EcoHIV-Infected Mice Show No Signs of Platelet Activation
Abstract
:1. Introduction
2. Methods
2.1. Preparation of EcoHIV Virions
2.2. EcoHIV Infection of Mice
2.3. Platelet Preparation
2.4. Flow Cytometry
2.5. RNA Isolation and RT-qPCR
2.6. Immunohistochemistry
2.7. Other Methods
2.8. Statistical Analysis
3. Results
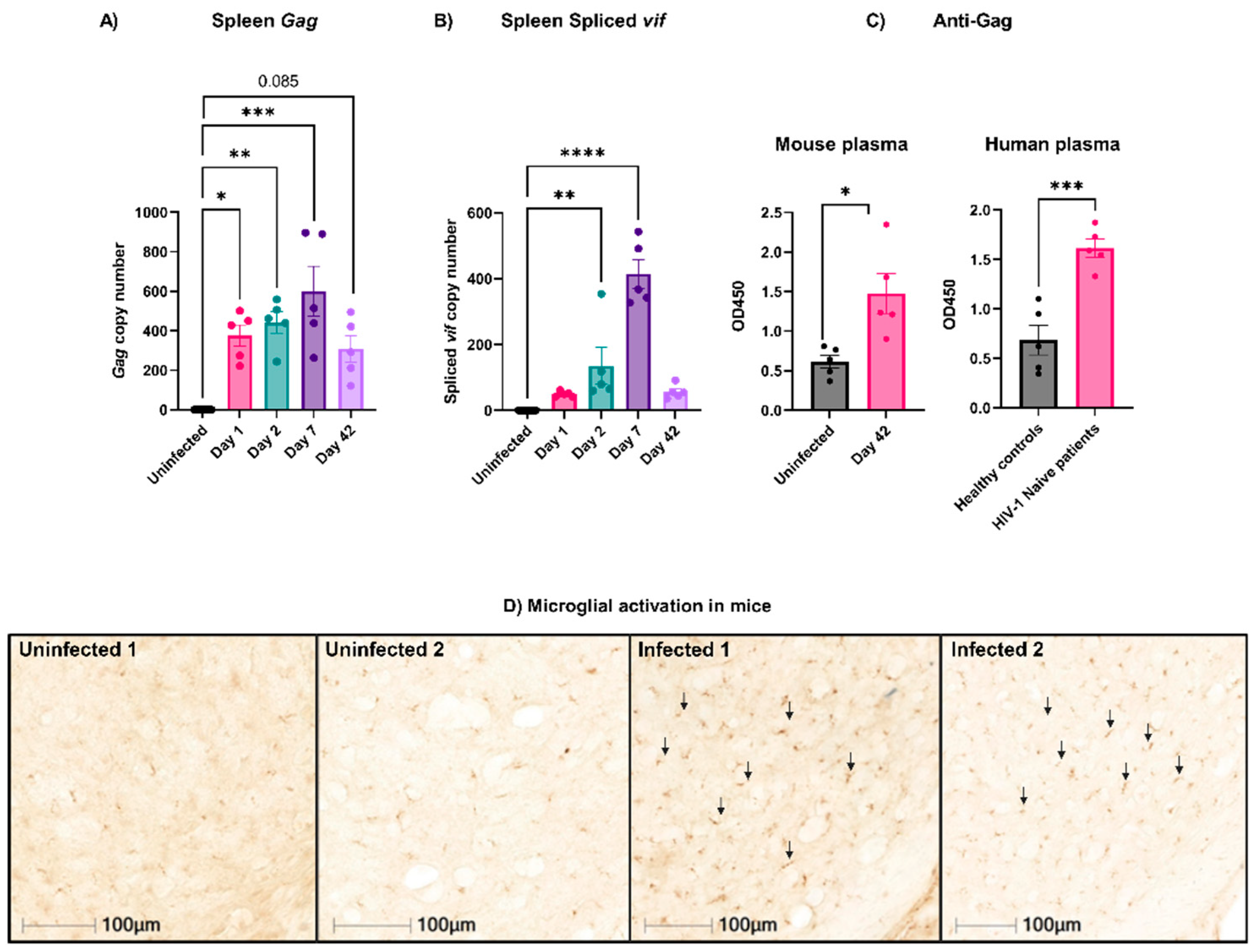
3.1. Active EcoHIV Was Detected in Mouse Splenocytes Weeks after Infection
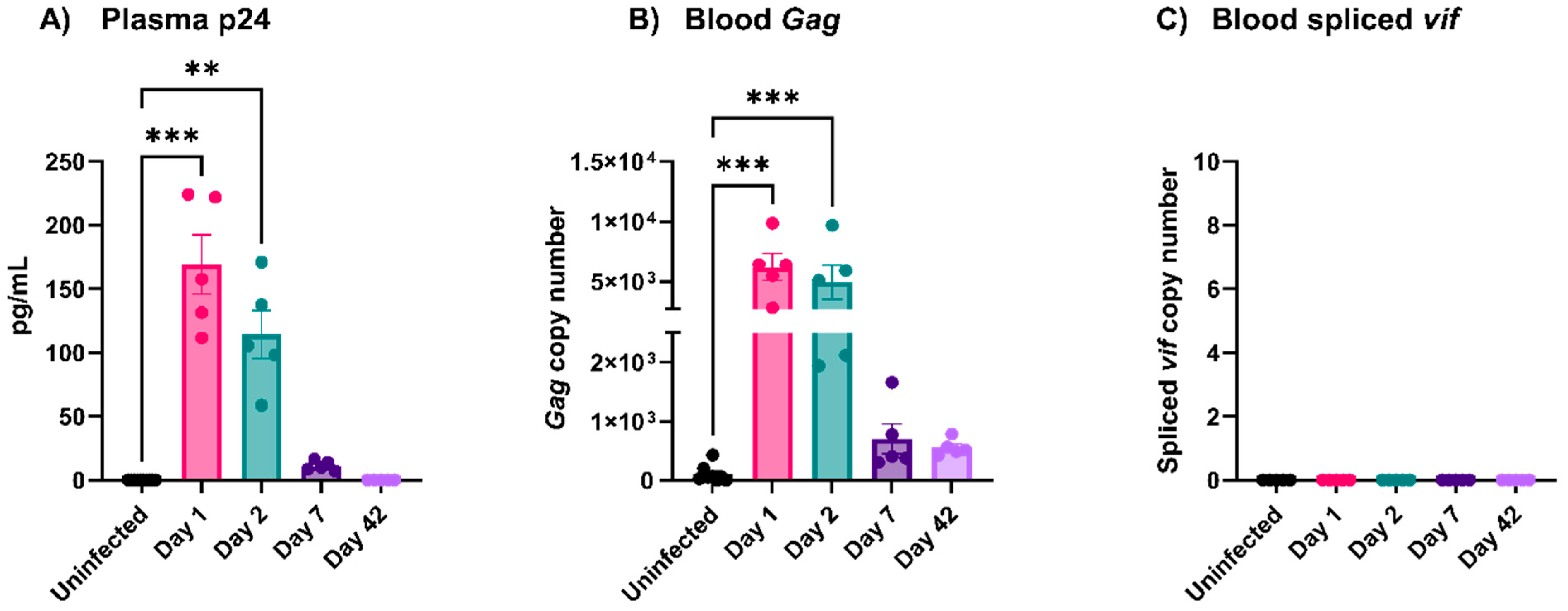
3.2. EcoHIV Was Rapidly Cleared from Circulation
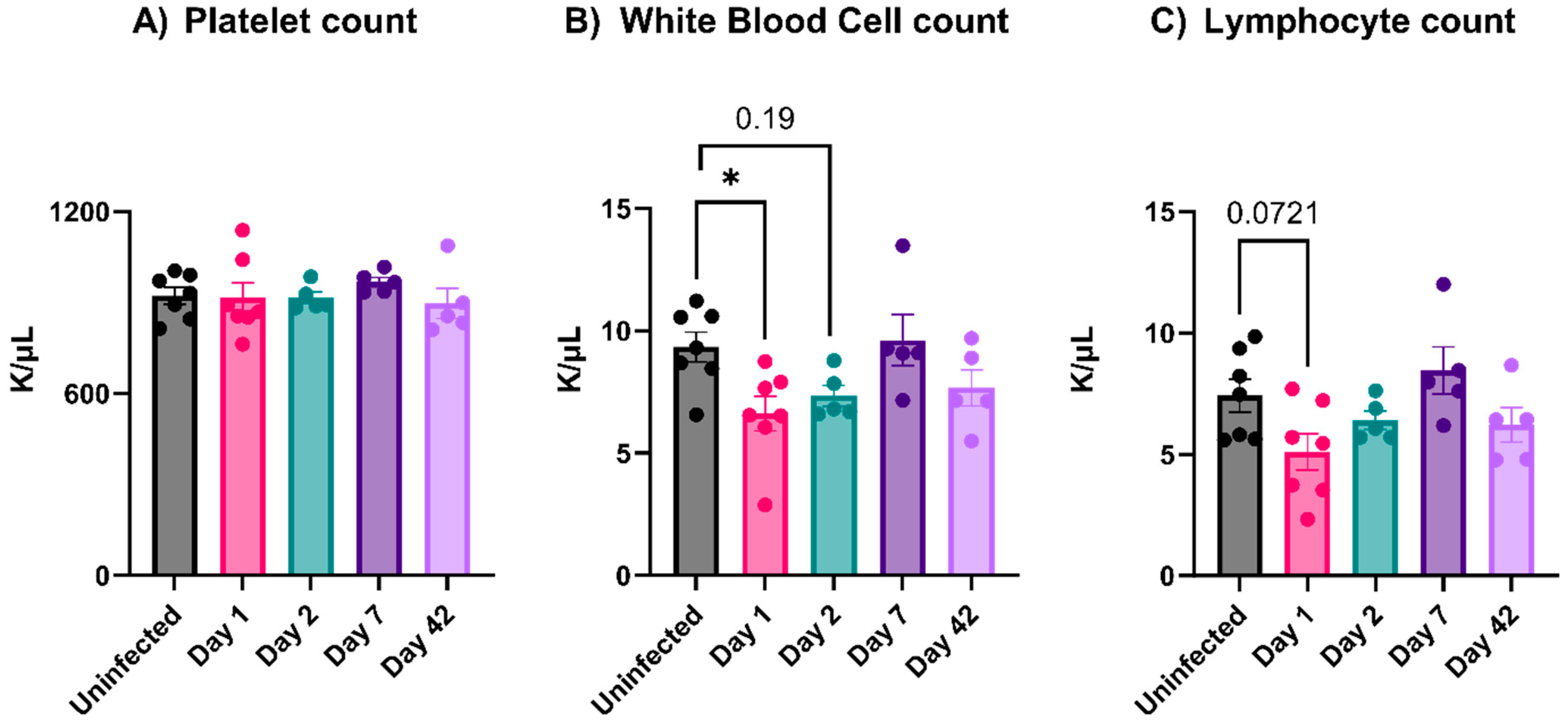
