Mechanisms of Yellow Fever Transmission: Gleaning the Overlooked Records of Importance and Identifying Problems, Puzzles, Serious Issues, Surprises and Research Questions
Abstract
:1. Introduction
2. The Evolution of the Concept of the Vector-Borne Transmission of Yellow Fever and Other Arboviral Diseases
2.1. A History of the Discovery of the Vector-Borne Transmission of YF
2.2. A Brief History of the Establishment of the Arbovirus Concept Based on Biological Transmission (BT)
3. Vectors
3.1. Ae. aegypti
3.2. The Extrinsic Incubation Period (EIP)
3.3. Vertical, Venereal and Direct Modes of Transmission in Vectors
3.4. Vectors as Reservoirs
3.5. The Minimal Size of the Vector Population Necessary for Viral Persistence
3.6. The Transoceanic or Transcontinental Dispersal of Vectors
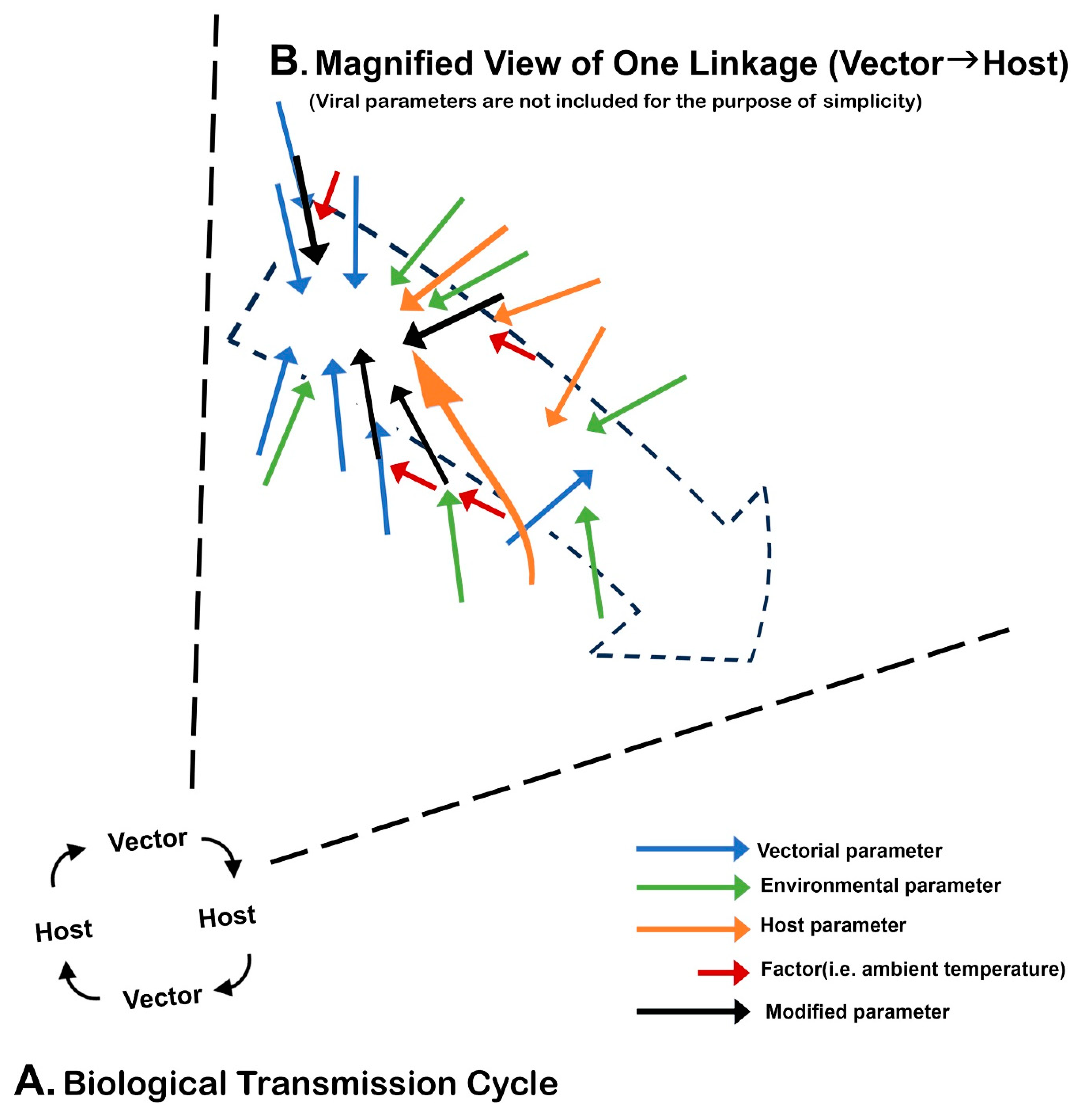
3.7. The Vectorial Capacity and Vector Competence
4. Hosts
4.1. Human
4.2. Nonhuman Primates (NHPs)
4.3. Incidental Hosts
4.4. Viremia
4.5. The Vertebrate Reservoir and Virus Persistence
4.6. Direct Transmission (DT) of YFV in Vertebrates
4.7. Viral Dispersal by Humans
4.7.1. Ship
4.7.2. Aircraft
4.8. Acquired Immunity and Social Distancing
5. Environments
5.1. Classification of the Environment Based on the Combination of the Ecosystem, the Landscape, and the Pattern of Human Habitation
5.2. Terms Used
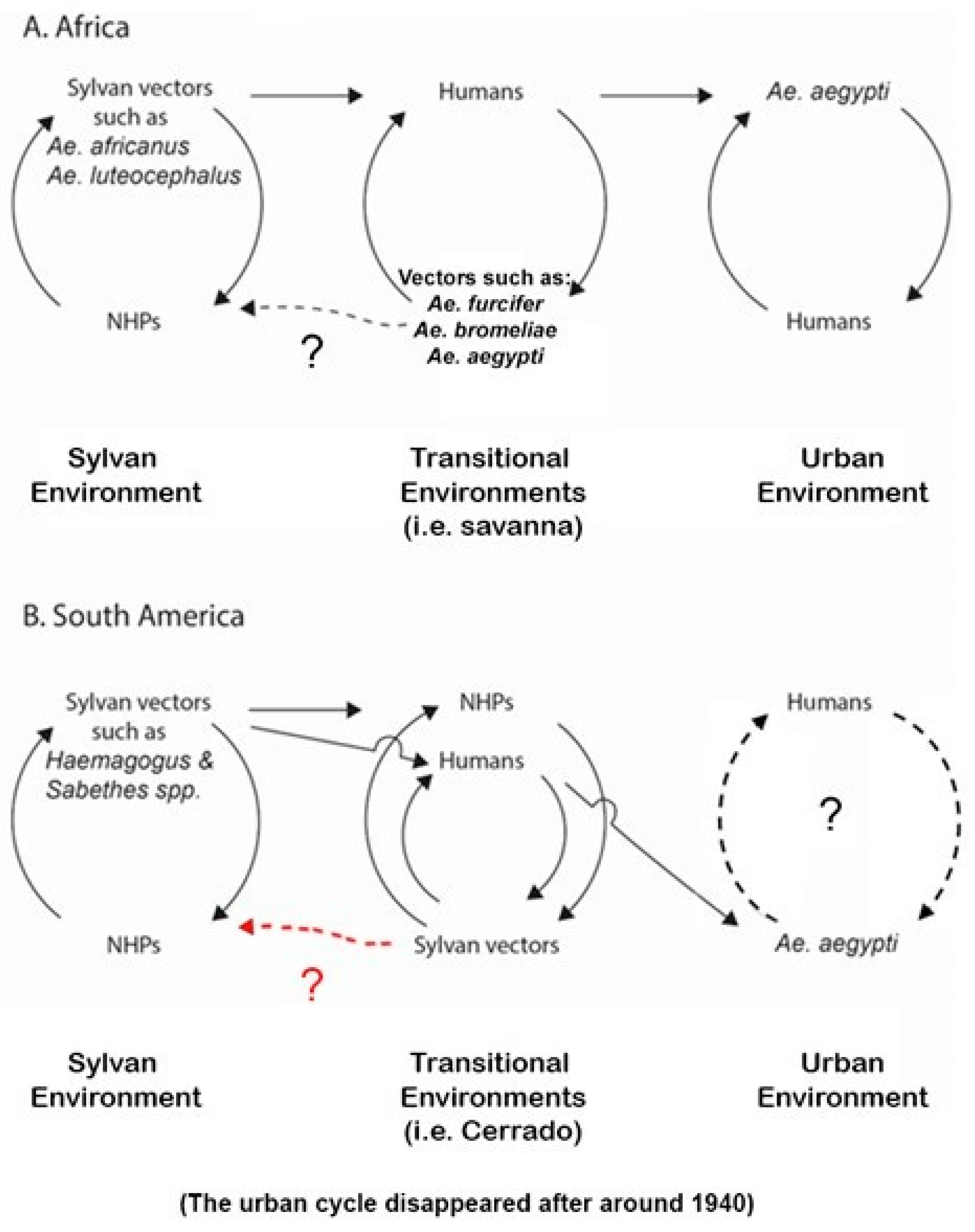
5.3. Sylvan Environments (Figure 1)
5.3.1. Sylvan Environments in the Americas
5.3.2. Sylvan Environments in Africa
5.4. Transitional Environments (Figure 1)
5.4.1. Transitional Environments in the Americas
5.4.2. Transitional Environments in Africa
5.5. The Urban Environment (Figure 1)
5.5.1. A Brief Review of the Urban Outbreaks in Europe, North America and South America before 1910
5.5.2. The Problems and Importance of the Definition of Urban Area
5.5.3. Urban YF Outbreaks in the Americas after 1910
5.5.4. Urban Outbreaks in Africa
6. Virus
6.1. A History of Early YFV Isolation
6.2. Differences between African and American Isolates of YFV and Virus Characterization
6.3. The Rate of Mutation and Phylogenetic Tree Topology
6.4. Host Adaptation (or Increased Host Resistance) Concept Regarding the Long-Term Relationship between the Pathogen and the Host
6.5. Virulence and Trade-Off Theory
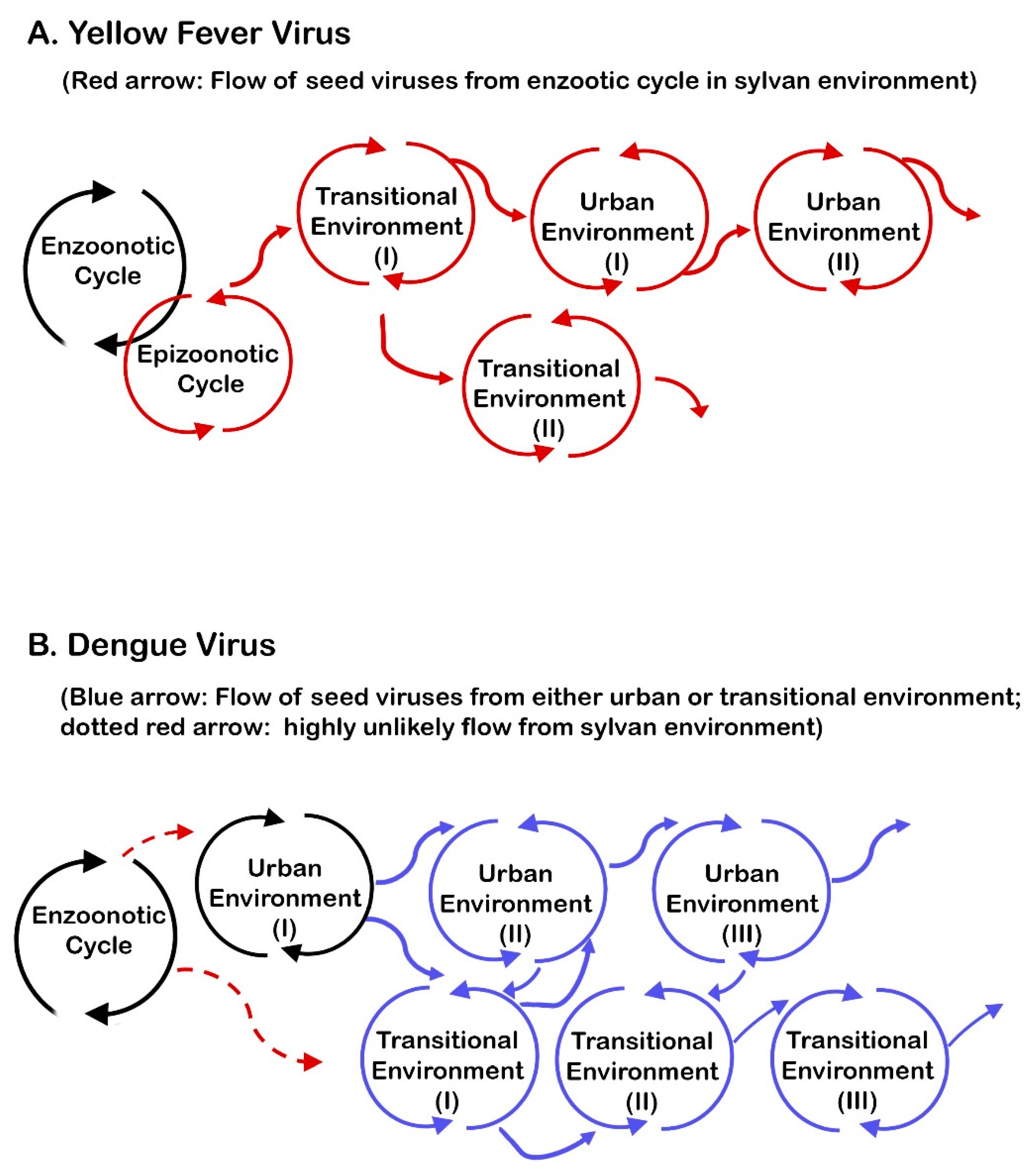
6.6. The Importance of Epizootic Outbreaks in Viral Transmission in Transitional and Urban Environments
6.7. Synthesizing the Uniqueness of the Requirements for YFV Transmission with an Emphasis on Geographic Distribution of YF
7. Relevance of Geologic Events in the Understanding of the Evolution of Flaviviruses, Vectors, and of Vector-Borne Viruses as Well as the Geographic Dispersal of Arboviruses Including YFV
7.1. The Evolution of Flaviviruses
7.2. The Evolution of Mosquitoes
7.3. Additional Information
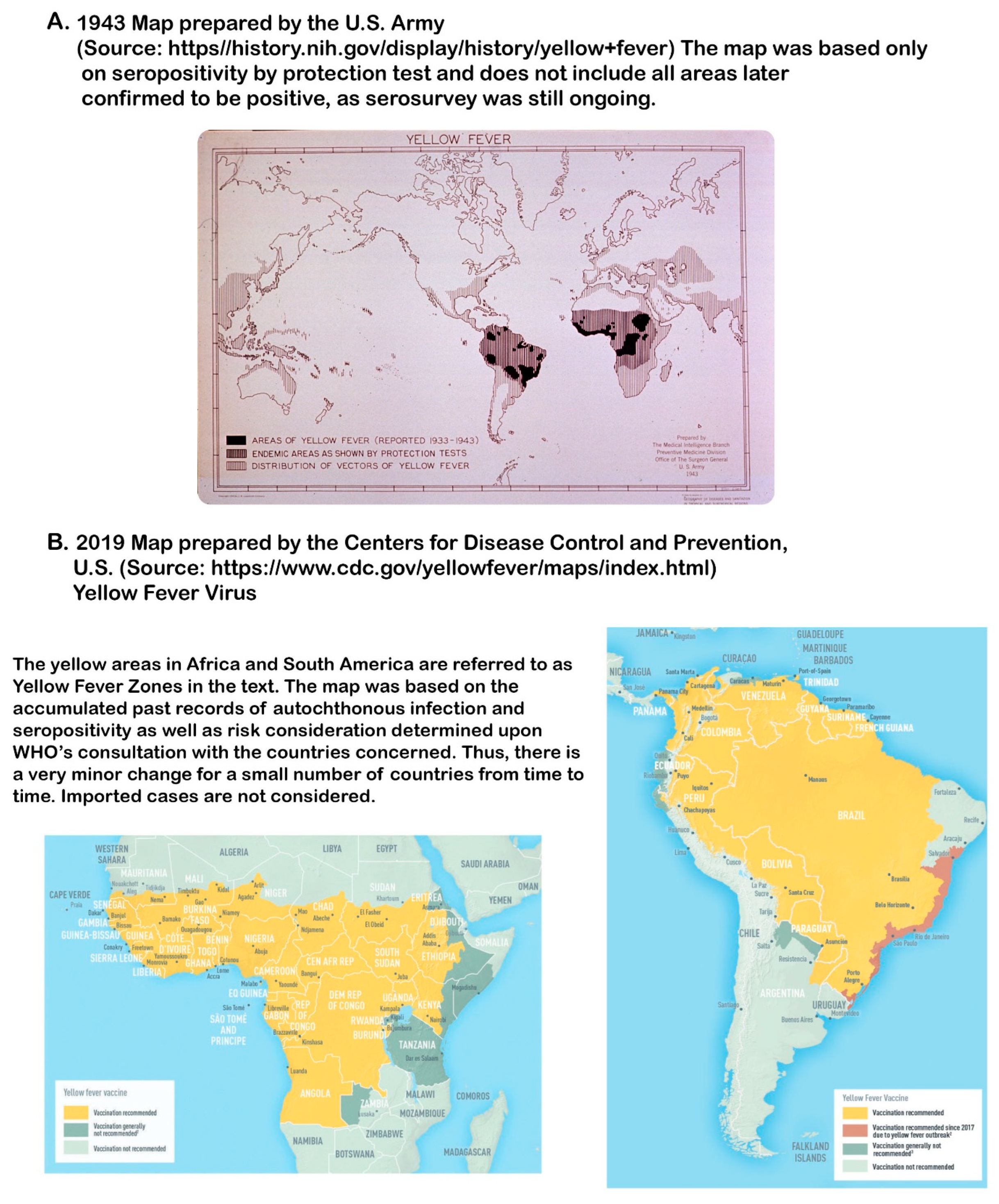
7.4. Understanding the Uniqueness of the Current Geographic Distribution of Viruses Including YFV
8. Issues Surrounding Research
8.1. Basic Sources of Problems
8.2. Puzzles/Unknown
- (i)
