UV-C Light Intervention as a Barrier against Airborne Transmission of SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Violett Air Sterilization Device
2.2. Animals
2.3. Study Design
2.4. Virus
2.5. Viral Challenge
2.6. Oropharyngeal Swabs
2.7. Clinical Observations
2.8. Tissue Collection
2.9. Virus Titration
2.10. Histopathology
2.11. Statistical Analysis
3. Results
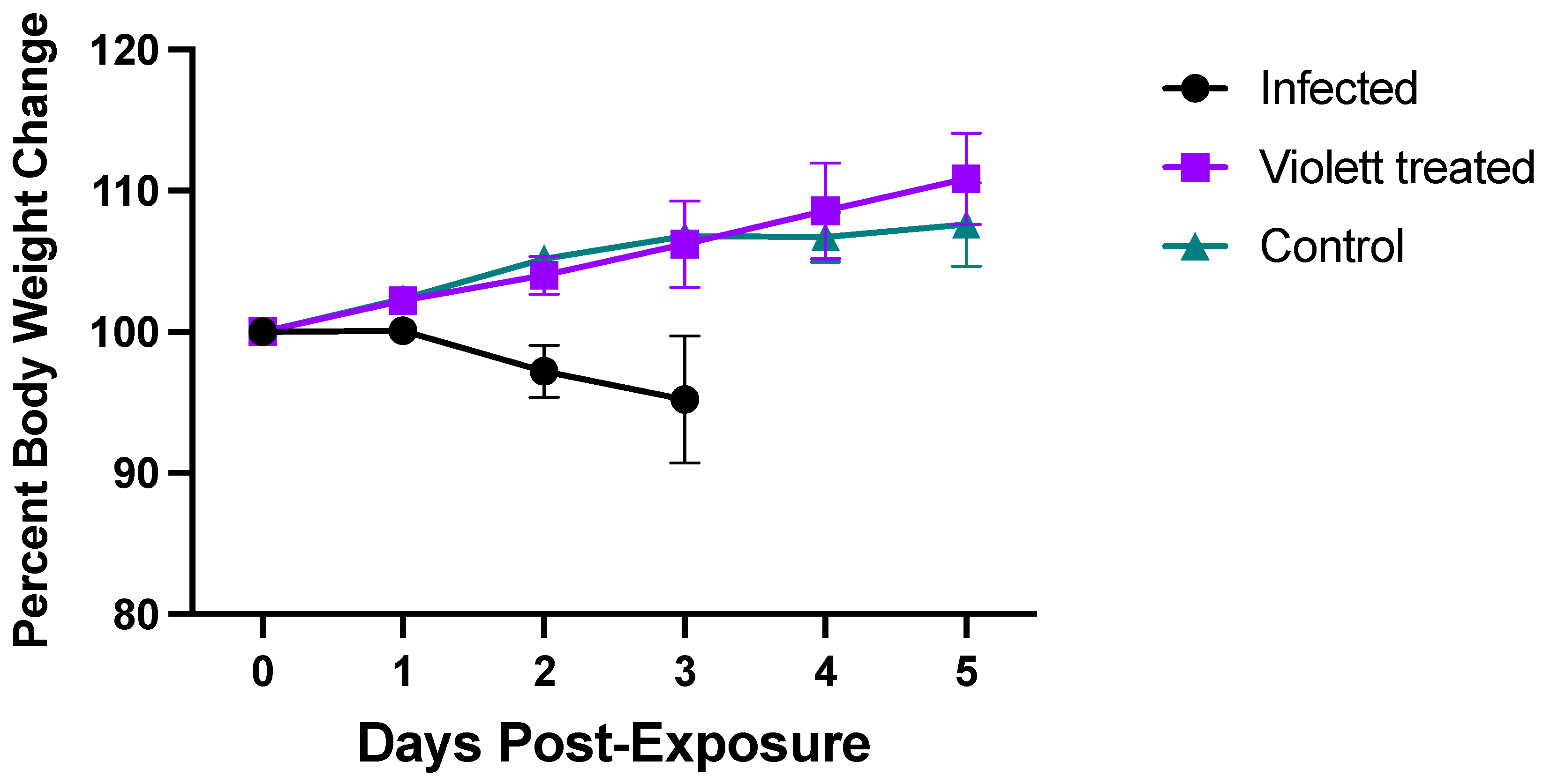
