studies to determine how ASFV infection induces immune responses and modulates inflammatory responses and cell death. What are the genes/proteins related to ASFV virulence/protection?
- (iii)
- Targets for ASFV attenuation may include interferon (IFN) inhibitors, cell adhesion molecules (such as CD2v), and inhibitors of programmed cell death. Safe and efficacious ASF LAV strains will most likely have multiple genes deleted to obtain a balance between virus replication and induction of immunity.
2.5. Session #4: Epidemiology and ASF Control on Swine Farms (Moderator: Llilianne Ganges)
- (i)
- Currently, our strongest weapon against ASF is good/enhanced biosecurity. Certain ASF features, such as ASFV is sensitive to heat, detergents, and a wide range of disinfectants; ASFV only survives in the environment when protected by organic material; and aerosols are only effective over distances of a few meters in an enclosed space, clearly indicate that good biosecurity measures can protect pigs from ASFV infection. Confining pigs, restricting access to them, providing dedicated and thoroughly disinfected footwear, assuring that newly introduced pigs are from “safe” sources, and ensuring the safety of feed will prevent an ASFV incursion.
- (ii)
- Prevention and control of ASF needs to involve all actors in the pig value chain, including various stakeholders, animal health service providers, communities in which pigs are kept, and farmer-based surveillance for early detection and reporting.
- (iii)
- In ASF-endemic areas, farmers raising pigs free of ASF need to strictly implement biosecurity measures on their farms, use ASF vaccines if licensed and available, and use point-of-care ASFV antigen diagnostics.
- (iv)
- Human factors (human behavior) should always be taken into consideration in science-based recommendations/policies for ASF control and prevention since a significant part of ASFV spread is based on anthropogenic factors.
3. Critical Issues That Need to Be Addressed for ASF Control and Prevention in the Future
3.1. Know the Enemy through Supporting Basic and Innovative Research
3.2. Develop Science-Based ASF Outbreak Emergency Management Policies
3.3. Collaborative Approach
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. African Swine Fever. Available online: https://www.woah.org/en/disease/african-swine-fever/#index-2 (accessed on 10 October 2023).
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W. Review of African swine fever: Transmission, spread and control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African swine fever virus isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever (ASF): Five years around Europe. Vet. Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular characterization of African swine fever virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef]
- Mighell, E.; Ward, M.P. African Swine Fever spread across Asia, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 2722–2732. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Qi, W.; Yang, Y.; Liu, Z.; An, T.; Wu, X.; Chen, J. Prevention and Control Strategies of African Swine Fever and Progress on Pig Farm Repopulation in China. Viruses 2021, 13, 2552. [Google Scholar] [CrossRef]