- Sylvan transmission in Muzo, Colombia: Muzo is a famed emerald mine town in the Department of Boyacá. YF outbreaks occurred there multiple times. Investigators discovered no evidence of the existence of either Ae. aegypti or NHPs. Only humans were the hosts. An obvious question raised was how YFV could persist if humans were not there. The repeated introduction of the virus by vectors to Muzo from other enzootic/epizootic foci and repeated introduction to Muzo by infected humans were two possible answers [167]. How YFV is maintained in the enzootic/epizootic foci year after year in the absence of NHPs was another puzzle. This is an enormously important question. Yet, research activity in the forest has dropped sharply in recent decades, except in Brazil and Sénégal.
- (ii)
- Why YF is absent in Asia: The warning of Manson over possible catastrophe in Asia if YF is introduced to Asia as a result of the completion of the Panama Canal [127] was instrumental in initiating a chain reaction to adopt preventive measures. As a result, quarantine inspection, ship fumigation, vector control at seaports, and a wireless international communication system were implemented in Asia. Through diplomatic negotiation, Anglo-Egyptian Sudan (now Sudan and South Sudan) was designated as a “buffer zone” [73].
- (iii)
- Possible return of YF to the countries in temperate climate: A similar question raised recently is a possibility of the return of YF to Europe (mostly around the Mediterranean) and to southern regions of North America where climate is warmer [278]. If this is still possible, a serious question is immediately raised about the validity of the proposed scheme of YFV transmission in the Section 6.6. The question concerns how extensive urban outbreaks in the locations far away from the sylvan environments in the YF zone in Africa or South America were possible in old days. Before arriving at an answer, it is first necessary to determine exactly how such a large epidemic occurred frequently before 1910 in the first place. Unfortunately, Hosack was the only one who recognized the importance of the abundance of mosquitoes during YF outbreaks [15]. He even did not know the name of the mosquitoes. Naturally, little is known about exactly how many infected passengers, infective vectors and uninfected mosquitoes disembarked at the ports of entry. All these blanks make estimation of the quantity of seed viruses introduced to port communities impossible.
9. Selected Issues with Laboratory Research
9.1. Virus Isolation
9.2. Differences between In Vitro and In Vivo
- (i)
- (ii)
- A combination of the following two reports provides a good example for exercising caution for anyone interested in determining the genetic determinants of the phenotypic traits of arboviruses. Earlier, researchers interested in the host range determinant of Sindbis virus (an alphavirus) developed a mutant that failed to replicate in mosquito cell cultures. Surprisingly, when adult mosquitoes were intrathoracically infected with this mutant, the mutant replicated normally in vivo [289]. A caveat in this episode is that intrathoracic inoculation does not simulate natural viral infection because it bypasses the midgut barrier. Accordingly, interpretation of similar reports based only on in vitro system is complicated [290]. This example serves as a good source of caution to exercise in deducing the virus–vector or virus–host interactions in vivo based only on in vitro results. Similarly, this caution is also applied when one deduces what happens under natural conditions based only on laboratory results.
10. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Arboviruses and human diseases. In WHO Technical Report Series; WHO: Geneva, Switzerland, 1967; p. 369. [Google Scholar]
- Ackerknecht, E.H. Anticontagionism between 1821 and 1867. The Fielding H. Garrison Lecture. Int. J. Epidemiol. 2009, 38, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.B. Yellow fever in eighteenth century America. Bull. N. Y. Acad. Med. 1968, 44, 673–686. [Google Scholar] [PubMed]
- Doetsch, R.N. John Crawford and his contribution to the doctrine of contagium vivum. Bacteriol. Rev. 1964, 28, 87–96. [Google Scholar] [CrossRef]
- Crawford, J. A Lecture, Introductory to a Course of Lectures in the Cause, Seat and Cure of Diseases; Edward, J., Ed.; Coale: Baltimore, MD, USA, 1811. [Google Scholar]
- Nott, J. Yellow fever contrasted with bilious fever: Reasons for believing it is a disease sui generis—Its mode of propagation-remote causes-probable insect or animalcular origin. New Orleans Med. Surg. J. 1848, 4, 563–601. [Google Scholar]
- Beauperthuy, L.-D. Fiebre amarilla. Gac. Of. De Cumaná 1854, 57, 1–12. [Google Scholar]
- Boyce, R.W. Mosquito or Man; E.P. Dutton & Co.: New York, NY, USA, 1909; pp. 41–48. [Google Scholar]
- Agramonte, A. An account of Dr. Louis-Daniel Beauperthuy, a pioneer in yellow fever research. Boston Med. Surg. J. 1908, 158, 927–930. [Google Scholar] [CrossRef]
- Wood, C.A. Louis Daniel Beauperthy. Ann. Med. Hist. 1922, 4, 166–174. [Google Scholar]
- Finlay, C. El mosquito Hipotéticamente Considerado Como Agente de Transmisión de la Fiebre Amarilla; Real Academia de Ciencias Médica y Naturales de la Habana, Ministerio de Cultura: Havana, Cuba, 1881. [Google Scholar]
- Manson, P. A further observations on filarial Sanginis hominis. China Imp. Cust. Med. Rep. 1877, 14, 1–35. [Google Scholar]
- Delaporte, F. The History of Yellow Fever: An Essay on the Birth of Tropical Medicine; MIT Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Williams, G. The Plague Killer; Charles Scribner’s Sons: New York, NY, USA, 1969. [Google Scholar]
- Hosack, D. Observations on the Laws Governing the Communication of Contagious Diseases and the Means of Arresting Their Progresses; Winkle and Wiley: New York, NY, USA, 1815; pp. 35–36. [Google Scholar]
- Carter, H.R. A note on the interval between infecting and secondary cases of yellow fever from the records of the yellow fever at Orwood and Taylor, Miss., in 1898. New Orleans Med. Sug. J. 1900, 52, 617–636. [Google Scholar] [CrossRef]
- Carter, H. A note on the spread of yellow fever in houses: Extrinsic incubation. Med. Rec. 1901, 59, 933–937. [Google Scholar]
- Marchoux, E.; Salimbeni, A.; Simond, P.L. La fièvre jaune. Rapports de la mission francaise. Ann. Inst. Pasteur 1903, 7, 665–731. [Google Scholar]
- Meyer, K.F. The animal kingdom- a reservoir of human disease. Proc. Int. Med. 1931, 8, 234–261. [Google Scholar]
- Reeves, W.C. Partners: Serendipity in arbovirus research. J. Vector Ecol. 2001, 26, 1–6. [Google Scholar]
- WHO. Arthropod-borne and rodent-borne viral diseases: Report of a WHO scientific group. In WHO Technical Report Series; WHO: Geneva, Switzerland, 1985; p. 719. [Google Scholar]
- Taylor, R.M. Epidemiology. In Yellow Fever; Strode, G.K., Ed.; McGraw-Hill Book Co.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1951; pp. 427–538. [Google Scholar]
- Wilson, A.J.; Morgan, E.R.; Booth, M.; Norman, R.; Perkins, S.E.; Hauffe, H.C.; Mideo, N.; Antonovics, J.; McCallum, H.; Fenton, A. What is a vector? Philos. Trans. R. Soc. B 2017, 372, 20160085. [Google Scholar] [CrossRef]
- Clements, A.N.; Harbach, R.E. Controversies over the scientific name of the principal mosquito vector of yellow fever virus-expediency versus validity. J. Vector Ecol. 2018, 43, 1–14. [Google Scholar] [CrossRef]
- Robertson, R.C.; Hu, S.M.K. The tiger mosquito in Shanghai. China J. 1935, 23, 299–306, (Reproduced and was made available in J. Am. Mosq. Contr. Assoc. 1988, 4, 179–183). [Google Scholar]
- Boyce, R. Yellow Fever and Its Prevention; John Murray: London, UK, 1911. [Google Scholar]
- Berkeley, W.N. (Ed.) Laboratory Work with Mosquitoes; Pediatrics Laboratory: New York, NY, USA, 1902. [Google Scholar]
- Maylsamy, M. Extremely long viability of Aedes aegypti (Diptera: Culicidae) eggs stored under normal conditions. J. Med. Entomol. 2019, 56, 878–880. [Google Scholar] [CrossRef]
- Hoffmann, W.H. Yellow fever as a Far Eastern problem. In Transactions of the Sixth Biennial Congress Eastern Association of Tropical Medicine Tokyo; Kyorinsha: Tokyo, Japan, 1925; pp. 143–157. [Google Scholar]
- Ramos Báez, P. Scientific contributions of Wilhelm H. Hoffmann and yellow fever. Rev. Méd. Cuba. 1950, 11, 697–701. [Google Scholar]
- Kuno, G. Early history of laboratory breeding of Aedes aegypti (Diptera: Culicidae) focusing on the origins and use of selected strains. J. Med. Entomol. 2010, 47, 957–971. [Google Scholar] [CrossRef]
- Kumm, H.W. The geographical distribution of the yellow fever vectors. Am. J. Hyg. Monogr. Ser. 1931, 12, 46. [Google Scholar]
- Rose, N.H.; Badolo, A.; Sylla, M.; Akorli, J.; Otoo, S.; Gloria-Soria, A.; Powell, J.R.; White, B.J.; Crawford, J.E.; McBride, C.S. Dating the origin and spread of specialization on human hosts in Aedes aegypti mosquitoes. eLife 2023, 12, e83524. [Google Scholar] [CrossRef]
- Wheetman, D.; Kamsang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulbaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes mosquitoes and Aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef]
- Captain-Esoah, M.; Baidoo, P.K.; Frempong, K.K.; Adabie-Gomez, D.; Chabi, J.; Obuobi, D.; Amlalo, G.K.; Veriegh, F.B.; Donkor, M.; Asoala, V.; et al. Biting behavior and molecular identification of Aedes aegypti (Diptera: Culicidae) subspecies in some selected recent yellow fever outbreak communities in northern Ghana. J. Med. Entomol. 2020, 57, 1239–1245. [Google Scholar] [CrossRef]
- Powell, J.R.; Gloria-Soria, A.; Kotsakiozi, P. Recent history of Aedes aegypti: Vector genetics and epidemiology records. BioScience 2018, 68, 854–860. [Google Scholar] [CrossRef]
- Christophers, S.R. Aedes aegypti (L.): The Yellow Fever Mosquito; Cambridge University Press: London, UK, 1960. [Google Scholar]
- Ruiz-López, F.; González-Mazo, A.; Vélez-Mira, A.; Gómez, G.F.; Zuleta, L.; Uribe, S.; Vélez-Bernal, I.D. Presence of Aedes (Stegomyia) aegypti (Linnaeus, 1762) and its natural infection with dengue virus at unrecorded heights in Colombia. Biomédica 2016, 36, 303–308. [Google Scholar] [CrossRef]
- Trpis, M.; Hausermann, W. Genetics of house-entering behavior in East African population of Aedes aegypti (L.) (Diptera: Culicidae) and its relevance to speciation. Bull. Entomol. Res. 1978, 68, 521–532. [Google Scholar] [CrossRef]
- Shannon, R.C. The environment and behavior of some Brazilian mosquitoes. Proc. Entomol. Soc. Wash. 1931, 33, 1–27. [Google Scholar]
- Tukei, P.M.; McCrae, A.W.R. Natural history of yellow fever vectors and reservoirs: Studies in East Africa. Cah. O.R.S.T.O.M. Ser. Ent. Med. Parasitol. 1972, 10, 159–161. [Google Scholar]
- Salvan, M.; Mouchet, J. Aedes albopictus et Aedes aegypti a l’Ile de la Réunion. Ann. Soc. Belge Med. Trop. 1994, 74, 323–326. [Google Scholar]
- Carter, H.R. Preferential and compulsory breeding places of Aedes (Stegomyia) aegypti and their limits. Ann. Trop. Med. Parasitol. 1924, 18, 493–503. [Google Scholar] [CrossRef]
- Nwoke, B.E.; Nduka, F.O.; Okereke, O.M.; Ehighibe, O.C. Sustainable urban development and human health: Septic tank as a major breeding habitat of mosquito vectors of human diseases in southeastern Nigeria. Appl. Parasitol. 1993, 34, 1–10. [Google Scholar]
- Burke, R.; Barrera, R.; Lewis, M.; Kluchinsky, T.; Claborn, D. Septic tanks as larval habitats for the mosquitoes Aedes aegypti and Culex quinquefasciatus in Playa Pleyita, Puerto Rico. Med. Vet. Entomol. 2010, 24, 117–123. [Google Scholar] [CrossRef]
- Bermudi, P.M.M.; Kowalski, F.; Menzato, M.M.; Ferreira, M.d.C.; dos Passos, W.B.S.; Oku, V.J.A.; Kumow, A.; Lucio, T.V.F.M.; Lima-Camara, T.N.; Urbinatti, P.R.; et al. Aedes aegypti breeding site in an underground rainwater reservoir: A warning. Rev. Saude Pub. 2017, 51, 122. [Google Scholar] [CrossRef] [PubMed]
- Diarrassouba, S.; Dossou-Yovo, S. Rhythme d’activité atypique chez Aedes aegypti en zone de savane sub-soudonienne de C’ote d’Ivoire. Bull. Soc. Pathol. Ex. 1997, 90, 361–363. [Google Scholar]
- Chadee, D.D.; Martinez, R. Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies. J. Vector Ecol. 2000, 25, 158–163. [Google Scholar] [PubMed]
- Carter, H.R. Some characteristics of the Stegomyia fasciata which affect its conveyance of yellow fever. Med. Rec. 1904, 65, 50–64. [Google Scholar]
- Bugher, J.C. The mammalian host in yellow fever. In Yellow Fever; Strode, G.K., Ed.; McGraw-Hill Book Co.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1951; pp. 299–384. [Google Scholar]
- Johansson, M.A.; Arana-Vizcarrondo, N.; Biggerstaff, B.J.; Staple, J.E. Incubation periods of yellow fever virus. Am. J. Trop. Med. Hyg. 2010, 83, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Bates, M. Saimiri monkey as experimental host for virus of yellow fever. Am. J. Trop. Med. 1944, 24, 83–89. [Google Scholar] [CrossRef]
- Fontenille, D.; Diallo, M.; Mundo, M.; Ndiage, M.; Thonnon, J. First evidence of natural vectorial transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 533–535. [Google Scholar] [CrossRef]
- de Beaurepaire Aragâo, M.; da Costa Lima, A. Possibility of infecting male Aedes aegypti with the virus of yellow fever. Brasil-Med. 1929, 43, 671. [Google Scholar]