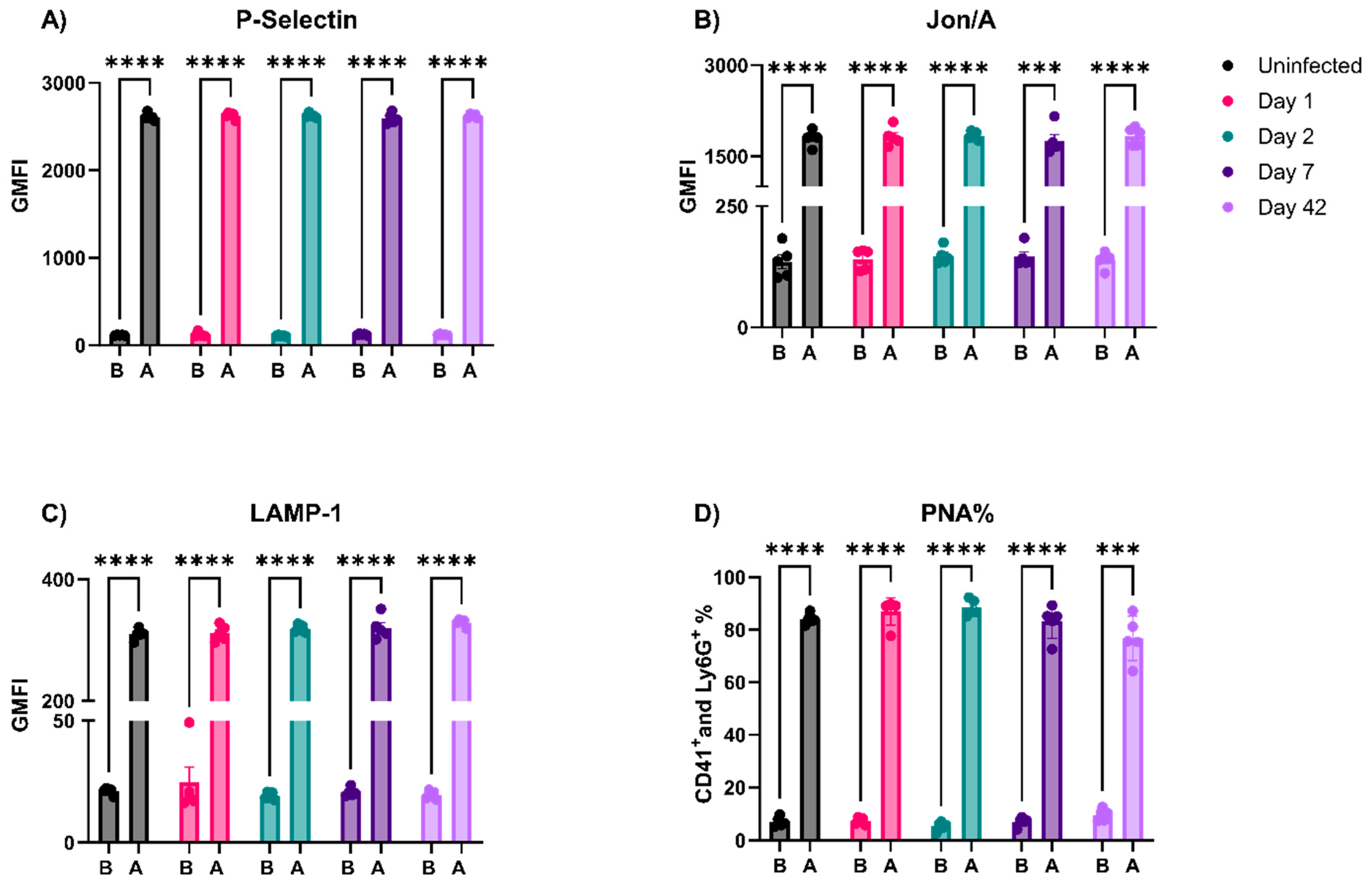
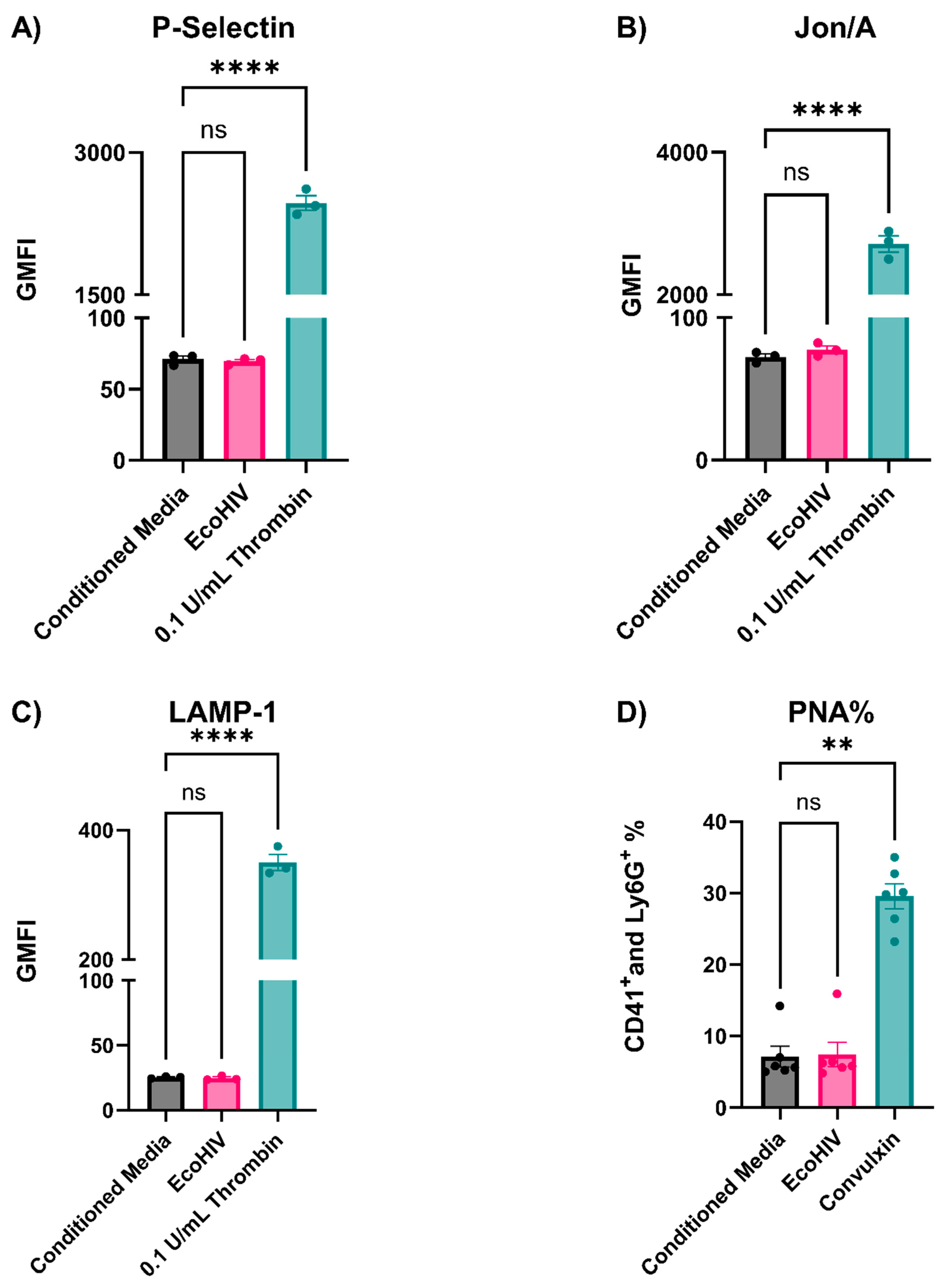
3.3. EcoHIV-Infected Mice Did Not Have Thrombocytopenia nor Show Signs of Platelet Activation
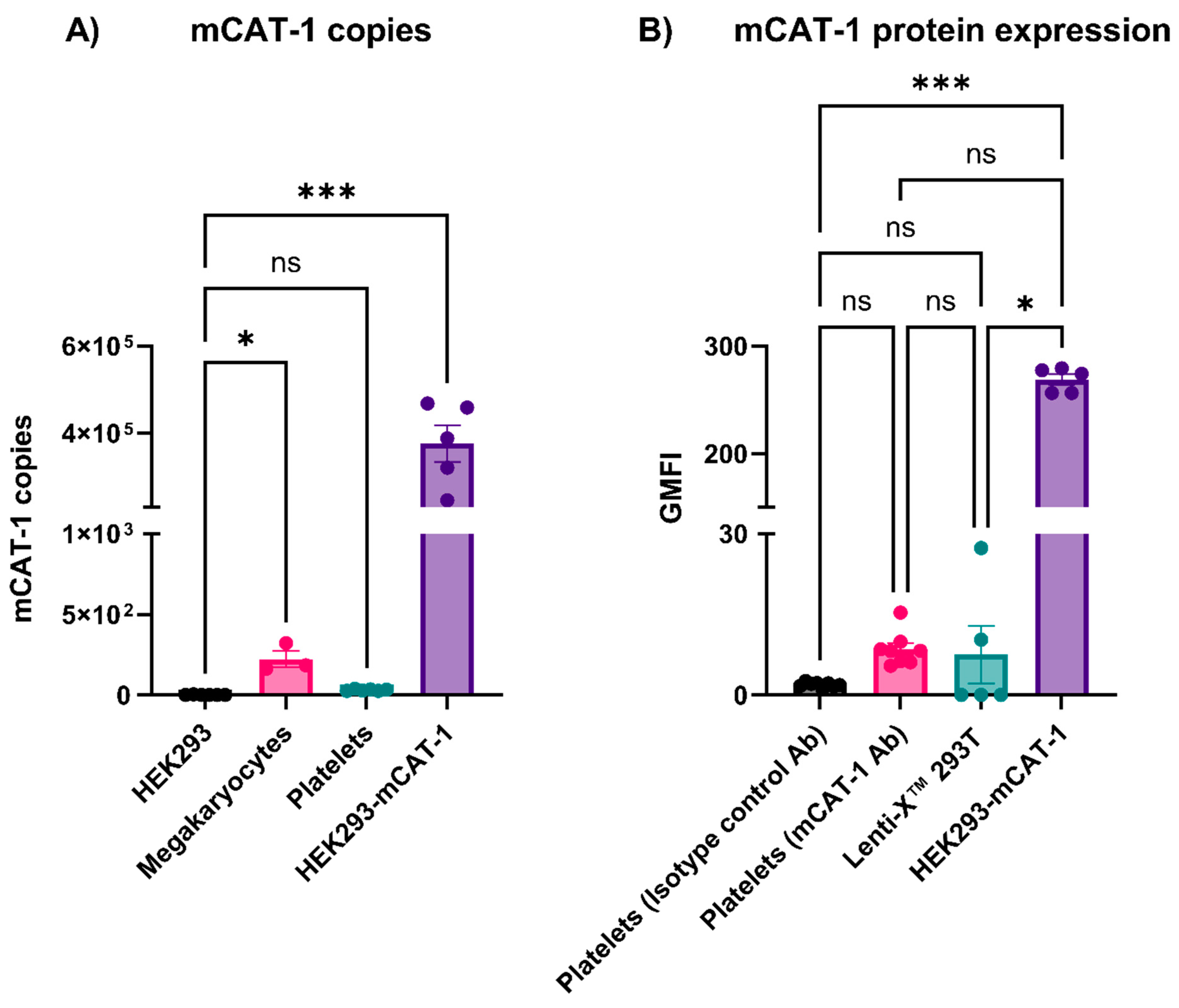
3.4. Mouse Platelets Did Not Express mCAT-1
3.5. Preparation Purity Affects How EcoHIV Affects Platelets
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Holly, S.P.; Gera, N.; Wang, P.; Wilson, A.; Guan, Z.; Lin, L.; Cooley, B.; Alfar, H.R.; Patil, R.G.; Piatt, R. Ether lipid metabolism by AADACL1 regulates platelet function and thrombosis. Blood Adv. 2019, 3, 3818–3828. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Bozza, F.A.; Bozza, P.T. Platelets in immune response to virus and immunopathology of viral infections. Front. Med. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Smith, A.N.; Prakhya, K.S.; Alfar, H.R.; Lykins, J.; Zhang, M.; Pokrovskaya, I.; Aronova, M.; Leapman, R.D.; Storrie, B. Ferric Chloride-Induced Arterial Thrombosis and Sample Collection for 3D Electron Microscopy Analysis. JoVE J. Vis. Exp. 2023, 193, e64985. [Google Scholar] [CrossRef] [PubMed]
- Matharu, S.S.; Nordmann, C.S.; Ottman, K.R.; Akkem, R.; Palumbo, D.; Cruz, D.R.; Campbell, K.; Sievert, G.; Sturgill, J.; Porterfield, J.Z. Deep learning, 3D ultrastructural analysis reveals quantitative differences in platelet and organelle packing in COVID-19/SARSCoV2 patient-derived platelets. Platelets 2023, 34, 2264978. [Google Scholar] [CrossRef] [PubMed]
- Scaradavou, A. HIV-related thrombocytopenia. Blood Rev. 2002, 16, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.C.; Hottz, E.D.; Amancio, R.T.; Carneiro, A.B.; Palhinha, L.; Coelho, L.E.; Grinsztejn, B.; Zimmerman, G.A.; Rondina, M.T.; Weyrich, A.S. Persistent platelet activation and apoptosis in virologically suppressed HIV-infected individuals. Sci. Rep. 2018, 8, 14999. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci. Rep. 2017, 7, 41697. [Google Scholar] [CrossRef]
- Flaujac, C.; Boukour, S.; Cramer-Bordé, E. Platelets and viruses: An ambivalent relationship. Cell. Mol. Life Sci. 2010, 67, 545–556. [Google Scholar] [CrossRef]
- Banerjee, M.; Huang, Y.; Joshi, S.; Popa, G.J.; Mendenhall, M.D.; Wang, Q.J.; Garvy, B.A.; Myint, T.; Whiteheart, S.W. Platelets endocytose viral particles and are activated via TLR (toll-like receptor) signaling. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1635–1650. [Google Scholar] [CrossRef]
- Assinger, A. Platelets and infection–an emerging role of platelets in viral infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.-C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Goetz, M.B.; Leaf, D.; Oursler, K.A. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet. 2020. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 6 June 2021).
