AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Flow Cytometry Analysis of Cell Surface and Intracellular Antigens
2.3. Cell Growth, Viability and Apoptosis Analysis, Cell Cycle Profile, DNA-Ploidy
2.4. AC-73 and Syrosingopine Treatment and Sensitivity
2.5. Quantitative Real-Time RT-PCR Analysis
2.6. Western Blot Analysis
2.7. Autophagy Detection
2.8. In Vitro SARS-CoV-2 Infection
2.9. Statistical Analysis
3. Results
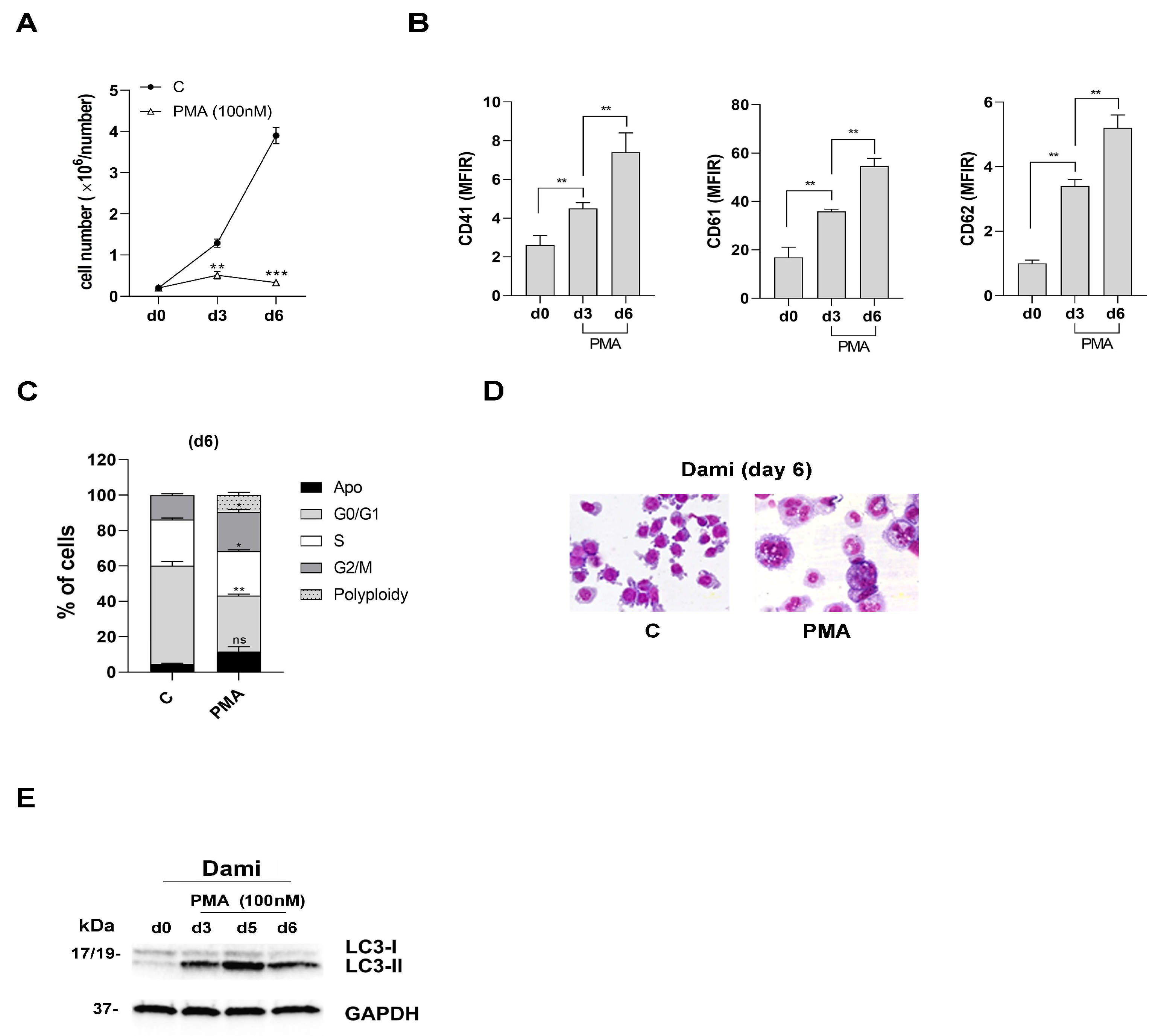
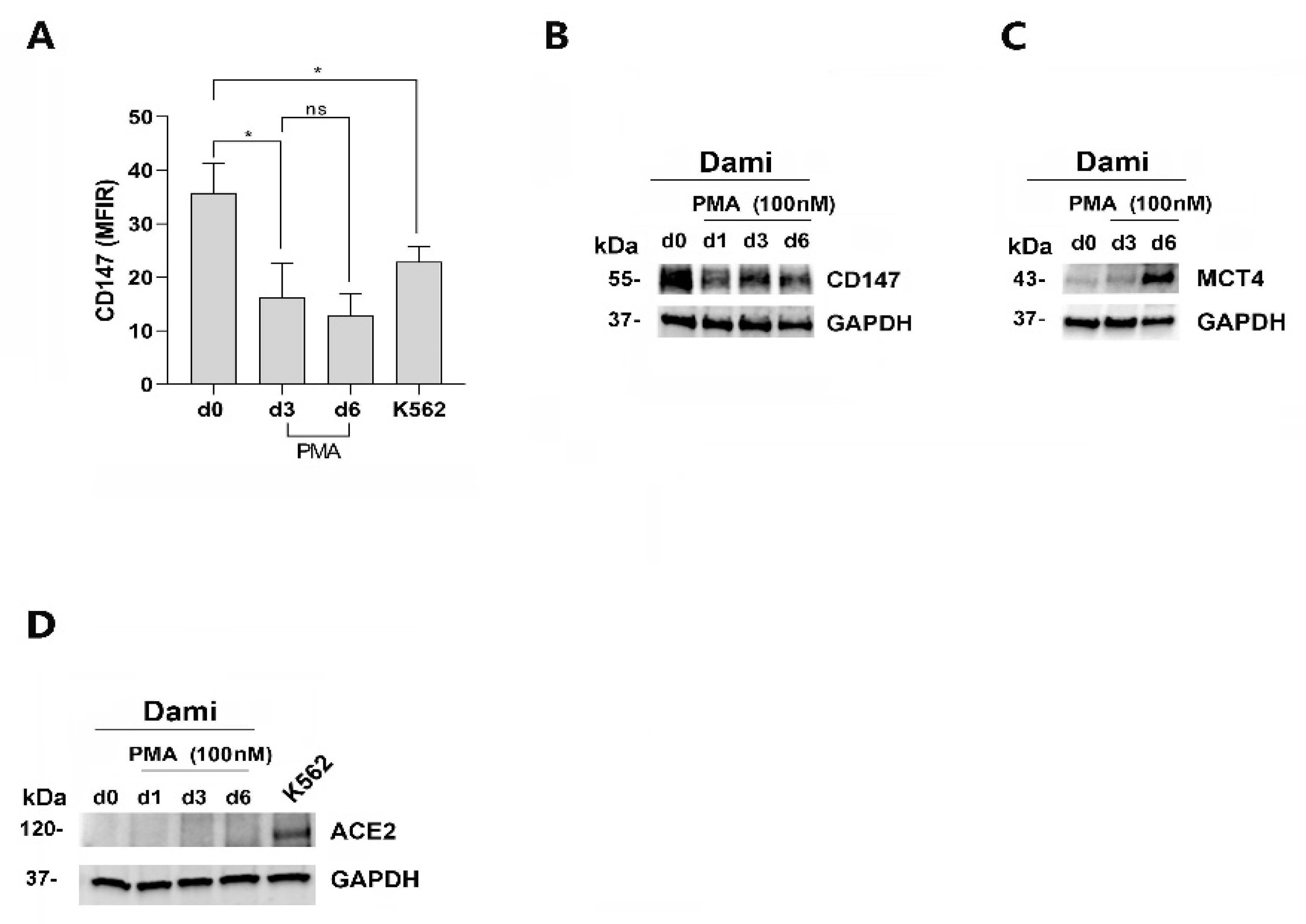
3.1. CD147 and MCT4 Expression during PMA-Induced Megakaryocytic Differentiation of Dami Cells, ACE-2-Deficient Cells
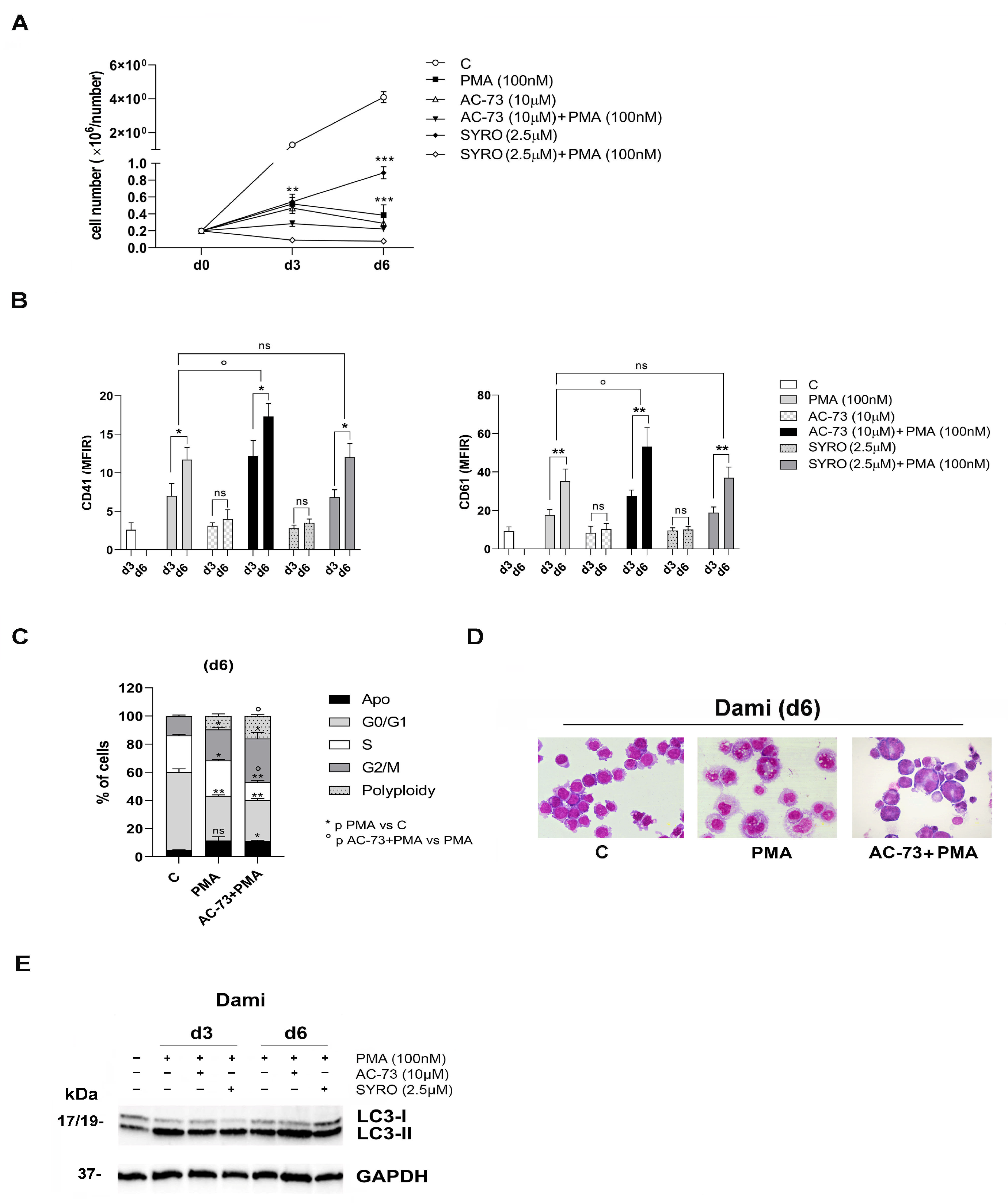
3.2. AC-73 and SYRO Have Both a Synergic Effect on the Autophagy Triggered by PMA during MK Differentiation of Dami Cells, but Only AC-73 Enhances MK Differentiation and Promotes Endomitosis in PMA-Dami Cells
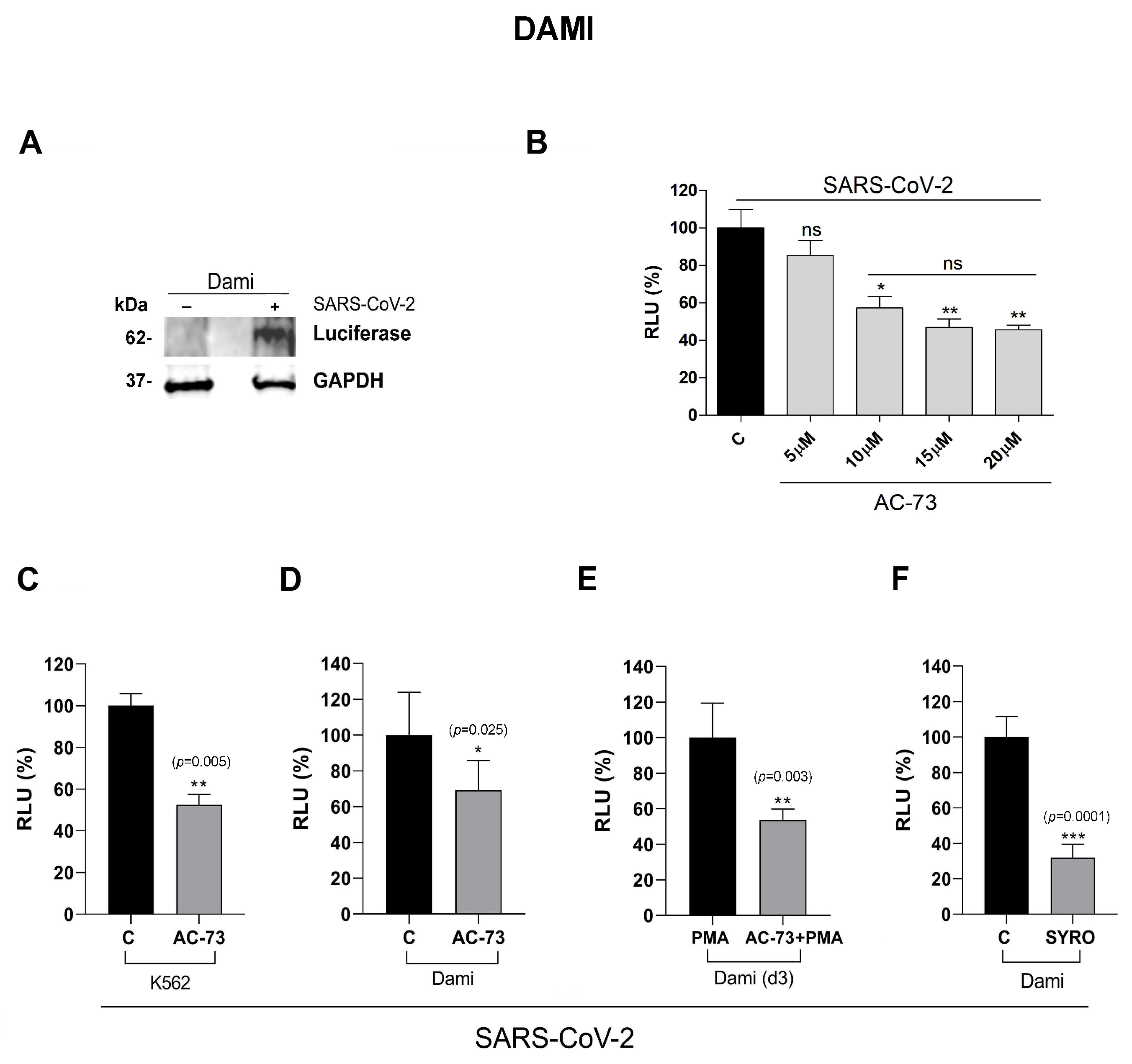
3.3. AC-73 and SYRO Are SARS-COV-2 Entry Inhibitors by Blocking CD147 and MCT4 Function in PMA-Dami Cells
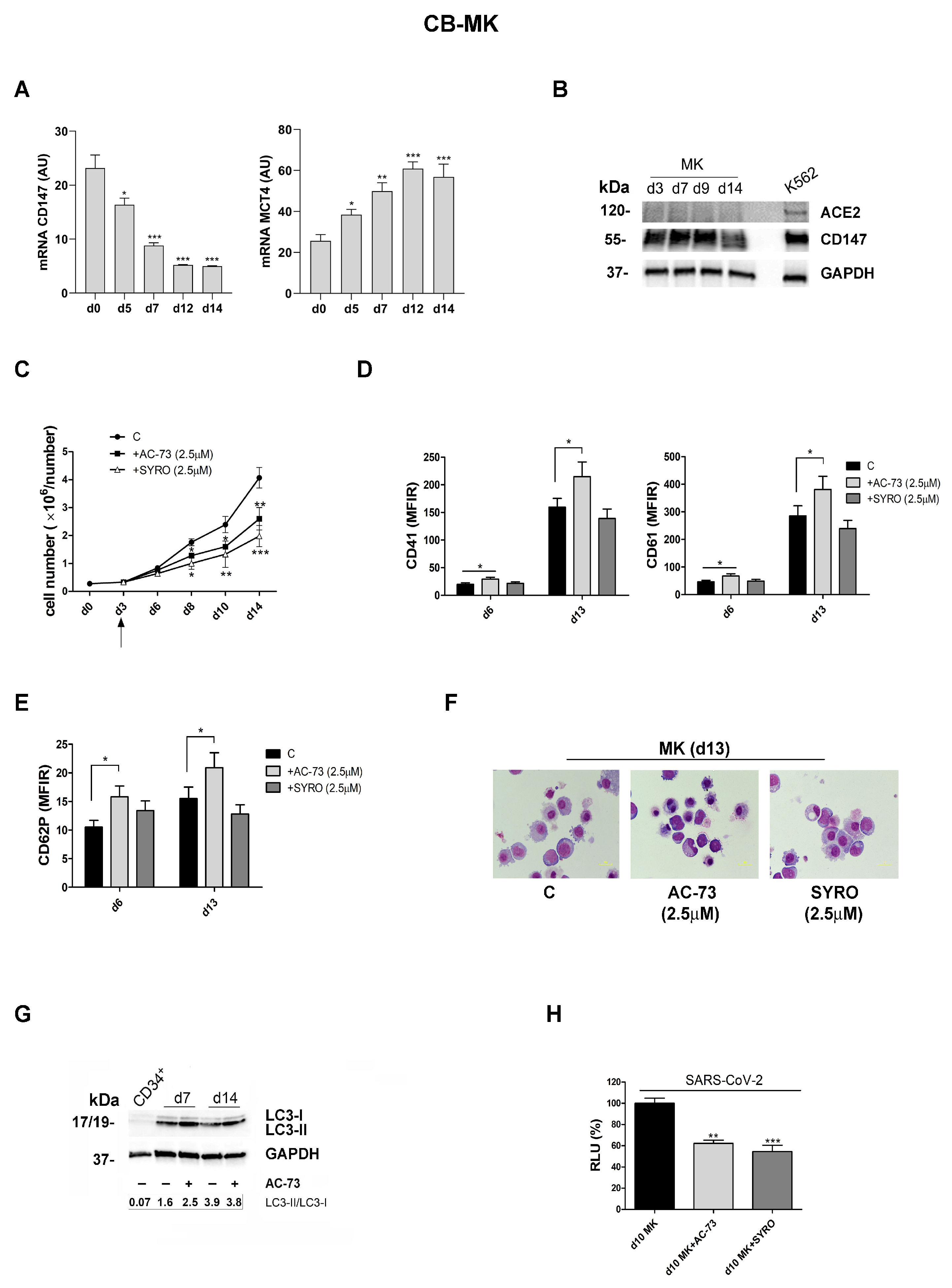
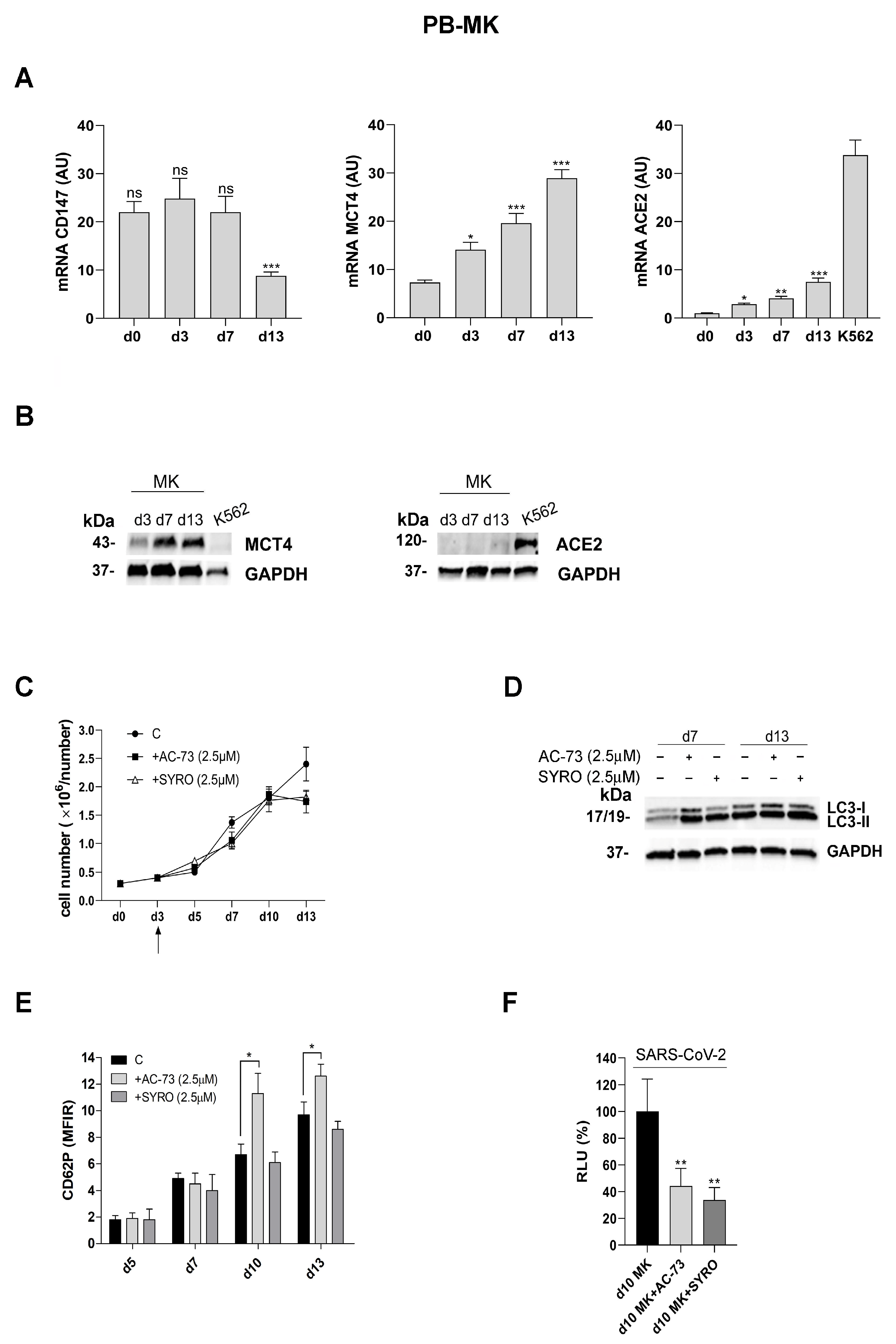
3.4. AC-73 and SYRO Are Inhibitors of SARS-COV-2 Entry into Megakaryocytes by Blocking Their Respective Targets CD147 and MCT4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Cron, R.Q.; Caricchio, R.; Chatham, W.W. Calming the cytokine storm in COVID-19. Nat. Med. 2021, 27, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, S.; Sattu, K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020, 260, 118431. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, M.G.; Piantoni, S.; Masneri, S.; Garrafa, E.; Martini, G.; Tincani, A.; Andreoli, L.; Franceschini, F. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021, 46, 100745. [Google Scholar] [CrossRef]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Al-Subaie, A.M. Coagulopathies in novel coronavirus (SARS-CoV-2) pandemic: Emerging evidence for hematologists. Saudi J. Biol. Sci. 2021, 28, 956–961. [Google Scholar] [CrossRef]
- Elahi, S. Hematopoietic responses to SARS-CoV-2 infection. Cell Mol. Life Sci. 2022, 79, 187. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Lazzaro, R.; Aityan, S.K.; Maggiore, M.E.; Mancini, A.; Laforgia, R.; et al. SARS-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte-Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 2021, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Thrombosis and thrombocytopenia in COVID-19 and after COVID-19 vaccination. Trends Cardiovasc. Med. 2022, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Estep, B.K.; Kuhlmann, C.J.; Osuka, S.; Suryavanshi, G.W.; Nagaoka-Kamata, Y.; Samuel, C.N.; Blucas, M.T.; Jepson, C.E.; Goepfert, P.A.; Kamata, M. Skewed fate and hematopoiesis of CD34+ HSPCs in umbilical cord blood amid the COVID-19 pandemic. iScience 2022, 25, 105544. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, J.; Fang, Y.; Lu, S.; Wu, J.; Zheng, X.; Deng, F. SARS-CoV-2 interacts with platelets and megakaryocytes via ACE2-independent mechanism. J. Hematol. Oncol. 2021, 14, 72. [Google Scholar] [CrossRef]