- Mondet, B.; Vasconcelos, P.F.C.; Travassos da Rosa, A.P.A.; Travassos da Rosa, E.S.; Rodrigues, S.G.; Travassos da Rosa, J.F.S.; Bicout, D.J. Isolation of yellow fever virus from nulliparous Haemagogus (Haemagogus) janthinomys in eastern Amazon. Vector. Borne Zoon. Dis. 2002, 2, 47–50. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; Ferreira-de-Brito, A.; dos Santos, A.A.C.; de Miranda, R.M.; de Souza Bonelly, I.; Falqueto, A.; Paupy, C.; Carvalho, G.; Moutailler, S.; Lourenço-Oliveira, R.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Cornet, M.; Robin, Y.; Hene, G.; Adam, C.; Renaudet, J.; Valade, M.; Eyroud, M. Une pouseé épizootique de fièvre jaune selvatique au Sénégal oriental. Isolement du virus de lots de moustiques adultlles males et femelles. Med. Malad. Infect. 1979, 9, 63–66. [Google Scholar] [CrossRef]
- Whitman, L.; Antunes, P.C.A. Studies on Aedes aegypti infected in larval stage with virus of yellow fever. Proc. Soc. Exp. Biol. Med. 1938, 37, 664–666. [Google Scholar] [CrossRef]
- Yuval, B. The other habit: Sugar feeding by mosquitos. Bull. Soc. Vector Ecol. 1992, 17, 150–156. [Google Scholar]
- Whitman, L. The arthropod vectors of yellow fever. In Yellow Fever; Strode, G.K., Ed.; McGraw-Hill Book Co.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1951; pp. 231–298. [Google Scholar]
- Meyer, K.F. The Zoonoses in Their Relation to Rural Health; University of California Press: Berkeley, CA, USA, 1955; p. 49. [Google Scholar]
- Saluzzo, J.F.; Hervé, J.P.; Germain, M.; Geoffroy, B.; Huard, M.; Fabre, J.; Salaün, J.J.; Hème, G.; Robin, Y. Seonde série d’isolement du virus de la fièvre jaune, á partir d’Aedes africanus (Theobald), dans une galerie forestière des savanos semi-humèdes du Sud de l’Empire Centrafricain. Cah. O.R.S.T.O.M. Ser Entom. Med. Parasitol. 1979, 17, 19–24. [Google Scholar]
- Carter, H.R. Mechanism of the spontaneous elimination of yellow fever from endemic centres. Ann. Trop. Med. 1920, 13, 299–311. [Google Scholar] [CrossRef]
- Kuno, G.; Mackenzie, J.S.; Junglen, S.; Hubálek, Z.; Plyusnin, A.; Gubler, D.J. Vertebrate reservoirs of arboviruses: Myth, synonym of amplifier, or reality? Viruses 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.F. Latent infections. J. Bcteriol. 1936, 31, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Ticks are not insects: Consequences of contrasting vector biology for transmission potential. Parasitol. Today 1998, 14, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P. Bionomics of Sabethes chloropterus Humboldt, a vector of yellow fever in Middle America. Am. J. Trop. Med. Hyg. 1958, 7, 429–440. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow Fever. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume V, pp. 139–231. [Google Scholar]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; Cardoso, J.d.C.; Goncalves-dos-Santos, M.E.; Oliveira, R.S.; Aguino-Teixeira, S.M.; Campos, A.A.S.; Almeida, M.A.B.; Simonini-Teixeira, D.; et al. Yellow fever virus maintained by Sabethes mosquitoes during dry season in Cerrado, a semiarid region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar] [CrossRef]
- Ross, R. The Prevention of Malaria; John Murray: London, UK, 1910. [Google Scholar]
- Scott, H.H. The influence of the slave trade in the spread of tropical disease. Tr. R. Soc. Trop. Med. Hyg. 1943, 37, 169–188. [Google Scholar] [CrossRef]
- Griffitts, T.H.D. Air traffic in relation to public health. Am. J. Trop. Med. 1933, 13, 283–296. [Google Scholar] [CrossRef]
- Kuno, G. The absence of yellow fever in Asia: History, hypotheses, vector dispersal, possibility of YF in Asia, and other enigmas. Viruses 2020, 12, 1349. [Google Scholar] [CrossRef]
- Findlay, G.M. Present position of yellow fever in Africa. Tr. R. Soc. Trop. Med. Hyg. 1941, 35, 51–76. [Google Scholar] [CrossRef]
- Eisler, R. Health risks of gold miners: A synoptic review. Environ. Geochem. Health 2003, 25, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, S.T.; Forshey, B.M.; Morrison, A.C.; Pazd-Soldan, V.A.; Vázquez-Prokopec, G.M.; Astete, H.; Reiner, R.C.; Vilcarromero, S.; Elder, J.P.; Halsey, E.S.; et al. House-to-house human movement drives dengue virus transmission. Proc. Nat. Acad. Sci. USA 2013, 110, 994–999. [Google Scholar] [CrossRef]
- Chungue, E.; Burucoa, C.; Boutin, J.P.; Philippon, G.; Laudon, F.; Plichart, R.; Barbazan, P.; Cardines, R.; Roux, J. Dengue-1 epidemic in French Polynesia, 1988–1989: Surveillance and clinical, epidemiological cases. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 193–197. [Google Scholar] [CrossRef]
- Ross, R. Some a priori pathometric equations. Br. Med. J. 1915, 1, 546–547. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G. The analysis of equilibrium in malaria. Trop. Dis. Bull. 1952, 49, 813–829. [Google Scholar] [PubMed]
- Legendre, J. Race de Stegomyia fasciata et fiévre jaune. Compt. Rend. Acad. Sci. 1927, 185, 1224–1226. [Google Scholar]
- Philip, C.B.; Hughes, L.E.; Darrow, D.I. Experimental transmission of yellow fever virus by Oriental mosquitoes. Proc. 10th Int. Congr. Entomol. 1958, 3, 587–592. [Google Scholar]
- Hindle, E. An experimental study of yellow fever. Tr. R. Soc. Trop. Med. Hyg. 1929, 22, 405–430. [Google Scholar] [CrossRef]
- Snijders, E.P. The yellow fever problem in the Far East. Tr. Far East Assoc. Trop. Med. 1931, 133–150. [Google Scholar]
- Dudley, S.F. Can yellow fever spread into Asia? An essay on the ecology of mosquito-borne disease. J. Trop. Med. Hyg. 1934, 37, 273–278. [Google Scholar]
- Hindle, E. Recent laboratory contributions to the control of yellow fever. Proc. R. Soc. Med. 1933, 27, 203–210. [Google Scholar] [CrossRef]
- Gubler, D.J.; Novak, R.; Mitchell, C.J. Arthropod vector competence-epidemiological, genetic, and biological considerations. In Recent Development in the Genetics of Insect Disease Vectors; Steiner, W., Tabachnick, W., Rai, K., Narang, S., Eds.; Stipes Publishing: Champaign, IL, USA, 1982; pp. 326–335. [Google Scholar]
- Laurenço-de-Oliveira, R.; Vazeille, M.; de Filippis, A.M.B.; Failloux, A.-B. Oral susceptibility to yellow fever virus of Aedes aegypti from Brazil. Mem. Inst. Oswaldo Cruz 2002, 97, 437–439. [Google Scholar] [CrossRef]
- de Lataillade, L.d.G.; Vazeille, M.; Obadia, T.; Madec, Y.; Mousson, L.; Kamgang, B.; Chen, C.-H.; Failloux, A.-B.; Yen, P.S. Risk of yellow fever virus transmission in the Asia-Pacific region. Nature Comm. 2020, 11, 5801. [Google Scholar] [CrossRef]
- Chamberlain, R.W.; Sikes, R.K.; Nelson, D.B.; Sudia, W.D. Studies on the North American arthropod-borne encephalitides. Part VI: Quantitative determinations of virus-vector relationships. Am. J. Hyg. 1954, 60, 278–285. [Google Scholar]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.P. Estimation of vectorial capacity: Pathogen extrinsic incubation and vector competence. Bull. Soc. Vector Ecol. 1989, 14, 60–66. [Google Scholar]
- Mitchell, C.J. Mosquito vector competence and arboviruses. In Current Topics in Vector Research; Harris, K.F., Ed.; Praeger: New York, NY, USA, 1983; pp. 63–92. [Google Scholar]
- Miller, B.R.; Monath, T.P.; Tabachnick, W.J.; Ezike, V.I. Epidemic yellow fever caused by an incompetent mosquito vector. Trop. Med. Parasitol. 1989, 40, 396–399. [Google Scholar] [PubMed]
- Agramonte, A. Yellow fever- a strictly human disease. N. Y. Med. J. 1912, 96, 465–468. [Google Scholar]
- Güereña-Burgueño, F. The centennial of the Yellow Fever Commission and the use of informed consent in medical research. Salud Pública De Mex. 2002, 44, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, C. At Last: A Christmas in the West Indies; Macmillan & Co.: London, UK; New York, NY, USA, 1871. [Google Scholar]
- Balfour, A. Wild monkeys as reservoir for virus of yellow fever. Lancet 1914, 1, 1176–1178. [Google Scholar] [CrossRef]
- Thomas, H.W. Yellow fever. Trans. R. Soc. Trop. Med. Hyg. 1909, 3, 59–62. [Google Scholar] [CrossRef]
- Barrie, H.J. Artifacts and archives. Diary notes on a trip to West Africa in relation to a yellow fever expedition under the auspice of the Rockefeller Foundation, 1926, by Oskar Klotz. Can. Bull. Med. Hist. 1997, 14, 153–163. [Google Scholar]
- Klotz, O. Diary of Oskar Klotz: Notes and Research Trip to West Coast of Africa: LAC Digital File: 3011072497; Library and Archives Canada (LAC): Ottawa, ON, Canada, 1926; 299p. [Google Scholar]
- Klotz, O. Diary Notes on a Trip to Africa in 1928; MG30, Series B61; Library Archives of Canada: Ottawa, ON, Canada, 1928. [Google Scholar]
- Davis, N.C. Transmission of yellow fever: Further experiments with members of New World. Am. J. Trop. Med. 1931, 11, 113–125. [Google Scholar] [CrossRef]
- Dinger, J.E.; Schüffner, W.A.P.; Swellengrebel, N.H. Onderzoek over gele koorts in Nederland (Derde Medeeling). Nederl. Tijdschr. V. Geneesk 1929, 73, 5982–5991. [Google Scholar]
- Davis, N.C. Transmission of yellow fever: On possibility of immunity in Stegomyia mosquitoes. Am. J. Trop. Med. 1931, 11, 31–42. [Google Scholar] [CrossRef]
- Downs, W.G. Epidemiological Notes in Connection with the 1954 Outbreak of Yellow Fever in Trinidad, B.W.I.; Jefferson Digital Common, Thomas Jefferson University: Philadelphia, PA, USA, 1955; 9-1955, Paper 4. [Google Scholar]
- Barnett, E.D. Yellow fever: Epidemiology and prevention. Clin. Infect. Dis. 2007, 44, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent end point. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Dulbecco, R. Production of plaques in monolayer tissue cultures by single particles of an animal virus. Proc. Nat. Acad. Sci. USA 1952, 38, 747–752. [Google Scholar] [CrossRef]
- Jones, L.D.; Davies, C.R.; Steele, G.M.; Nuttall, P.A. A novel mode of arbovirus transmission involving a nonviremic host. Science 1987, 237, 775–777. [Google Scholar] [CrossRef]
- Labuda, M.; Nuttall, P.A.; Kozuch, O.; Eleckova, E.; Williams, T.; Zuttová, E.; Sabó, A. Non-viremic transmission of tick-borne encephalitis virus: A mechanism for arbovirus survival in nature. Experientia 1993, 49, 802–805. [Google Scholar] [CrossRef]
- Higgs, S.; Schneider, B.S.; Vanlandingham, D.L.; Klingler, K.A.; Gould, E.A. Nonviremic transmission of West Nile virus. Proc. Nat. Acad. Sci. USA 2005, 124, 8871–8874. [Google Scholar] [CrossRef]
- Rosen, L.; Gubler, D.J. The use of mosquitoes to detect and propagate dengue viruses. Am. J. Trop. Med. Hyg. 1974, 23, 1153–1160. [Google Scholar] [CrossRef]