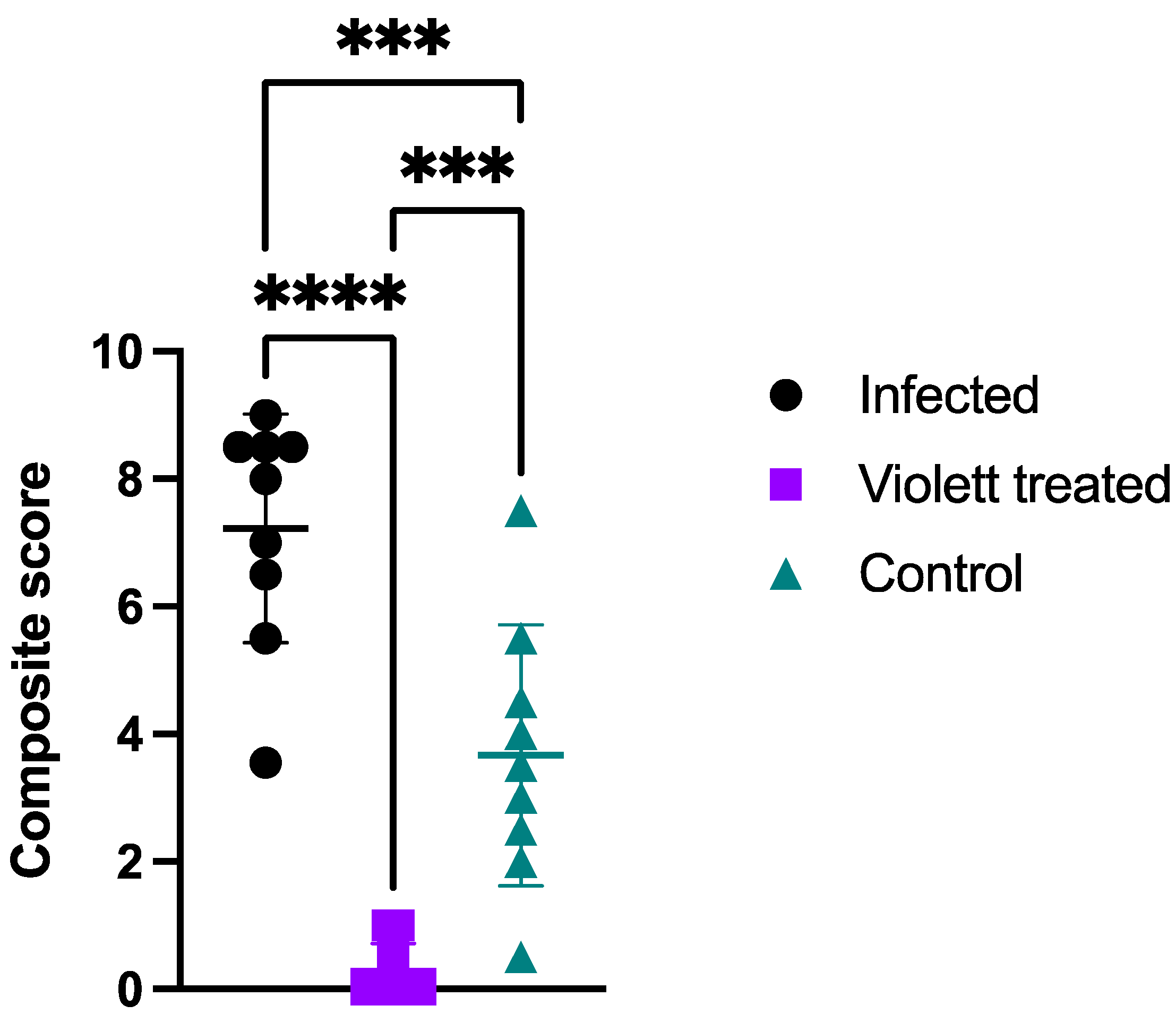
3.1. Clinical Parameters
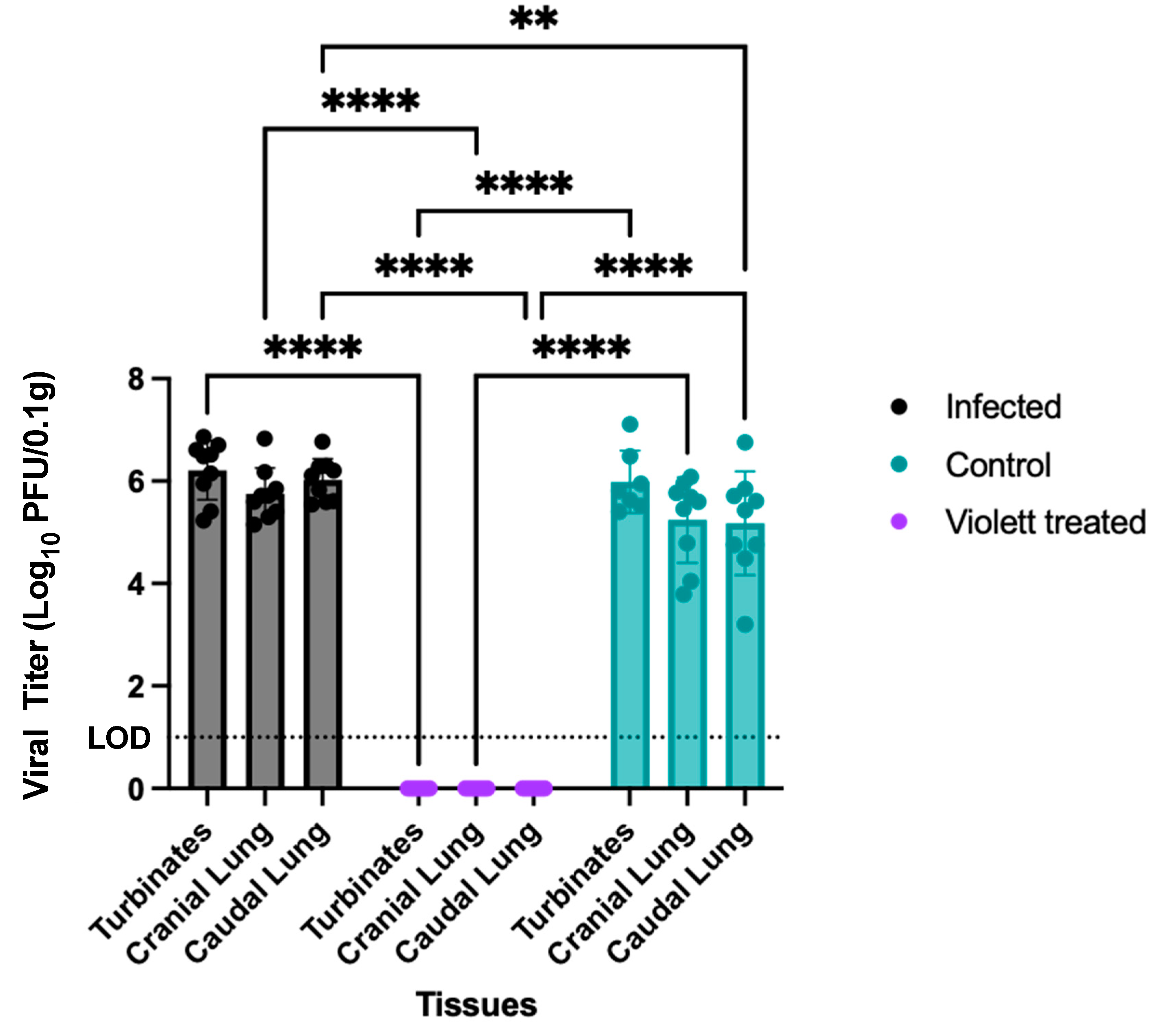
3.2. Virus Titration
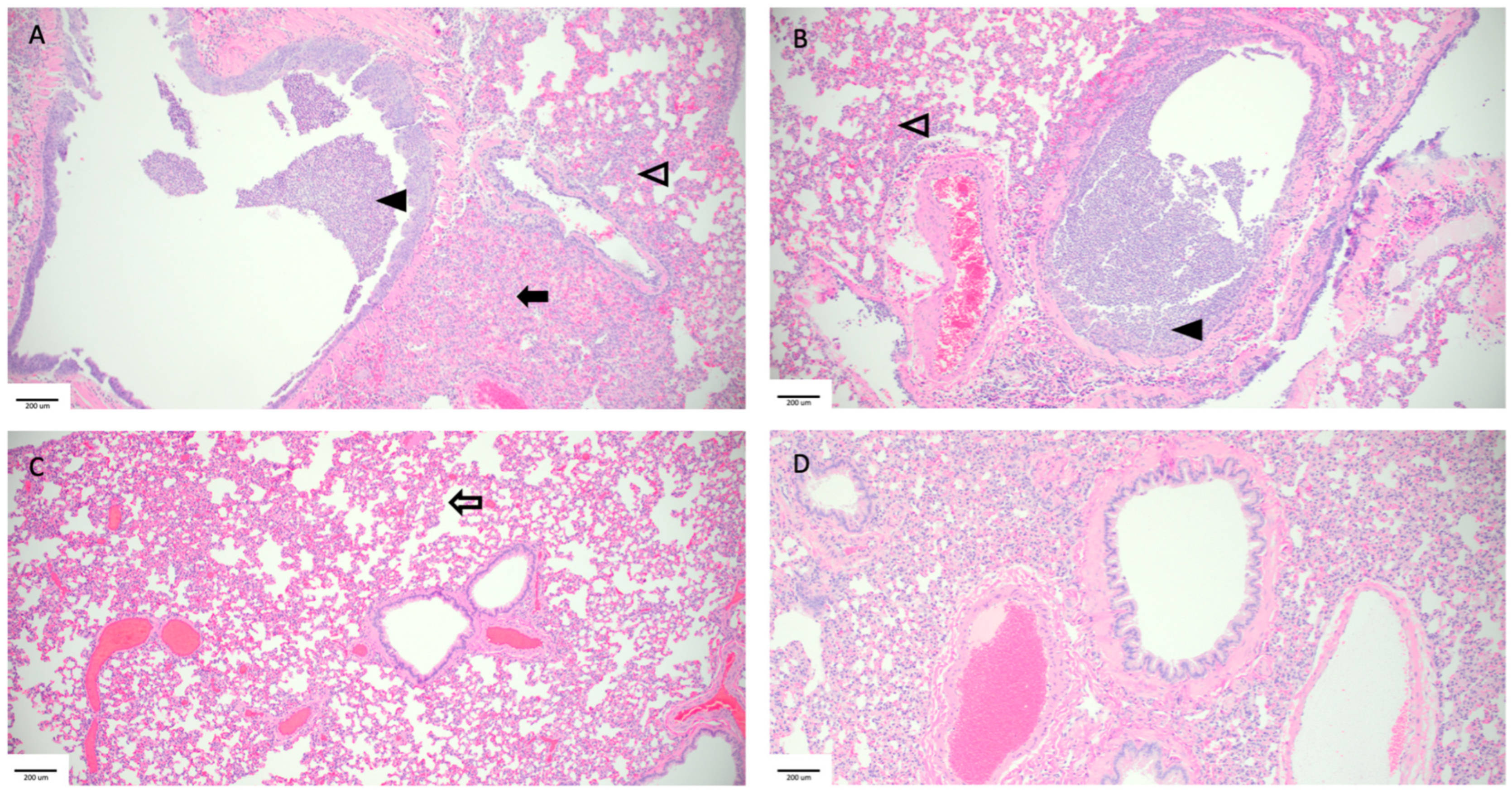
3.3. Histopathology
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization WHO Coronavirus (COVID-19) Dashboard 2020. Available online: https://data.who.int/dashboards/covid19/deaths?n=c (accessed on 31 July 2023).