- Maugeri, N.; De Lorenzo, R.; Clementi, N.; Antonia Diotti, R.; Criscuolo, E.; Godino, C.; Tresoldi, C.; Angels for Covid-BioB Study Group B; Bonini, C.; Clementi, M.; et al. Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 2022, 20, 434–448. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Li, F.; Qin, S.; Song, X.; Wu, Y.; Zeng, Q. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int. J. Med. Sci. 2020, 17, 1522–1531. [Google Scholar] [CrossRef]
- Kazemi, S.; López-Muñoz, A.D.; Hollý, J.; Jin, L.; Yewdell, J.W.; Dolan, B.P. Variations in Cell Surface ACE2 Levels Alter Direct Binding of SARS-CoV-2 Spike Protein and Viral Infectivity: Implications for Measuring Spike Protein Interactions with Animal ACE2 Orthologs. J. Virol. 2022, 96, e0025622. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016, 159, 481–490. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Xiong, L.; Edwards, C.K., 3rd; Zhou, L. The biological function and clinical utilization of CD147 in human diseases: A review of the current scientific literature. Int. J. Mol. Sci. 2014, 15, 17411–17441. [Google Scholar] [CrossRef]
- Spinello, I.; Saulle, E.; Quaranta, M.T.; Pasquini, L.; Pelosi, E.; Castelli, G.; Ottone, T.; Voso, M.T.; Testa, U.; Labbaye, C. The small-molecule compound AC-73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica 2019, 104, 973–985. [Google Scholar] [CrossRef]