- Domingo, C.; Charrel, R.N.; Schmitt-Chanasit, J.; Zeller, H.; Reusken, C. Yellow fever in the diagnostic laboratory. Emerg. Infect. Dis. 2018, 7, 129. [Google Scholar] [CrossRef]
- Mares-Guia, M.A.M.d.M.; Horta, M.A.; Romano, A.; Rodrigues, C.D.S.; Mendonça, M.C.L.; dos Santos, C.C.; Torres, M.C.; Araujo, E.S.M.; Fabri, A.; de Souza, E.R.; et al. Yellow fever epizootics in non-human primates, Southeast and Northeast Brazil (2017 and 2018). Parasites Vectors 2020, 13, 90. [Google Scholar] [CrossRef]
- Reusken, C.; Knoester, M.; Geurtsvankessel, C.; Koopmans, M.; Knapen, D.G.; Bierman, W.F.W.; Pas, S. Urine as a sample type for molecular diagnosis of natural yellow fever virus infections. J. Clin. Microbiol. 2017, 55, 3294–3296. [Google Scholar] [CrossRef] [PubMed]
- Honigsbaum, M. Tipping the balance? Karl Friedrich Meyer, latent infections, and the birth of modern ideas of disease ecology. J. Hist. Biol. 2016, 49, 261–309. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.J. Landmarks in the conquest of yellow fever. In Yellow Fever; Strode, G.K., Ed.; McGraw-Hill Book Co.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1951; pp. 1–37. [Google Scholar]
- Bates, M.; Roca-Garcia, M. Laboratory studies of the Saimiri-Haemagogus cycle of jungle yellow fever. Am. J. Trop. Med. 1945, 25, 203–216. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Haddow, A.J. The susceptibility of African wild animals to yellow fever. 1. Monkeys. Am. J. Trop. Med. 1949, 29, 389–423. [Google Scholar] [CrossRef] [PubMed]
- Gillett, J.D. The monkey myth. Lancet 1994, 343, 126. [Google Scholar] [CrossRef]
- Burke, A.W.; Davis, N.C. Notes on laboratory infections with yellow fever. Am. J. Trop. Med. 1930, 10, 419–426. [Google Scholar] [CrossRef]
- Berry, G.P.; Kitchen, S.F. Yellow fever accidentally contracted in the laboratory. A study of seven cases. Am. J. Med. 1931, 11, 365–434. [Google Scholar]
- Findlay, G.M.; MacCallum, F.O. Epidemiology of yellow fever. Nature 1939, 143, 289. [Google Scholar] [CrossRef]
- Audouard, M.F.M. Relation historique de la fièvre jaune qui a regne au Port-du-Passage en 1829. Rev. Med. Fr. Et Etrangère 1824, 3, 224–264. [Google Scholar]
- Kodama, K. Antislavery and epidemic: Mathieu François Maxime Audouardo’s “The negro slave trade considered as the cause of yellow fever” and the city of Rio de Janeiro in 1850. Hist. Cienc. Saúde-Manguinhos Rio De Jan. 2009, 16, 2. [Google Scholar]
- Eager, J.M. Yellow fever in France, Italy, Great Britain, and Austria and bibliography of yellow fever in Europe. Yellow Fever Inst. Bull. 1902, 8, 25–35. [Google Scholar]
- Manson, P. The relation of the Panama Canal to the introduction of yellow fever into Asia. Trans. Epidemiol. Soc. Lond. 1903, 22, 60–100. [Google Scholar] [PubMed]
- Morris, A.D. The epidemic that never was: Yellow fever in Hawaii. Hawaii Med. J. 1995, 54, 781–784. [Google Scholar] [PubMed]
- Mouchet, J.; Giacomin, T.; Julvez, J. La diffusion anthropique des arthropods vecteurs de maladie dans le monde. Cah. Santé 1995, 5, 293–298. [Google Scholar]
- Sawchuk, L.A.; Burke, S.D.A. Gibraltar’s 1804 yellow fever scourge: The search for scapegoats. J. Hist. Med. Allied Sci. 1998, 53, 3–42. [Google Scholar] [CrossRef] [PubMed]
- Sawchuk, L.A.; Tripp, L. Managing epidemic in imperfect times: Encampment and immunity passes in 19th century Gibraltar. BMJ Glob. Health 2021, 6, 006713. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Epidemiological update for dengue, chikungunya.a and Zika. In Weekly Epidemiological Update for Dengue and Other Arboviruses; Update: 10 June 2023; PAHO: Washington, DC, USA, 2023. [Google Scholar]
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef]
- Powell, J.R. An evolutionary perspective on vector-borne diseases. Front. Genet. 2019, 10, 1266. [Google Scholar] [CrossRef]
- French, R.K.; Holmes, E.C. An ecosystems perspective on virus evolution and emergence. Trends Microbiol. 2020, 28, 165–175. [Google Scholar] [CrossRef]
- Juntes, E.S.; Poumerol, G.; Gershman, M.D.; Hill, D.R.; Lemarchand, J.; Lewis, R.F.; Staples, J.E.; Tomori, O.; Wilder-Smith, A.; Monath, T.P. The revised global yellow fever risk map and recommendations for vaccination, 2010. Consensus of the informal WHO Working group on geographic risk for yellow fever. Lancet Infect. Dis. 2011, 11, 622–632. [Google Scholar] [CrossRef]
- Soper, F.L. Rural and jungle yellow fever: New public health problem in Colombia. Rev. Hig. Bogotá 1935, 4, 49–84. [Google Scholar]
- Jones, J. Original investigations on the natural history (symptoms and pathology) of yellow fever. 1854–1894. J. Am. Med. Assoc. 1894, 23, 885–893. [Google Scholar] [CrossRef]
- Mondet, B. Considerations sur l’epidemiologie de la fièbre jaune au Brésil. Bull. Pathol. Ex. 2001, 94, 260–267. [Google Scholar]
- Franco, R.; Martínez Santamaria, J.; Torovilla, G. Fiebre Amarilla y Fiebre Espiroquetal; Academia Nacional de Medicinas, Sesiónes Scientíficas del Centenario: Bogotá, Colombia, 1911. [Google Scholar]
- Pena, C.A.; Serpa, R.; Beaver, G. Yellow fever in Colombia with special reference in the epidemic in Socorro in 1929. J. Prev. Med. 1930, 4, 417–457. [Google Scholar]
- Soper, F.L. The newer epidemiology of yellow fever. Am. J. Public Health Nation’s Health 1937, 27, 1–14. [Google Scholar] [CrossRef]
- Soper, F.L.; Penna, H.; Cardoso, E.; Serafim, J.; Frobisher, M.; Pinheiro, J. Yellow fever without Aedes aegypti: Study of rural epidemic in Valle do Chanaan, Espírito Santo, Brazil, 1932. Am. J. Hyg. 1933, 18, 555–587. [Google Scholar] [CrossRef]
- Lutz, A. Reminiscências de febre amarela no Estado de Sâo Paulo. Mem. Inst. Oswaldo Cruz 1930, 24, 127–142. [Google Scholar] [CrossRef]
- Li, S.L.; Acosta, A.L.; Hill, S.C.; Brady, O.J.; de Almeid, M.A.B.; Cardoso, J.d.C.; Hamlet, A.; Mucci, L.F.; de Deus, J.T.; Iani, F.C.M.; et al. Mapping environmental suitability of Haemagogus and Sabethes spp. mosquitoes to understand sylvatic transmission risk of yellow fever virus in Brazil. PLoS Negl. Trop. Dis. 2022, 16, e0010019. [Google Scholar] [CrossRef]
- Causey, O.R.; Kumm, H.W.; Laemmert, H.W. Dispersion of forest mosquitoes in Brazil: Further studies. Am. J. Trop. Med. Hyg. 1950, 30, 301–312. [Google Scholar] [CrossRef]
- Downs, W.G. History on the natural history of yellow fever in Trinidad. In Studies on the Natural History of Yellow Fever in Trinidad; Tikasingh, E.S., Ed.; Caribbean Epidemiology Centre: Port of Spain, Trinidad, 1991; Series 1; pp. 2–5. [Google Scholar]
- Downs, W.G. The known and the unknown in yellow fever ecology and epidemiology. Ecol. Dis. 1982, 1, 103–110. [Google Scholar]
- Davis, N.C. Susceptibility of marmosets to yellow fever virus. J. Exp. Med. 1930, 52, 405–415. [Google Scholar] [CrossRef]
- de Azevedo Fernandes, N.C.C.; Guerra, J.M.; Diaz-Delgado, J.; Cunha, M.S.; Saad, L.d.C.; Iglezias, S.D.; Ressio, R.A.; Cirqueira, C.d.S.; Yanamura, C.T.; Jesus, I.P.; et al. Differential yellow fever susceptibility in New World nonhuman primates, comparison with humans, and implications for surveillance. Emerg. Infect. Dis. 2021, 27, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Muench, H. Derivation of rates from summation data by catalytic curve. J. Am. Stat. Assoc. 1934, 29, 25–38. [Google Scholar] [CrossRef]
- Auguste, A.J.; Lemey, P.; Pybus, O.G.; Suchard, M.A.; Salas, R.A.; Adesiyun, A.A.; Barrett, A.D.; Tesh, R.B.; Weaver, S.C.; Carrington, C.V.F. Yellow fever virus maintenance in Trinidad and its dispersal throughout the Americas. J. Virol. 2010, 84, 9967–9977. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fevers. Infect. Genet. Evol. 2013, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Hernandez Acosta, E.; Chaves, B.A.; Fé, N.F.; Valerio, D.; Mendonca, C.; De Lacerda, M.V.G.; Buenemann, M.; Vasilakis, N.; Hanley, K.A. Into the woods: Changes in mosquito community composition and presence of key vectors at increasing distances from the urban edge in urban forest parks in Manaus, Brazil. Acta Trop. 2020, 206, 105449. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; Ferreira-de-Brito, A.; Azevedo, A.d.S.; Linhares, J.H.R.; Santos, V.d.O.; Miranda, E.H.; Neves, M.S.A.S.; Yousfi, L.; Ribeiro, I.P.; dos Santos, A.A.C.; et al. Survey on non-human primates and mosquitoes does not provide evidences of spillover/spill back between the urban and sylvatic cycles of yellow fever and Zika viruses following severe outbreaks in southeast Brazil. Viruses 2020, 12, 364. [Google Scholar] [CrossRef]
- Fontenille, D.; Powell, J.R. From anonymous to public enemy: How does a mosquito become a feared arbovirus vector. Pathogens 2020, 9, 265. [Google Scholar] [CrossRef]
- Haddow, A.J. The natural history of yellow fever in Africa. Proc. R. Soc. Edinb. 1968, 70, 191–227. [Google Scholar] [CrossRef]
- Lumsden, W.H.R. The crepuscular biting activity of insects in the forest canopy in Bwamba, Uganda. A study in relation to the sylvan epidemiology of yellow fever. Bull. Entom. Res. 1952, 42, 721–760. [Google Scholar] [CrossRef]
- Pettit, A.; Stefanopoulo, G.J. Fièvre jaune chez un singe du nord-africain, Macacus inuus L. Compt. Rend. Soc. Biol. 1930, 104, 63–65. [Google Scholar]
- Smith, T. Brief sketch of the fever which prevailed at Gibraltar in the Autumn of 1829; together with observations on the answers of Sir William Pym to queries from the Royal Medico-Chirurgical Society of Cadiz, addressed to him on the origin and nature of that disease. Edinburgh Med. Surg. J. 1931, 35, 12–52. [Google Scholar]
- Hindle, E. Filterable virus. Proc. R. Soc. Med. 1929, 22, 823–826. [Google Scholar] [PubMed]
- Germain, M.; Cornet, M.; Mouchet, J.; Herve, J.P.; Robert, V.; Camicas, J.L.; Cordellier, R.; Hervy, J.P.; Digoutte, J.P.; Monath, T.P.; et al. La fièvre jaune selvetique en Afrique: Donees recentres et conceptions actuelles. Med. Trop. 1981, 41, 31–43. [Google Scholar]
- Smith, C.E.G. Factors influencing the hehaviour of viruses in their arthropod hosts. In Host-Parasite Relationship in Invertebrate Hosts; Taylor, A.E.R., Ed.; Blackwell Science Press: Oxford, UK, 1964; pp. 1–31. [Google Scholar]