- Li, H.; Wang, Y.; Ji, M.; Pei, F.; Zhao, Q.; Zhou, Y.; Hong, Y.; Han, S.; Wang, J.; Wang, Q.; et al. Transmission Routes Analysis of SARS-CoV-2: A Systematic Review and Case Report. Front. Cell Dev. Biol. 2020, 8, 618. [Google Scholar] [CrossRef]
- Ahlawat, A.; Mishra, S.K.; Birks, J.W.; Costabile, F.; Wiedensohler, A. Preventing Airborne Transmission of SARS-CoV-2 in Hospitals and Nursing Homes. Int. J. Environ. Res. Public. Health 2020, 17, 8553. [Google Scholar] [CrossRef] [PubMed]
- Addleman, S.; Leung, V.; Asadi, L.; Sharkawy, A.; McDonald, J. Mitigating Airborne Transmission of SARS-CoV-2. CMAJ 2021, 193, E1010–E1011. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Bundgaard, J.S.; Raaschou-Pedersen, D.E.T.; von Buchwald, C.; Todsen, T.; Norsk, J.B.; Pries-Heje, M.M.; Vissing, C.R.; Nielsen, P.B.; Winsløw, U.C.; et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers : A Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 335–343. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.P.M.; In ’t Veen, J.C.C.M. SARS-Cov-2: The Relevance and Prevention of Aerosol Transmission. J. Occup. Environ. Med. 2021, 63, e395–e401. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Gribben, C.; Bishop, J.; Hanlon, P.; Caldwell, D.; Wood, R.; Reid, M.; McMenamin, J.; Goldberg, D.; Stockton, D.; et al. Effect of Vaccination on Transmission of SARS-CoV-2. N. Engl. J. Med. 2021, 385, 1718–1720. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M.; Hill, H.R. Role of Host Immune and Inflammatory Responses in COVID-19 Cases with Underlying Primary Immunodeficiency: A Review. J. Interferon Cytokine Res. 2020, 40, 549–554. [Google Scholar] [CrossRef]
- Liu, B.M.; Martins, T.B.; Peterson, L.K.; Hill, H.R. Clinical Significance of Measuring Serum Cytokine Levels as Inflammatory Biomarkers in Adult and Pediatric COVID-19 Cases: A Review. Cytokine 2021, 142, 155478. [Google Scholar] [CrossRef]
- Bahl, P.; Doolan, C.; De Silva, C.; Chughtai, A.A.; Bourouiba, L.; MacIntyre, C.R. Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? J. Infect. Dis. 2022, 225, 1561–1568. [Google Scholar] [CrossRef]
- Duval, D.; Palmer, J.C.; Tudge, I.; Pearce-Smith, N.; O’Connell, E.; Bennett, A.; Clark, R. Long Distance Airborne Transmission of SARS-CoV-2: Rapid Systematic Review. BMJ 2022, 377, e068743. [Google Scholar] [CrossRef]
- Mallach, G.; Kasloff, S.B.; Kovesi, T.; Kumar, A.; Kulka, R.; Krishnan, J.; Robert, B.; McGuinty, M.; den Otter-Moore, S.; Yazji, B.; et al. Aerosol SARS-CoV-2 in Hospitals and Long-Term Care Homes during the COVID-19 Pandemic. PLoS ONE 2021, 16, e0258151. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Binka, M.; Adu, P.A.; Jeong, D.; Vadlamudi, N.K.; Velásquez García, H.A.; Mahmood, B.; Buller-Taylor, T.; Otterstatter, M.; Janjua, N.Z. The Impact of Mask Mandates on Face Mask Use During the COVID-19 Pandemic: Longitudinal Survey Study. JMIR Public Health Surveill. 2023, 9, e42616. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Ratzan, S.C.; Kamarulzaman, A.; El-Mohandes, A. A Survey of COVID-19 Vaccine Acceptance across 23 Countries in 2022. Nat. Med. 2023, 29, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Raj, A.; Gupta, A.; Gautam, S.; Kumar, M.; Bherwani, H.; Anshul, A. Pollution Free UV-C Radiation to Mitigate COVID-19 Transmission. Gondwana Res. 2023, 114, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Jureka, A.S.; Williams, C.G.; Basler, C.F. Pulsed Broad-Spectrum UV Light Effectively Inactivates SARS-CoV-2 on Multiple Surfaces and N95 Material. Viruses 2021, 13, 460. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C Irradiation Is Highly Effective in Inactivating SARS-CoV-2 Replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef]
- Inagaki, H.; Saito, A.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid Inactivation of SARS-CoV-2 with Deep-UV LED Irradiation. Emerg. Microbes Infect. 2020, 9, 1744–1747. [Google Scholar] [CrossRef]
- Fischer, R.J.; Port, J.R.; Holbrook, M.G.; Yinda, K.C.; Creusen, M.; Ter Stege, J.; de Samber, M.; Munster, V.J. UV-C Light Completely Blocks Aerosol Transmission of Highly Contagious SARS-CoV-2 Variants WA1 and Delta in Hamsters. Environ. Sci. Technol. 2022, 56, 12424–12430. [Google Scholar] [CrossRef]
- FDA Ultraviolet (UV) Radiation. Available online: https://www.fda.gov/radiation-emitting-products/tanning/ultraviolet-uv-radiation#UVC (accessed on 20 December 2023).