- Chuliá-Peris, L.; Carreres-Rey, C.; Gabasa, M.; Alcaraz, J.; Carretero, J.; Pereda, J. Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play. Int. J. Mol. Sci. 2022, 23, 6894. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Quaranta, M.T.; Crippa, L.; Spinello, I.; Saulle, E.; Di Carlo, N.; Campanile, D.; Boirivant, M.; Labbaye, C. CD147 Targeting by AC-73 Induces Autophagy and Reduces Intestinal Fibrosis Associated with TNBS Chronic Colitis. J. Crohns Colitis 2022, 16, 1751–1761. [Google Scholar] [CrossRef]
- Liu, C.; von Brunn, A.; Zhu, D. Cyclophilin A and CD147: Novel therapeutic targets for the treatment of COVID-19. Med. Drug Discov. 2020, 7, 100056. [Google Scholar] [CrossRef]
- Contreras-Baeza, Y.; Sandoval, P.Y.; Alarcón, R.; Galaz, A.; Cortés-Molina, F.; Alegría, K.; Baeza-Lehnert, F.; Arce-Molina, R.; Guequén, A.; Flores, C.A.; et al. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J. Biol. Chem. 2019, 294, 20135–20147. [Google Scholar] [CrossRef]
- Icard, P.; Lincet, H.; Wu, Z.; Coquerel, A.; Forgez, P.; Alifano, M.; Fournel, L. The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie 2021, 180, 169–177. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Rosenthal, D.S.; Greeley, T.A.; Tantravahi, R.; Handin, R.I. Characterization of a new megakaryocytic cell line: The Dami cell. Blood 1988, 72, 1968–1977. [Google Scholar] [CrossRef]
- Besancenot, R.; Roos-Weil, D.; Tonetti, C.; Abdelouahab, H.; Lacout, C.; Pasquier, F.; Willekens, C.; Rameau, P.; Lecluse, Y.; Micol, J.B.; et al. JAK2 and MPL protein levels determine TPO-induced megakaryocyte proliferation vs differentiation. Blood 2014, 124, 2104–2115. [Google Scholar] [CrossRef]