- Real, F.; Capron, C.; Sennepin, A.; Arrigucci, R.; Zhu, A.; Sannier, G.; Zheng, J.; Xu, L.; Massé, J.-M.; Greffe, S. Platelets from HIV-infected individuals on antiretroviral drug therapy with poor CD4+ T cell recovery can harbor replication-competent HIV despite viral suppression. Sci. Transl. Med. 2020, 12, eaat6263. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.R.; Singh, M.V.; Dewhurst, S.; Schifitto, G.; Maggirwar, S.B. Platelets function as an acute viral reservoir during HIV-1 infection by harboring virus and T-cell complex formation. Blood Adv. 2020, 4, 4512–4521. [Google Scholar] [CrossRef] [PubMed]
- Rozmyslowicz, T.; Majka, M.; Kijowski, J.; Murphy, S.L.; Conover, D.O.; Poncz, M.; Ratajczak, J.; Gaulton, G.N.; Ratajczak, M.Z. Platelet-and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. Aids 2003, 17, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hatziioannou, T.; Evans, D.T. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 2012, 10, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Potash, M.J.; Chao, W.; Bentsman, G.; Paris, N.; Saini, M.; Nitkiewicz, J.; Belem, P.; Sharer, L.; Brooks, A.I.; Volsky, D.J. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. USA 2005, 102, 3760–3765. [Google Scholar] [CrossRef] [PubMed]
- Alfar, H.R.; Pariser, D.N.; Chanzu, H.; Joshi, S.; Coenen, D.M.; Lykins, J.; Prakhya, K.S.; Potash, M.J.; Chao, W.; Kelschenbach, J. Protocol for optimizing production and quality control of infective EcoHIV virions. STAR Protoc. 2023, 4, 102368. [Google Scholar] [CrossRef]
- Gu, C.-J.; Borjabad, A.; Hadas, E.; Kelschenbach, J.; Kim, B.-H.; Chao, W.; Arancio, O.; Suh, J.; Polsky, B.; McMillan, J.; et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 2018, 14, e1007061. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A.; Mactutus, C.F.; Booze, R.M. A rat model of EcoHIV brain infection. JoVE J. Vis. Exp. 2021, 167, e62137. [Google Scholar]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model. Sci. Rep. 2019, 9, 9428. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.D.; Jackson, J.W.; Maggirwar, S.B. Modeling HIV-1 induced neuroinflammation in mice: Role of platelets in mediating blood-brain barrier dysfunction. PLoS ONE 2016, 11, e0151702. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Joshi, S.; Chanzu, H.; Alfar, H.R.; Shravani Prakhya, K.; Whiteheart, S.W. α-Synuclein is the major platelet isoform but is dispensable for activation, secretion, and thrombosis. Platelets 2023, 34, 2267147. [Google Scholar] [CrossRef] [PubMed]
- Prakhya, K.S.; Vekaria, H.; Coenen, D.M.; Omali, L.; Lykins, J.; Joshi, S.; Alfar, H.R.; Wang, Q.J.; Sullivan, P.; Whiteheart, S.W. Platelet glycogenolysis is important for energy production and function. Platelets 2023, 34, 2222184. [Google Scholar] [CrossRef] [PubMed]
- Kizhakke Madathil, S.; Evans, H.N.; Saatman, K.E. Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma 2010, 27, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Hadas, E.; Borjabad, A.; Chao, W.; Saini, M.; Ichiyama, K.; Potash, M.J.; Volsky, D.J. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. Aids 2007, 21, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Méroth, F.; Tournebize, M.; Leda, A.R.; Sun, E.; Toborek, M. Targeting the HIV-infected brain to improve ischemic stroke outcome. Nat. Commun. 2019, 10, 2009. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A.; Illenberger, J.M.; Mactutus, C.F.; Booze, R.M. Microglial HIV-1 expression: Role in HIV-1 associated neurocognitive disorders. Viruses 2021, 13, 924. [Google Scholar] [CrossRef]