- Garret-Jones, C. A dispersion of mosquitoes by wind. Nature 1950, 165, 285. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Sureau, P.; Herve, J.P.; Fabre, J.; Mouchet, J.; Robin, Y.; Geoffroy, B. Isolements du virus de la fièvre jaune a partir du groupe A. africanus (Theobald) en Républíque Centrafricaine: Importance des savanes humides et semi-humides en tanto que zone d’emergence du virus amaril. Cah. O.R.S.T.O.M. Ent. Med. Parasitol. 1979, 14, 125–139. [Google Scholar]
- Soper, F.L. The geographical distribution of immunity to yellow fever in man in South America. Am. J. Trop. Med. 1937, 17, 457–511. [Google Scholar] [CrossRef]
- Kerr, J.A.; Patiño-Camargo, L. Investigaciones sobre fiebre amarilla en Muzo y Santander. Rev. De Hig. Bogotâ 1933, 2, 63–83. [Google Scholar]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.d.P.; Pissinatti, A.; da Cunha, R.V.; Freire, M.; Martins, R.M.; Homma, A. The puzzle of rapid viral spread and challenges for immunization. Mem. Inst. Oswaldo Cruz 2018, 113, 1–12. [Google Scholar] [CrossRef]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; Goes de Jesus, J.; de Aguiar, R.S.; Iani, F.C.; Xavier, J.; Quick, J.; DuPlessis, L.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 2019, 361, 894–899. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; de Andreazzi, C.S.; Neves, M.S.A.S.; Meneguete, P.S.; Ribeiro, M.S.; Dias, C.M.G.; Motta, M.d.A.; Brucellos, C.; Româo, A.R.; Magalháes, M.d.A.F.M.; et al. Ecological and environmental factors affecting transmission of sylvatic yellow fever in the 2017-2019 outbreak in the Atlantic Forest, Brazil. Parasites Vectors 2022, 15, 23. [Google Scholar] [CrossRef]
- Galindo, P.; Srihongse, S. Evidence of recent jungle yellow-fever activity in Eastern Panamá. Bull. Wld. Health Org. 1967, 36, 157–161. [Google Scholar]
- Prist, P.R.; Tambosi, L.R.; Mucci, L.F.; Pinter, A.; de Souza, R.P.; Muylaert, R.d.L.; Rhodes, J.R.; Comin, C.H.; Costa, L.d.F.; D’Agostini, T.L.; et al. Roads and forest edges facilitate yellow fever virus dispersion. J. Appl. Ecol. 2022, 59, 4–17. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; Delatorre, E.; Ferreira-de-Brito, A.; de Castro, M.G.; Ribeiro, I.P.; Furtado, N.D.; Vargas, W.P.; Ribeiro, M.S.; Menequete, P.; Ronaldo, M.D.; et al. Combination of surveillance tools reveals that yellow fever virus can remain in the same Atlantic Forest area at least for three transmission seasons. Mem. Oswaldo Cruz 2019, 114, e190076. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R. An epidemic of yellow fever in the Nuba Mountains, Anglo-Egyptian Sudan. Ann. Trop. Med. Parasitol. 1941, 35, 67–108. [Google Scholar] [CrossRef]
- Cordellier, R.; Akoliba, P. Les moustiques de la sempervirente du sud-ouest ivoirien: I. Etude du contact entre l’homme et les vecteurs potentiels de fièvre jaune au niveau du sol. Cah. O.R.S.T.O.M. Ser. Ent. Med. Parasitol. 1981, 19, 297–301. [Google Scholar]
- Nwachukwu, W.E.; Yusuff, A.; Nwangwu, U.; Okon, A.; Ogunniyi, A.; Imuetiyan-Clement, J.; Besong, M.; Ayo-Ajayi, P.; Nikau, J.; Baloa, A.; et al. The response to re-emergence of yellow fever in Nigeria, 2017. Int. J. Infect. Dis. 2020, 92, 189–196. [Google Scholar] [CrossRef]
- Eckert, J. In the days of the epidemic: The 1793 yellow fever outbreak in Philadelphia as seen by physicians. Trans. Stud. Coll. Physicians Phila. 1993, 15, 31–38. [Google Scholar]
- Seaman, V. An inquiry into the cause of the prevalence of the yellow fever in New York. Med. Reposit. 1798, 1, 315–332. [Google Scholar]
- Soler, J.C.; Fusté, M.R.P.; Herrandis, R.A.; Adell, C.N.; Lawrence, R.S. A mortality study of the last outbreak of yellow fever in Barcelona City (Spain) in 1870. Gac. Sanit. 2009, 23, 295–299. [Google Scholar] [CrossRef]
- Shannon, G.W. Disease mapping and early theories of yellow fever. Prof. Geogr. 1981, 33, 221–227. [Google Scholar] [CrossRef]
- Snow, J. On the Mode of Transmission of Cholera; John Churchill: London, UK, 1855. [Google Scholar]
- Hughes, G. Natural History of Barbados, in Ten Books; (Originally published by author in London in 1750); Arno Press: New York, NY, USA, 1972. [Google Scholar]
- Nogueira, P. The early history of yellow fever. Jefferson Digit. Commons 1955, 9–1955. [Google Scholar]
- Sawchuk, L.; Benady, S. Diary of an Epidemic: Yellow Fever in Gibraltar 1828; Gibraltar Government Heritage Division: Gibraltar, UK, 2003. [Google Scholar]
- Jankilevich, A. La epidemia de fiebre amarilla de Buenos Aires, 1871. Hosp. Y Comunidad 1999, 2, 108–118. [Google Scholar]
- Duffy, J. Yellow fever in the Continental United States during the nineteenth century. Bull. N. Y. Acad. Med. 1968, 44, 687–701. [Google Scholar] [PubMed]
- Anglotti, E. La fiebre amarilla. Historia y situación actual. La fiebre amarilla en la Barcelona de 1821. Rev. De Sanid. E Hig. Pública 1980, 54, 89–102. [Google Scholar]
- Chastel, C. La “peste” de Barcelone. Epidémie de fièvre jaune de 1821. Bull. Soc. Pathol. Ex. 1999, 92, 405–407. [Google Scholar]
- Mackellar, F.L. Early mortality data: Sources and difficulties of interpretation. In The Cambridge World History of Human Diseases; Kiple, K.F., Ed.; Cambridge University Press: Cambridge, UK, 1993; pp. 209–213. [Google Scholar]
- McNeil, J.R. Ecology, epidemics and empires: Environmental change and the geopolitics of tropical America, 1600–1825. Environ. Hist. 1999, 5, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C. The yellow fever epidemic at Rio de Janeiro. Public Health Rept. 1928, 43, 3079–3083. [Google Scholar] [CrossRef]
- Anonymous. Yellow fever at Rio de Janeiro. Public Health Rept. 1929, 44, 1657–1659. [Google Scholar]
- Salomón, O.D.; de Arias, A.R. The second coming of urban yellow fever in the Americas: Looking the past to see the future. An. Acad. Bras. Cienc. 2022, 94, e20201252. [Google Scholar] [CrossRef]
- Walcott, A.M.; Cruz, E.; Paoliello, A.; Serafim, J. An epidemic of urban yellow fever which originated from a case contracted in the jungle. Am. J. Trop. Med. 1937, 17, 677–688. [Google Scholar] [CrossRef]
- Lewis, D.J. Mosquitoes in relation to yellow fever in the Nuba Mountains, Anglo-Egyptian Sudan. Ann. Trop. Med. Parasitol. 1943, 37, 65–76. [Google Scholar] [CrossRef]
- Ngalamulume, K. Keeping the city totally clean: Yellow fever and the politics of prevention in colonial Saint-Louis-du-Sénégal, 1850–1914. J. Afr. Hist. 2004, 45, 183–202. [Google Scholar] [CrossRef]
- Nwaiwu, A.U.; Musekiwa, A.; Tamuzi, J.L.; Sambala, E.Z.; Nyasulu, P.S. The incidence and mortality of yellow fever in Africa: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 1089. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Faria, N.R.; Reiner, R.C.; Golding, N.; Kolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.R.W.; et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of Congo 2015–2016: A modeling study. Lancet Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, F.; Frosch, P. Berichte der Kommission zur Erfoschung der Maulund-Klauenseude bei den Institut für Infections Krankheiten in Berlin. Zbl. Bakteriol. Abt. I. Orig. 1898, 23, 371–391. [Google Scholar]
- Reed, W.; Carroll, J. The etiology of yellow fever. A supplemental note. Am. Med. 1902, 3, 301–305. [Google Scholar] [CrossRef]
- Mahaffy, A.F. Progress in the conquest of yellow fever during the period 1905-1930. In Thomas Jefferson University Digital Commons, Jefferson History and Publications; Thomas Jefferson University: Philadelphia, PA, USA, 1955; Paper 9. [Google Scholar]
- Mathis, C.; Sellards, A.W.; Laigret, J. Sensibilité du Macaca rhesus au virus de la fièvre jaune. C.R. Acad. Sci. 1928, 188, 604–606. [Google Scholar]
- Aragâo, H. de Beaurepaire. Report upon some researches on yellow fever. Mem. Inst. Oswaldo Cruz 1928, 2, 35–46. [Google Scholar]
- Stokes, A.; Bauer, J.H.; Hudson, N.P. Experimental transfer of yellow fever virus to laboratory animals. Am. J. Trop. Med. Hyg. 1928, 8, 103–164. [Google Scholar] [CrossRef]
- Councilman, W.T. Report on etiology and prevention of yellow fever: Pathological anatomy and histology. U.S. Marine Hosp. Serv. Publ. Health Bull. 1890, 2, 151–159. [Google Scholar]
- Sawyer, W.A.; Lloyd, W.; Kitchen, S.F. Preservation of yellow fever virus. J. Exp. Med. 1929, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.H. Antigenic analysis of certain group B arthropod-borne viruses by antibody adsorption. J. Exp. Med. 1960, 111, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.M.; Lenches, E.M.; Eddy, S.R.; Shin, S.J.; Sheets, R.L.; Strauss, J.H. Nucleotide sequence of yellow fever virus: Implications for flavivirus gene expression and evolution. Science 1985, 229, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Reagan, R.L. Electron microscopy of yellow fever virus (17D strain). Am. J. Pathol. 1953, 29, 1157–1159. [Google Scholar] [PubMed]
- Deubel, V.; Digoutte, J.P.; Monath, T.P.; Givart, M. Genetic heterogeneity of yellow fever virus strains from Africa and the Americas. J. Gen. Virol. 1986, 67, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, R.D.V.; Montoya-Diaz, E.; Khera, T.; Welsch, K.; Tegtemeyer, B.; Hoeht, S.; Cieseki, S.; Brown, R.J.P. Yellow fever: Integrating current knowledge with technological innovations to identify strategies for controlling a re-emerging virus. Viruses 2019, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D.T. Out of Africa: A molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007, 3, e75. [Google Scholar] [CrossRef]
- Sall, A.A.; Faye, O.; Diallo, M.; Firth, C.; Kitchen, A.; Holmes, E.C. Yellow fever virus exhibits slower evolutionary dynamics than dengue virus. J. Virol. 2010, 84, 765–772. [Google Scholar] [CrossRef]
- Metky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Beniecki, M.L.; Gladden-Young, A.; et al. Zika virus evolution and spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef]