- Mardente, S.; Mari, E.; Massimi, I.; Tafani, M.; Guerriero, R.; Morsilli, O.; Pulcinelli, F.M.; Bianchi, M.E.; Zicari, A. From Human Megakaryocytes to Platelets: Effects of Aspirin on High-Mobility Group Box 1/Receptor for Advanced Glycation End Products Axis. Front. Immunol. 2018, 8, 1946. [Google Scholar] [CrossRef]
- Saulle, E.; Guerriero, R.; Petronelli, A.; Coppotelli, E.; Gabbianelli, M.; Morsilli, O.; Spinello, I.; Pelosi, E.; Castelli, G.; Testa, U.; et al. Autocrine role of angiopoietins during megakaryocytic differentiation. PLoS ONE 2012, 7, e39796. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.G.; Wang, L.; Cui, H.Y.; Peng, J.L.; Wang, S.J.; Geng, J.J.; Liu, J.D.; Feng, F.; Song, F.; Li, L.; et al. A novel small-molecule compound targeting CD147 inhibits the motility and invasion of hepatocellular carcinoma cells. Oncotarget 2016, 7, 9429–9447. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Saulle, E.; Spinello, I.; Quaranta, M.T.; Pasquini, L.; Pelosi, E.; Iorio, E.; Castelli, G.; Chirico, M.; Pisanu, M.E.; Ottone, T.; et al. Targeting Lactate Metabolism by Inhibiting MCT1 or MCT4 Impairs Leukemic Cell Proliferation, Induces Two Different Related Death-Pathways and Increases Chemotherapeutic Sensitivity of Acute Myeloid Leukemia Cells. Front. Oncol. 2021, 10, 621458. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cai, J.; Zhang, S.; Yuan, N.; Li, X.; Fang, Y.; Song, L.; Shang, M.; Liu, S.; Zhao, W.; et al. Loss of autophagy leads to failure in megakaryopoiesis, megakaryocyte differentiation, and thrombopoiesis in mice. Exp. Hematol. 2015, 43, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.J.; Shan, N.N. Megakaryocytic dysfunction in immune thrombocytopenia is linked to autophagy. Cancer Cell Int. 2019, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Xu, M.; Pradhan, M.; Gorshkov, K.; Petersen, J.D.; Straus, M.R.; Zhu, W.; Shinn, P.; Guo, H.; Shen, M.; et al. Identifying SARS-CoV-2 Entry Inhibitors through Drug Repurposing Screens of SARS-S and MERS-S Pseudotyped Particles. ACS Pharmacol. Transl. Sci. 2020, 3, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Anguissola, M.; Pugliese, S.; Falco, M.; Menicanti, L. The Long Term Residual Effects of COVID-Associated Coagulopathy. Int. J. Mol. Sci. 2023, 24, 5514. [Google Scholar] [CrossRef]
- Goh, D.; Lim, J.C.T.; Fernaíndez, S.B.; Joseph, C.R.; Edwards, S.G.; Neo, Z.W.; Lee, J.N.; Caballero, S.G.; Lau, M.C.; Yeong, J.P.S. Case report: Persistence of residual antigen and RNA of SARS-CoV-2 virus in tissues of two patients with long COVID. Front. Immunol. 2022, 13, 1036894. [Google Scholar] [CrossRef]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Cangemi, R.; Pignatelli, P.; Loffredo, L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thromb. Haemost. 2020, 120, 949–956. [Google Scholar] [CrossRef]