- Cohen, M.S.; Shaw, G.M.; McMichael, A.J.; Haynes, B.F. Acute HIV-1 infection. N. Engl. J. Med. 2011, 364, 1943–1954. [Google Scholar] [CrossRef]
- Nascimento, F.G.; Tanaka, P.Y. Thrombocytopenia in HIV-infected patients. Indian J. Hematol. Blood Transfus. 2012, 28, 109–111. [Google Scholar] [CrossRef]
- Getawa, S.; Aynalem, M.; Bayleyegn, B.; Adane, T. The global prevalence of thrombocytopenia among HIV-infected adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Livada, A.C.; Morrell, C.N. Platelet and megakaryocyte roles in innate and adaptive immunity. Circ. Res. 2022, 130, 288–308. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Alfar, H.; Hollifield, M.; Chung, D.; Fu, X.; Banerjee, M.; Li, X.; Thornton, A.; Porterfield, J.; Sturgill, J. HIV-1 and SARS-CoV2 both cause protein s, but through different mechanisms. Res. Pract. Thromb. Haemost. 2021, 5, 1509117. [Google Scholar]
- Sim, M.; Alfar, H.; Hollifield, M.; Chung, D.W.; Fu, X.; Banerjee, M.; Peng, C.; Li, X.; Thornton, A.; Porterfield, J.Z. Unfolded Von Willebrand Factor Interacts with Protein S and Limits Its Anticoagulant Activity. Blood 2022, 140 (Suppl. S1), 2710–2711. [Google Scholar] [CrossRef]
- Sim, M.M.; Banerjee, M.; Hollifield, M.; Alfar, H.; Li, X.; Thornton, A.; Porterfield, J.Z.; Sturgill, J.; Sievert, G.A.; Barton-Baxter, M. Inflammation drives coagulopathies in SARS-CoV-2 Patients. Blood 2020, 136, 34–35. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P. The role of platelets in mediating a response to human influenza infection. Nat. Commun. 2019, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Vitseva, O.; MacKay, C.R.; Beaulieu, L.M.; Benjamin, E.J.; Mick, E.; Kurt-Jones, E.A.; Ravid, K.; Freedman, J.E. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood J. Am. Soc. Hematol. 2014, 124, 791–802. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Zeiler, M.; Moser, M.; Mann, M. Copy number analysis of the murine platelet proteome spanning the complete abundance range. Mol. Cell. Proteom. 2014, 13, 3435–3445. [Google Scholar] [CrossRef]
- Cognasse, F.; Nguyen, K.A.; Damien, P.; McNicol, A.; Pozzetto, B.; Hamzeh-Cognasse, H.; Garraud, O. The inflammatory role of platelets via their TLRs and Siglec receptors. Front. Immunol. 2015, 6, 83. [Google Scholar] [CrossRef]
- López, J.A. The platelet Fc receptor: A new role for an old actor. Blood J. Am. Soc. Hematol. 2013, 121, 1674–1675. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfar, H.R.; Nthenge-Ngumbau, D.N.; Saatman, K.E.; Whiteheart, S.W. EcoHIV-Infected Mice Show No Signs of Platelet Activation. Viruses 2024, 16, 55. https://doi.org/10.3390/v16010055
Alfar HR, Nthenge-Ngumbau DN, Saatman KE, Whiteheart SW. EcoHIV-Infected Mice Show No Signs of Platelet Activation. Viruses. 2024; 16(1):55. https://doi.org/10.3390/v16010055
Chicago/Turabian StyleAlfar, Hammodah R., Dominic Ngima Nthenge-Ngumbau, Kathryn E. Saatman, and Sidney W. Whiteheart. 2024. "EcoHIV-Infected Mice Show No Signs of Platelet Activation" Viruses 16, no. 1: 55. https://doi.org/10.3390/v16010055