- Baer, A.; Kehn-Hall, K. Viral Concentration Determination Through Plaque Assays: Using Traditional and Novel Overlay Systems. JoVE 2014, 93, 52065. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian Hamsters as a Small Animal Model for SARS-CoV-2 Infection and Countermeasure Development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.E.; Goncin, U.; Kroeker, A.; Swan, C.; Ralph, R.; Lu, Y.; Etzioni, A.L.; Falzarano, D.; Gerdts, V.; Machtaler, S.; et al. SARS-CoV-2 Infection in the Syrian Hamster Model Causes Inflammation as Well as Type I Interferon Dysregulation in Both Respiratory and Non-Respiratory Tissues Including the Heart and Kidney. PLoS Pathog. 2021, 17, e1009705. [Google Scholar] [CrossRef]
- Reed, N.G. The History of Ultraviolet Germicidal Irradiation for Air Disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Lindsley, W.G.; Brooks, J.T. Reducing SARS-CoV-2 in Shared Indoor Air. JAMA 2022, 328, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; et al.; COVID-19 Genomics UK Consortium SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xie, C.; Bu, G.-L.; Zhong, L.-Y.; Zeng, M.-S. Molecular Characteristics, Immune Evasion, and Impact of SARS-CoV-2 Variants. Sig Transduct. Target. Ther. 2022, 7, 202. [Google Scholar] [CrossRef]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Singson, J.R.C.; Kirley, P.D.; Pham, H.; Rothrock, G.; Armistead, I.; Meek, J.; Anderson, E.J.; Reeg, L.; Lynfield, R.; Ropp, S.; et al. Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19—COVID-NET, 10 States, March 2020-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 878–884. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The Immunology and Immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.-M.; Chin, A.W.H.; Fung, K.; Choy, K.-T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.A.P.M.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and Transmission of SARS-CoV-2 in Golden Hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Cao, Z.; Yao, Y.; Yu, J.; Zhou, J.; Gao, G.; He, P.; Dong, Z.; Zhong, J.; et al. Increased Pathogenicity and Aerosol Transmission for One SARS-CoV-2 B.1.617.2 Delta Variant over the Wild-Type Strain in Hamsters. Virol. Sin. 2022, 37, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.M.; Darling, T.L.; Halfmann, P.J.; Franks, J.; Webby, R.J.; Barouch, D.H.; Port, J.R.; Munster, V.J.; Diamond, M.S.; Kawaoka, Y. Reduced Airborne Transmission of SARS-CoV-2 BA.1 Omicron Virus in Syrian Hamsters. PLoS Pathog. 2022, 18, e1010970. [Google Scholar] [CrossRef] [PubMed]
- Port, J.R.; Yinda, C.K.; Owusu, I.O.; Holbrook, M.; Fischer, R.; Bushmaker, T.; Avanzato, V.A.; Schulz, J.E.; Martens, C.; van Doremalen, N.; et al. SARS-CoV-2 Disease Severity and Transmission Efficiency Is Increased for Airborne Compared to Fomite Exposure in Syrian Hamsters. Nat. Commun. 2021, 12, 4985. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.A.; Gilgunn, P.; Hartwig, A.E.; Mullen, J. Prevention of Airborne Transmission of SARS-CoV-2 by UV-C Illumination of Airflow. COVID 2021, 1, 602–607. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Health and Medicine Division; Board on Behavioral, Cognitive and Sensory Sciences; Board of Health Sciences Policy; Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults. Health Impacts of Social Isolation and Loneliness on Morbidity and Quality of Life. In Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System; National Academies Press (US): Washington, DC, USA, 2020; Chapter 3. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragan, I.; Perez, J.; Davenport, W.; Hartson, L.; Doyle, B. UV-C Light Intervention as a Barrier against Airborne Transmission of SARS-CoV-2. Viruses 2024, 16, 89. https://doi.org/10.3390/v16010089
Ragan I, Perez J, Davenport W, Hartson L, Doyle B. UV-C Light Intervention as a Barrier against Airborne Transmission of SARS-CoV-2. Viruses. 2024; 16(1):89. https://doi.org/10.3390/v16010089
Chicago/Turabian StyleRagan, Izabela, Jessie Perez, Wilson Davenport, Lindsay Hartson, and Branden Doyle. 2024. "UV-C Light Intervention as a Barrier against Airborne Transmission of SARS-CoV-2" Viruses 16, no. 1: 89. https://doi.org/10.3390/v16010089