- Wang, C.; Yu, C.; Jing, H.; Wu, X.; Novakovic, V.A.; Xie, R.; Shi, J. Long COVID: The Nature of Thrombotic Sequelae Determines the Necessity of Early Anticoagulation. Front. Cell. Infect. Microbiol. 2022, 12, 861703. [Google Scholar] [CrossRef]
- Zaid, Y.; Guessous, F. The ongoing enigma of SARS-CoV-2 and platelet interaction. Res. Pr. Thromb. Haemost. 2022, 6, e12642. [Google Scholar] [CrossRef] [PubMed]
- Lefrançais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.N.; Hilt, Z.T.; Ture, S.K.; Blick-Nitko, S.K.; Looney, M.R.; Cleary, S.J.; Roman-Pagan, E.; Saunders, J., 2nd; Georas, S.N.; Veazey, J.; et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Investig. 2021, 131, e137377. [Google Scholar] [CrossRef] [PubMed]
- Gelon, L.; Fromont, L.; Lefrançais, E. Occurrence and role of lung megakaryocytes in infection and inflammation. Front. Immunol. 2022, 13, 1029223. [Google Scholar] [CrossRef]
- Heinzmann, D.; Noethel, M.; Ungern-Sternberg, S.V.; Mitroulis, I.; Gawaz, M.; Chavakis, T.; May, A.E.; Seizer, P. CD147 is a Novel Interaction Partner of Integrin αMβ2 Mediating Leukocyte and Platelet Adhesion. Biomolecules 2020, 10, 541. [Google Scholar] [CrossRef]
- Merezhinskaya, N.; Ogunwuyi, S.A.; Fishbein, W.N. Expression of monocarboxylate transporter 4 in human platelets, leukocytes, and tissues assessed by antibodies raised against terminal versus pre-terminal peptides. Mol. Genet. Metab. 2006, 87, 152–161. [Google Scholar] [CrossRef][Green Version]