- Vasilakis, N.; Fokam, E.B.; Hanson, C.T.; Weinberg, E.; Sall, A.A.; Whitehead, S.S.; Hanley, K.A.; Weaver, S.C. Genetic and phenotypic characterization of sylvatic dengue virus type 2 strains. Virology 2008, 377, 296–307. [Google Scholar] [CrossRef]
- Kuno, G. Review of the factors modulating dengue transmission. Epidemiol. Rev. 1995, 17, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Freire, C.C.M.; Lamarino, A.; Faye, O.; de Olivera, J.V.C.; Diallo, M.; Zanotto, P.M.A.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Tomazella, M.V.; Neto, O.A.L.; Junior, F.F.D.; Jorge, F.A.; Moreira, D.d.C.; Junior, A.D.L.; Presibella, M.M.; Riedigerm, I.N.; da Silava, R.A.; de Carvalho, I.M.V.G.; et al. Insights on Zika virus envelope gene conservation in American outbursts. Braz. J. Micobiol. 2020, 51, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.-J.J. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005, 18, 608–637. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: The attributes of virulence. J. Infect. Dis. 2001, 184, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.D. Yellow fever epidemics and mortality in the United States, 1693–1905. Soc. Sci. Med. 1992, 34, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Curtin, P.D. Death by Migration: Europe’s Encounter with the Tropical World in the Nineteenth Century; Cambridge University Press: New York, NY, USA; Melbourne, Australia, 1989. [Google Scholar]
- Curtin, P.D. The end of the “White Man’s Grave”? Nineteenth century mortality in West Africa. J. Interdisc. Hist. 1990, 21, 63–88. [Google Scholar] [CrossRef]
- Lewis, M.J. Yellow fever activity in Trinidad: An historical review, 1620–1978. In Studies on the Natural History of Yellow Fever in Trinidad; Tikasingh, E.S., Ed.; Epidemiology Centre: Port of Spain, Trinidad, 1991; Series 1; pp. 6–13. [Google Scholar]
- Carey, D.E. Chikungunya and dengue: A case of mistaken identity? J. Hist. Med. 1971, 26, 243–262. [Google Scholar] [CrossRef]
- Kuno, G. A re-examination of the history of etiologic confusion between dengue and chikungunya. PLoS Negl. Trop. Dis. 2015, 9, e0004101. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever: Its history and resurgence as a global public health problem. In Dengue and Dengue Hemorrhagic Fever; Gubler, D.J., Kuno, G., Eds.; CAB International: Wallingford, UK, 1977; pp. 1–22. [Google Scholar]
- Alfnes, K.; Eldholm, V.; Gaunt, M.W.; de Lamballerie, X.; Gould, E.A.; Pettersson, J.H.-O. Tracing and tracking the emergence, epidemiology and dispersal of dengue virus to Africa during the 20th century. One Health 2021, 13, 100337. [Google Scholar]
- Twiddy, S.S.; Holmes, E.C.; Rambaut, A. Inferring the rate and time-scale of dengue virus evolution. Mol. Biol. Evol. 2003, 20, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Dick, O.B.; San Martin, J.L.; Montoya, R.H.; del Diego, J.; Zambrano, B.; Doyan, G.H. The history of dengue outbreaks in the Americas. Am. J. Trop. Med. Hyg. 2012, 87, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Selemane, I. Epidemiological monitoring of the last outbreak of yellow fever in Brazil-An outlook from Portugal. Travel Med. Infect. Dis. 2019, 28, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Servadio, J.L.; Muñoz-Zanzi, C.; Convertino, M. Estimating case fatality risk of severe yellow fever cases: Systematic literature review and meta-analysis. BMC Infect. Dis. 2021, 21, 819. [Google Scholar] [CrossRef]
- Alizon, S.; Hurford, A.; Mideo, N.; Van Boalan, M. Virulence evolution and the trade-off hypothesis: History, current state of affairs and future. J. Evol. Biol. 2009, 22, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Marianneau, P.; Steffan, A.M.; Royer, C.; Drouet, M.T.; Kirn, A.; Deubel, V. Differing infection patterns of dengue and yellow fever viruses in a human hepatoma cell line. J. Infect. Dis. 1998, 178, 1270–1278. [Google Scholar] [CrossRef]
- Cardosa, J.; Ooi, M.H.; Tio, P.H.; Perera, D.; Holmes, E.C.; Bibi, K.; Manap, Z.A. Dengue virus serotype 2 from a sylvatic lineage isolated from a patient with dengue hemorrhagic fever. PLoS Negl. Trop. Dis. 2009, 3, e423. [Google Scholar] [CrossRef]
- Vasilakis, N.; Cardosa, J.; Hanley, K.A.; Holmes, E.C.; Weaver, S.C. Fever from the forest: Prospects for the continual emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011, 9, 532–541. [Google Scholar] [CrossRef]
- Man, O.; Kraay, A.; Thomas, R.; Trostle, S.; Lee, G.O.; Robbins, C.; Morrison, A.C.; Colona, J.; Eisenberg, J.N.S. Characterizing dengue transmission in rural areas: A systematic review. PLoS Negl. Trop. Dis. 2023, 17, e0011333. [Google Scholar] [CrossRef]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its history in Africa and its spread to new regions in 2013-2014. J. Infect. Dis. 2016, 24 (Suppl. S5), S436–S440. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bugallo, G.; Piedra, L.A.; Rodriguez, M.; Bisset, J.A.; Lourenço-de-Oliveira, R.; Weaver, S.C.; Vasilakis, N.; Vega-Rúa, A. Vector-borne transmission and evolution of Zika virus. Nature Ecol. Evol. 2019, 3, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Scanlon, J.E.; Umparivit, P.; Udomsakdi, S. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am. J. Trop. Med. Hyg. 1969, 18, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.H.O.; Eldholm, V.; Seligman, S.J.; Lundkvist, A.; Falconar, A.K.; Gaunt, M.W.; Musso, D.; Nougairéde, A.; Charrel, R.; Gould, E.A.; et al. How did Zika virus evolve in the Pacific islands and Latin America. MBio 2016, 7, e01239-16. [Google Scholar] [CrossRef]
- Alonso-Palomares, L.A.; Moreno-Garcia, M.; Lanz-Mendoza, H.; Salazar, M.I. Molecular basis for arbovirus transmission by Aedes aegypti mosquitoes. Intervirology 2018, 61, 255–266. [Google Scholar] [CrossRef]
- Dennis, L.H.; Reisberg, B.E.; Crosbie, J.; Crozier, D.; Conrad, M.E. The original haemorrhagic fever: Yellow fever. Br. J. Haematol. 1969, 17, 455–462. [Google Scholar] [CrossRef]
- Soper, F.L. The 1957 status of yellow fever in the Americas. Mosq. News 1958, 18, 203–216. [Google Scholar]
- Elton, N.W. Yellow fever in Central America: The imminent threat to Mexico and the United States. Am. J. Publ. Health 1956, 46, 1259–1265. [Google Scholar] [CrossRef]
- Boshell, J. Yellow fever in Central America. In Yellow Fever, a Symposium in Commemoration of Carlos Juan Finlay; Thomas Jefferson University: Philadelphia, PA, USA, 1955; Paper 5. [Google Scholar]
- de Almeida, M.A.B.; dos Santos, E.; Cardoso, J.d.C.; da Silva, L.G.; Rabelo, R.M.; Bicca-Marques, C. Predicting yellow fever through species distribution modeling of virus, vector, and monkeys. EcoHealth 2019, 16, 95–108. [Google Scholar] [CrossRef]
- Hamrick, P.N.; Aldighieri, S.; Machado, G.; Leonel, D.G.; Vilca, L.M.; Uriona, S.; Schneider, M.C. Geographic patterns and environmental factors associated with human yellow fever presence in the Americas. PLoS. Negl. Trop. Dis. 2017, 11, e0005897. [Google Scholar] [CrossRef]
- Ellice, B.R.; Barrett, A.D.T. The enigma of yellow fever in East Africa. Rev. Med. Virol. 2008, 18, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, X.-D.; Vasilakis, N.; Tian, J.-H.; Li, C.-X.; Chen, L.-J.; Eastwood, g.; Diao, X.-N.; Chen, M.-H.; Chen, X.; et al. Divergent viruses discovered in arthropods and vertebrates revise the evolutionary history of the flaviviruses and related viruses. J. Virol. 2016, 90, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.G.G.; de Souza, W.M.; Parry, R.; Gifford, R.J. Comparative analysis of genome-encoded viral sequences reveals the evolutionary history of flavivirids (Family Flaviviridae). Virus Evol. 2022, 8, veac085. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.-J.J.; Tuschiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the Genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Host range specificity of flaviviruses: Correlation with in vitro replication. J. Med. Entomol. 2007, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Forrester, N.L.; Palacios, G.; Tesh, R.B.; Savji, N.; Guzman, H.; Sherman, M.; Weaver, S.C.; Lipkin, W.I. Genome-scale phylogeny of the Alphavirus Genus suggests a marine origin. J. Virol. 2012, 86, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Bell-Sakyi, L.; Weisheit, S.; Rückert, C.; Barry, G.; Fazakerley, J.; Fragkoudis, R. Microscopic visualization of zoonotic arbovirus replication in tick cell line and organ cultures using Semliki Forest virus reporter systems. Vet. Sci. 2016, 3, 28. [Google Scholar] [CrossRef]
- Varelas-Wesley, L.; Calisher, C.H. Antigenic relationship of flaviviruses with undetermined arthropod-borne status. Am. J. Trop. Med. Hyg. 1982, 31, 1273–1284. [Google Scholar] [CrossRef]
- Holmes, E.C. Molecular clocks and the puzzle of RNA virus origins. J. Virol. 2003, 77, 3893–3897. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Fiz-Palacios, O. Dating the origin of the Genus Flaviivrus in the light of Beringian biogeography. J. Gen. Virol. 2014, 95, 1969–1982. [Google Scholar] [CrossRef]
- Cook, S.; Holmes, E.C. A multi gene analysis of the phylogenetic relationships among the flaviviruses (Family Flaviviridae) and the evolution of vector transmission. Arch. Virol. 2005, 151, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Soghigian, J.; Sither, C.; Justi, S.A.; Morinaga, G.; Cassel, B.K.; Vitek, C.J.; Livdahl, T.; Xia, S.-Y.; Gloria-Soria, A.; Powell, J.R.; et al. Phylogenomics reveals the history of host use in mosquitoes. Nat. Commun. 2023, 14, 6252. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Valencia, J.C.; Muñoz-Laiton, P.; Gómez, G.F.; Correa, M.M. a systematic revision on the viruses of Anopheles mosquitoes: The potential importance for public health. Trop. Med. Infect. Dis. 2023, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Replication of dengue, yellow fever, St. Louis encephalitis, and vesicular stomatitis viruses in a cell line (TRA-171) derived from Toxorhynchites amboinensis. In Vitro 1981, 17, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.; Shroyer, D.A. Comparative susceptibility of five species of Toxorhynchites mosquitoes to parenteral infection with dengue and other flaviviruses. Am. J. Trop. Med. Hyg. 1985, 34, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. The boundaries of arboviruses: Complexities revealed in their host ranges, virus-host interactions and evolutionary relationships. In Arboviruses: Molecular Biology, Evolution and Control; Vasilakis, N., Gubler, D.J., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 219–268. [Google Scholar]