- Le, V.P.; Jeong, D.G.; Yoon, S.W.; Kwon, H.M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Berends, J.; Bendita da Costa Jong, J.; Cooper, T.L.; Dizyee, K.; Morais, O.; Pereira, A.; Smith, D.; Rich, K.M. Investigating the Socio-Economic and Livelihoods Impacts of African Swine Fever in Timor-Leste: An Application of Spatial Group Model Building. Front. Vet. Sci. 2021, 8, 687708. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, R.P.; Hagerman, A.D.; Rich, K.M. An analysis of African Swine Fever consequences on rural economies and smallholder swine producers in Haiti. Front. Vet. Sci. 2022, 9, 960344. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Medina, E.; O’Donnell, V.; Silva, E.; Espinoza, N.; Velazquez-Salinas, L.; Moran, K.; Daite, D.A.; Barrette, R.; Faburay, B.; Holland, R.; et al. Experimental Infection of Domestic Pigs with an African Swine Fever Virus Field Strain Isolated in 2021 from the Dominican Republic. Viruses 2022, 14, 1090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Rathakrishnan, A.; Reis, A.L.; Petrovan, V.; Goatley, L.C.; Moffat, K.; Lui, Y.; Vuong, M.T.; Ikemizu, S.; Davis, S.J.; Dixon, L.K. A protective multiple gene-deleted African swine fever virus genotype II, Georgia 2007/1, expressing a modified non-haemadsorbing CD2v protein. Emerg. Microbes Infect. 2023, 12, 2265661. [Google Scholar] [CrossRef]
- Truong, Q.L.; Wang, L.; Nguyen, T.A.; Nguyen, H.T.; Tran, S.D.; Vu, A.T.; Le, A.D.; Nguyen, V.G.; Hoang, P.T.; Nguyen, Y.T.; et al. A Cell-Adapted Live-Attenuated Vaccine Candidate Protects Pigs against the Homologous Strain VNUA-ASFV-05L1, a Representative Strain of the Contemporary Pandemic African Swine Fever Virus. Viruses 2023, 15, 2089. [Google Scholar] [CrossRef]
- Sunwoo, S.Y.; Pérez-Núñez, D.; Morozov, I.; Sánchez, E.G.; Gaudreault, N.N.; Trujillo, J.D.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-Protein Vaccination Strategy Does Not Protect from Challenge with African Swine Fever Virus Armenia 2007 Strain. Vaccines 2019, 7, 12. [Google Scholar] [CrossRef]
- Lv, C.; Yang, J.; Zhao, L.; Zou, Z.; Kang, C.; Zhang, Q.; Wu, C.; Yang, L.; Cheng, C.; Zhao, Y.; et al. Bacillus subtilis partially inhibits African swine fever virus infection in vivo and in vitro based on its metabolites arctiin and genistein interfering with the function of viral topoisomerase II. J. Virol. 2023, 97, e0071923. [Google Scholar] [CrossRef]
- Schäfer, A.; Franzoni, G.; Netherton, C.L.; Hartmann, L.; Blome, S.; Blohm, U. Adaptive Cellular Immunity against African Swine Fever Virus Infections. Pathogens 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, E.; Mehinagic, K.; Wüthrich, T.; Hilty, M.; Posthaus, H.; Summerfield, A.; Ruggli, N.; Benarafa, C. The baseline immunological and hygienic status of pigs impact disease severity of African swine fever. PLoS Pathog. 2022, 18, e1010522. [Google Scholar] [CrossRef] [PubMed]
- Bohorquez, J.A.; Lanka, S.; Rosell, R.; Pérez-Simó, M.; Alberch, M.; Rodriguez, F.; Ganges, L.; Maddox, C.W. Efficient detection of African Swine Fever Virus using minimal equipment through a LAMP PCR method. Front Cell Infect Microbiol. 2023, 13, 1114772. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Zhou, S.; Li, S.; Liu, J.; Li, T.; Zhang, Z.; Zhang, X.; He, X.; Chen, W.; et al. Development of a new effective African swine fever virus vaccine candidate by deletion of the H240R and MGF505-7R genes results in protective immunity against the Eurasia strain. J. Virol. 2023, 97, e0070423. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Liu, H.; Liu, X.; Chen, W.; Li, J.; Zhao, D.; Wang, G.; Feng, C.; Zhang, Z.; Zhou, Q.; et al. African Swine Fever Virus H240R Protein Inhibits the Production of Type I Interferon through Disrupting the Oligomerization of STING. J. Virol. 2023, 97, e0057723. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.L.; Rathakrishnan, A.; Goulding, L.V.; Barber, C.; Goatley, L.C.; Dixon, L.K. Deletion of the gene for the African swine fever virus BCL-2 family member A179L increases virus uptake and apoptosis but decreases virus spread in macrophages and reduces virulence in pigs. J. Virol. 2023, 97, e0110623. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, L.; Wu, J.; Weng, W.; Wang, H.; Ye, M.; Qu, Y.; Hao, Y.; Zhang, Y.; Ge, X.; et al. Riding apoptotic bodies for cell-cell transmission by African swine fever virus. Proc. Natl. Acad. Sci. USA 2023, 120, e2309506120. [Google Scholar] [CrossRef]
- Penrith, M.-L.; Bastos, A.; Chenais, E. With or without a vaccine—Complementary and alternative approaches to managing African swine fever is resource-constrained smallholder settings. Vaccines 2021, 9, 116. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Ebata, A.; Penrith, M.-L.; Pfeiffer, D.U.; Price, C.; Ståhl, K.; Fischer, K. Exploring the hurdles that remain for control of African swine fever in smallholder farming settings. Transbound. Emerg. Dis. 2022, 69, e3370–e3378. [Google Scholar] [CrossRef]
- Chenais, E.; Sternberg-Lewerin, S.; Aliro, T.; Ståhl, K.; Fischer, K. Co-created community contracts support biosecurity changes in a region where African swine fever is endemic—Part I: The methodology. Prev. Vet. Med. 2023, 212, 105840. [Google Scholar] [CrossRef]
- Chenais, E.; Fischer, K.; Aliro, T.; Ståhl, K.; Sternberg-Lewerin, S. Co-created community contracts support biosecurity changes in a region where African swine fever is endemic—Part II: Implementation of biosecurity measures. Prev. Vet. Med. 2023, 214, 105902. [Google Scholar] [CrossRef] [PubMed]
- Ragland, D.; Pogranichniy, R.M.; Yurchenko, O.S.; Bashinskiy, V.V.; Gerilovych, A.P.; Brown, D. Assessment of biosecurity policies and practices for the control of African swine fever virus on Ukrainian pig farms. J. Vet. Med. Biotechnol. Biosaf. 2020, 6, 17–24. [Google Scholar] [CrossRef]
- Tzu, S. The Art of War; Capstone Publishing: Chichester, UK, 2010. [Google Scholar]
- Shi, J.; Wang, L.; McVey, D.S. Of pigs and men: The best-laid plans for prevention and control of swine fevers. Anim. Front. 2021, 11, 6–13. [Google Scholar] [CrossRef] [PubMed]

| Outbreaks | Cases | Losses * | |||
|---|---|---|---|---|---|
| Domestic Pigs | Wild Boar | Domestic Pigs | Wild Boar | Domestic Pigs | |
| Africa | 212 | 0 | 22,786 | 0 | 24,321 |
| Americas | 278 | 0 | 9957 | 0 | 18,857 |
| Asia | 918 | 1284 | 68,339 | 1962 | 388,035 |
| Europe | 3815 | 16,214 | 85,2500 | 26,716 | 1,076,951 |
| Oceania | 0 | 0 | 0 | 0 | 0 |
| Total | 5229 | 17,498 | 953,582 | 28,678 | 1,508,164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ganges, L.; Dixon, L.K.; Bu, Z.; Zhao, D.; Truong, Q.L.; Richt, J.A.; Jin, M.; Netherton, C.L.; Benarafa, C.; et al. 2023 International African Swine Fever Workshop: Critical Issues That Need to Be Addressed for ASF Control. Viruses 2024, 16, 4. https://doi.org/10.3390/v16010004
Wang L, Ganges L, Dixon LK, Bu Z, Zhao D, Truong QL, Richt JA, Jin M, Netherton CL, Benarafa C, et al. 2023 International African Swine Fever Workshop: Critical Issues That Need to Be Addressed for ASF Control. Viruses. 2024; 16(1):4. https://doi.org/10.3390/v16010004
Chicago/Turabian StyleWang, Lihua, Llilianne Ganges, Linda K. Dixon, Zhigao Bu, Dongming Zhao, Quang Lam Truong, Juergen A. Richt, Meilin Jin, Christopher L. Netherton, Charaf Benarafa, and et al. 2024. "2023 International African Swine Fever Workshop: Critical Issues That Need to Be Addressed for ASF Control" Viruses 16, no. 1: 4. https://doi.org/10.3390/v16010004