- Aibibula, M.; Naseem, K.M.; Sturmey, R.G. Glucose metabolism and metabolic flexibility in blood platelets. J. Thromb. Haemost. 2018, 16, 2300–2314. [Google Scholar] [CrossRef]
- Kulkarni, P.P.; Tiwari, A.; Singh, N.; Gautam, D.; Sonkar, V.K.; Agarwal, V.; Dash, D. Aerobic glycolysis fuels platelet activation: Small-molecule modulators of platelet metabolism as anti-thrombotic agents. Haematologica 2019, 104, 806–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinello, I.; Saulle, E.; Quaranta, M.T.; Pelosi, E.; Castelli, G.; Cerio, A.; Pasquini, L.; Morsilli, O.; Dupuis, M.L.; Labbaye, C. AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4. Viruses 2024, 16, 82. https://doi.org/10.3390/v16010082
Spinello I, Saulle E, Quaranta MT, Pelosi E, Castelli G, Cerio A, Pasquini L, Morsilli O, Dupuis ML, Labbaye C. AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4. Viruses. 2024; 16(1):82. https://doi.org/10.3390/v16010082
Chicago/Turabian StyleSpinello, Isabella, Ernestina Saulle, Maria Teresa Quaranta, Elvira Pelosi, Germana Castelli, Annamaria Cerio, Luca Pasquini, Ornella Morsilli, Maria Luisa Dupuis, and Catherine Labbaye. 2024. "AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4" Viruses 16, no. 1: 82. https://doi.org/10.3390/v16010082
APA StyleSpinello, I., Saulle, E., Quaranta, M. T., Pelosi, E., Castelli, G., Cerio, A., Pasquini, L., Morsilli, O., Dupuis, M. L., & Labbaye, C. (2024). AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4. Viruses, 16(1), 82. https://doi.org/10.3390/v16010082