- Gaunt, M.W.; Pettersson, J.H.-O.; Kuno, G.; Gaunt, B.; de Lamballerie, X.; Gould, E.A. Widespread interspecific phylogenetic tree incongruence between mosquito-borne and insect-specific flaviviruses at hotspots originally identified in Zika virus. Virus Evol. 2022, 8, veac027. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H. Antigenic relationships of the arboviruses: An ecological and evolutionary approach. In International Symposium on New Aspects in Ecology of Arboviruses. Proceedings Institute of Virology; Slovak Academy of Sciences: Bratislava, Slovakia, 1980; pp. 117–152. [Google Scholar]
- Díaz, L.A.; Flores, F.S.; Quaglia, A.; Contigiani, M.S. Intertwined arbovirus transmission activity: Reassessing the transmission cycle paradigm. Front. Physiol. 2013, 3, 493. [Google Scholar] [CrossRef]
- Hinman, E.H. the medical entomologist in public health. Public Health Rep. 1952, 67, 753–758. [Google Scholar] [CrossRef]
- Spielman, A.; Pollack, R.J.; Kiszewski, A.E.; Telford, S.R. Issues in public health entomology. Vector-Borne Zoon. Dis. 2001, 1, 3–19. [Google Scholar] [CrossRef]
- Connelly, R. Highlights of medical entomology 2018: The importance of sustainable surveillance of vectors and vector-borne pathogens. J. Med. Entomol. 2019, 56, 1183–1187. [Google Scholar] [CrossRef]
- Calisher, C.H.; Blair, C.D.; Bowen, M.D.; Casals, J.H.; Drebot, M.A.; Henbcghal, E.A.; Karabatsos, N.; LeDuc, J.H.W.; Repik, P.N.M.; Roehrig, J.T.; et al. Identification of arboviruses and certain rodent-borne viruses: Reevaluation of the paradigm. Emerg. Infect. Dis. 2001, 7, 756–758. [Google Scholar] [CrossRef]
- Tourney, S.; Cameron, E.R.; Cloutier, C.A.; Buddle, C.M. Non-repeatable science: Assessing the frequency of voucher specimen deposition reveals that most arthropod research cannot be verified. PearJ 2015, 3, e11678. [Google Scholar] [CrossRef]
- Braack, L.; Gouveira de Almeida, A.P.; Cornel, A.L.; Swanepoel, R.; de Jager, C. Mosquito-borne arboviruses of Africa origin: Review of key viruses and vectors. Parasitol. Vectors 2018, 11, 29. [Google Scholar] [CrossRef]
- Thompson, C.W.; Phelps, K.L.; Allard, M.W.; Cook, J.A.; Dunnum, J.L.; Ferguson, A.W.; Gelang, M.N.; Khan, F.A.A.; Paul, D.L.; Reader, D.M.; et al. Preserve a voucher specimen! The critical need for integrating natural history collections in infectious disease studies. mBio 2021, 12, e02698-20. [Google Scholar] [CrossRef]
- Gryseels, S.; Watts, T.D.; Kabongo Mpolesha, J.M.; Larsen, B.B.; Lemey, P.; Muyembe-Tamfum, J.J.; Teuwen, D.E.; Worobey, M. A near full-length HIV-1 genome from 1966 recovered from formalin-fixed paraffin-embedded tissue. Proc. Nat. Acad. Sci. USA 2020, 117, 12222–12229. [Google Scholar] [CrossRef]
- Fantini, B. Anophelism without malaria: An ecological and epidemiological puzzle. Parasitology 1994, 36, 83–106. [Google Scholar]
- Olson, M.F.; Juarez, J.G.; Kraemer, M.U.G.; Messina, J.P.; Hamer, G.L. Global patterns of aegyptism without arbovirus. PLoS Negl. Trop. Dis. 2021, 15, e0009397. [Google Scholar] [CrossRef]
- Hotez, P.J.; LaBeaud, A.D. Yellow Jack’s potential return to the American South. N. Engl. J. Med. 2023, 389, 1445–1447. [Google Scholar] [CrossRef]
- Rodrigo, F.S. The influence of meteorological conditions on the yellow fever epidemic in Cádiz (Southern Spain) in 1800: A historical scientific controversy. Atmosphere 2020, 11, 405. [Google Scholar] [CrossRef]
- Ajybtamento de Barcelona. Barcelona and 1821 Epidemic. Arxiu Historia de la Ciutat de Barcelona. Available online: https://www.barcelona.cat/ca (accessed on 4 December 2023).
- Geleano, D. Médicos y policías durante la epidemia de fiebre amarilla (Buenos Aires, 1871). Rev. Salud. Colectiva 2009, 5, 107–120. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yamaguchi, T.; Yamamoto, N.; Nishiura, H. Modeling the elevated risk of yellow fever among travelers visiting Brazil, 2018. Theor. Biol. Med. Model. 2018, 15, 9. [Google Scholar] [CrossRef]
- Dorigatti, I.; Morrison, S.; Donnelly, C.A.; Garske, T.; Bowden, S.; Grills, A. Risk of yellow fever virus importation into the United States from Brazil, outbreak years 2010–2017 and 2017–2018. Sci. Rep. 2019, 9, 20420. [Google Scholar] [CrossRef]
- Ding, S.; Xiong, Y.; Pan, H.H.; Li, Z.-G.; Tang, S.; Huang, X.-K.; Li, J.-X.; Shi, Y.; Fu, W.-J.; Cheng, H.-J.; et al. Notes from the field: Suspected yellow fever case determined to be adverse vaccine rection. Chin. CDC Wkly. 2019, 1, 54–55. [Google Scholar] [CrossRef]
- Hendy, A.; Fé, N.F.; Valério, D.; Fernándes-Acosta, E.; Chaves, B.A.; da Silv, L.F.; Santana, R.A.G.; Paz, A.d.C.; Soares, M.M.M.; Assunçao, F.P.; et al. Towards the laboratory maintenance of Haemagogus janthinomys (Dyar, l921), the major neotropical vector of sylvatic yellow fever. Viruses 2023, 15, 45. [Google Scholar] [CrossRef]
- Brackney, D.E.; Scott, J.C.; Sagawa, F.; Woodward, J.E.; Miller, N.A.; Schilkey, F.D.; Mudge, J.; Wilusz, J.; Olson, K.E.; Blair, C.D.; et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl. Trop. Dis. 2010, 4, e856. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Contamin, H.; Decelle, T.; Fournier, C.; Lang, J.; Deubel, V.; Marianneau, P. Host-cell interaction of attenuated wild-type strains of yellow fever virus can be differentiated at early stages of hepatocyte infection. Microbes Infect. 2006, 8, 1530–1538. [Google Scholar] [CrossRef]
- Woodson, S.E.; Holbrook, M.R. Infection of hepatocytes with 17D vaccine strain of yellow fever virus induces a strong pro-inflammatory host response. J. Gen. Virol. 2011, 92, 2262–2271. [Google Scholar] [CrossRef]
- Stollar, V.; Hardy, J.L. Host-dependent mutants of Sindbis virus whose growth is restricted in cultured Aedes albopictus cells produce normal yields of virus in intact mosquitoes. Virology 1984, 134, 177–183. [Google Scholar] [CrossRef]
- Ciota, A.T.; Kramer, L.D. Insights into arbovirus evolution and adaptation from experimental studies. Viruses 2010, 2, 2594–2617. [Google Scholar] [CrossRef]
- Philip, C.B.; Burgdorfer, W. Arthropod vectors as reservoirs of microbial disease agents. Annu. Rev. Entomol. 1961, 6, 391–412. [Google Scholar] [CrossRef]
- Gershman, M.; Staples, J.E. Yellow fever. CDC Yellow Book 2024; National Center for Emerging and Zoonotic Diseases, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Shinde, D.P.; Plante, J.A.; Plante, K.S.; Weaver, S.C. Yellow fever: Roles of animal models and arthropod vector studies in understanding epidemic emergence. Microorganisms 2022, 10, 1578. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.S.; Hamer, G.L.; Diallo, M.; Kitron, U.; Ko, A.I.; Weaver, S.C. Influence of herd immunity in the cyclical nature of arboviruses. Clin. Opin. Virol. 2020, 40, 1010. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.A.; Huber, J.H.; Tran, Q.M.; Oidtman, R.J.; Walters, M.K.; Siraj, A.S.; Moore, S.M. Burden is in the eye of the beholder: Sensitivity of yellow fever disease burden to modeling assumptions. Sci. Adv. 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Maki, J.; Guiot, A.-L.; Aubert, M.; Brochier, B.; Cliquet, F.; Hanlon, C.A.; King, R.; Oertli, E.H.; Rupprecht, C.E.; Schumacher, C.; et al. Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORALV-RG®): A global review. Vet. Res. 2017, 48, 57. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.O. Report on the recent outbreak of jungle yellow fever in Panama. Am. J. Public Health 1950, 40, 417–426. [Google Scholar] [CrossRef]
- Rosser, J.I.; Nielsen-Saines, K.; Saad, E.; Fuller, T. Reemergence of yellow fever virus in southeastern Brazil, 2017-2018: What sparked the spread? PLoS. Negl. Trop. Dis. 2022, 16, e0010133. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.O.; Mello, C.R. Meteorological droughts in part of Southeastern Brazil: Understanding the last 100 years. An. Da Academ.Bras. De Cienc. 2021, 93 (Suppl. 4), e20201130. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, W.M. Aedes aegypti in Malaya. I. Distribution and dispersal. Ann. Trop. Med. Parasitol. 1956, 50, 385–398. [Google Scholar] [CrossRef]
- Shekhar, K.C.; Huat, O.L. Epidemiology of dengue/dengue haemorrhagic fever in Malaysia-A retrospective epidemiological study 1973-1987. Part 1. Dengue hemorrhagic fever (DHF). Asia-Pac. J. Public Health 1992, 6, 15–25. [Google Scholar] [CrossRef]
- Mattingly, P.F. The Biology of Mosquito-Borne Diseases; George Allen and Unwin: London and American Elsevier Publishing Co.: New York, NY, USA, 1969. [Google Scholar]
- Azar, D.; Nel, A.; Huang, D.; Engell, M.S. The earliest fossil mosquito. Curr. Biol. 2023, 33, 5240–5246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuno, G. Mechanisms of Yellow Fever Transmission: Gleaning the Overlooked Records of Importance and Identifying Problems, Puzzles, Serious Issues, Surprises and Research Questions. Viruses 2024, 16, 84. https://doi.org/10.3390/v16010084
Kuno G. Mechanisms of Yellow Fever Transmission: Gleaning the Overlooked Records of Importance and Identifying Problems, Puzzles, Serious Issues, Surprises and Research Questions. Viruses. 2024; 16(1):84. https://doi.org/10.3390/v16010084
Chicago/Turabian StyleKuno, Goro. 2024. "Mechanisms of Yellow Fever Transmission: Gleaning the Overlooked Records of Importance and Identifying Problems, Puzzles, Serious Issues, Surprises and Research Questions" Viruses 16, no. 1: 84. https://doi.org/10.3390/v16010084
APA StyleKuno, G. (2024). Mechanisms of Yellow Fever Transmission: Gleaning the Overlooked Records of Importance and Identifying Problems, Puzzles, Serious Issues, Surprises and Research Questions. Viruses, 16(1), 84. https://doi.org/10.3390/v16010084




