The Current Epizootiological Situation of Three Major Viral Infections Affecting Cattle in Egypt
Abstract
:1. Introduction
2. Foot and Mouth Disease
2.1. General Information
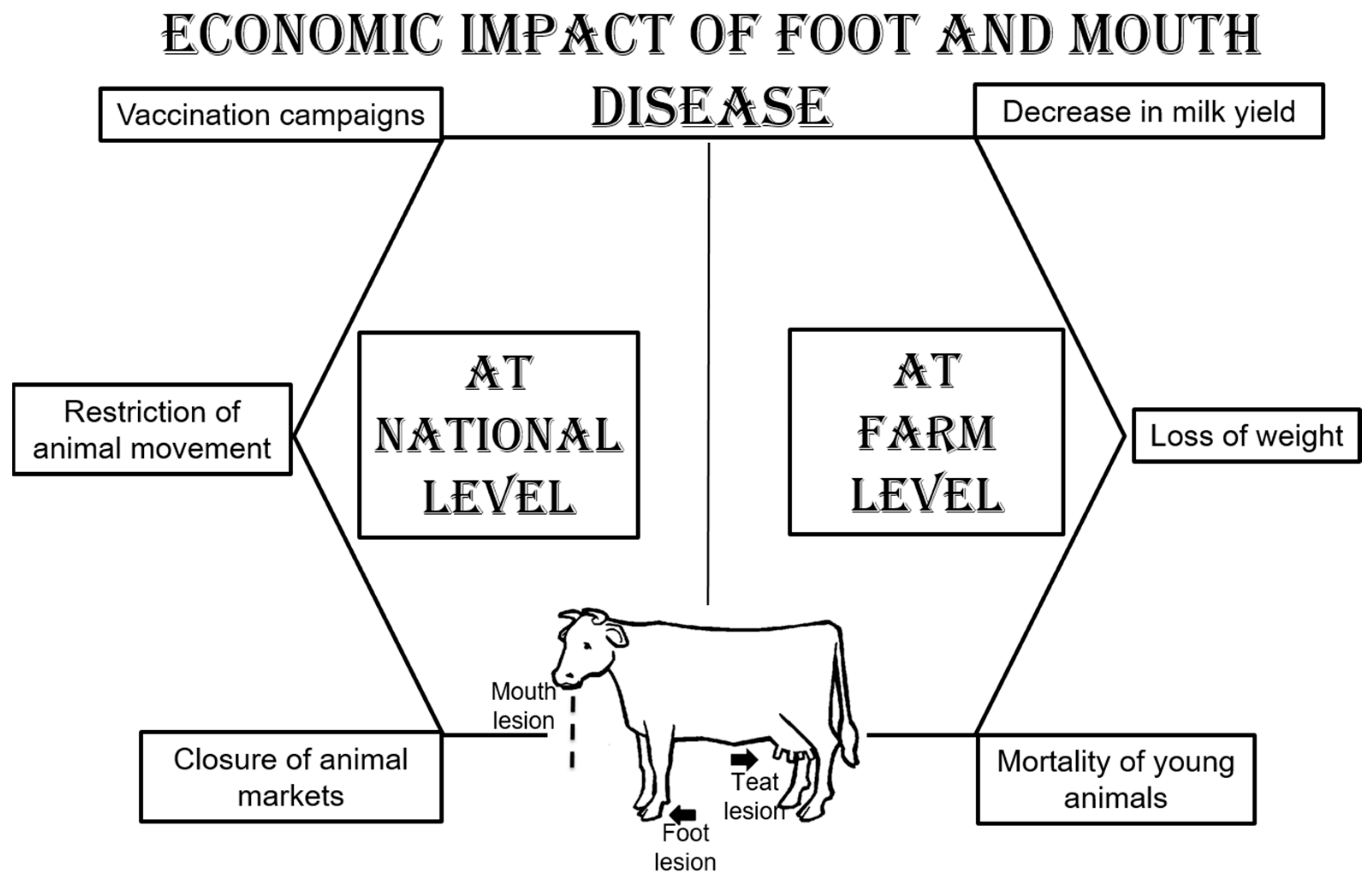
2.2. Economic Impact
2.3. The Virus
2.4. Global Burden
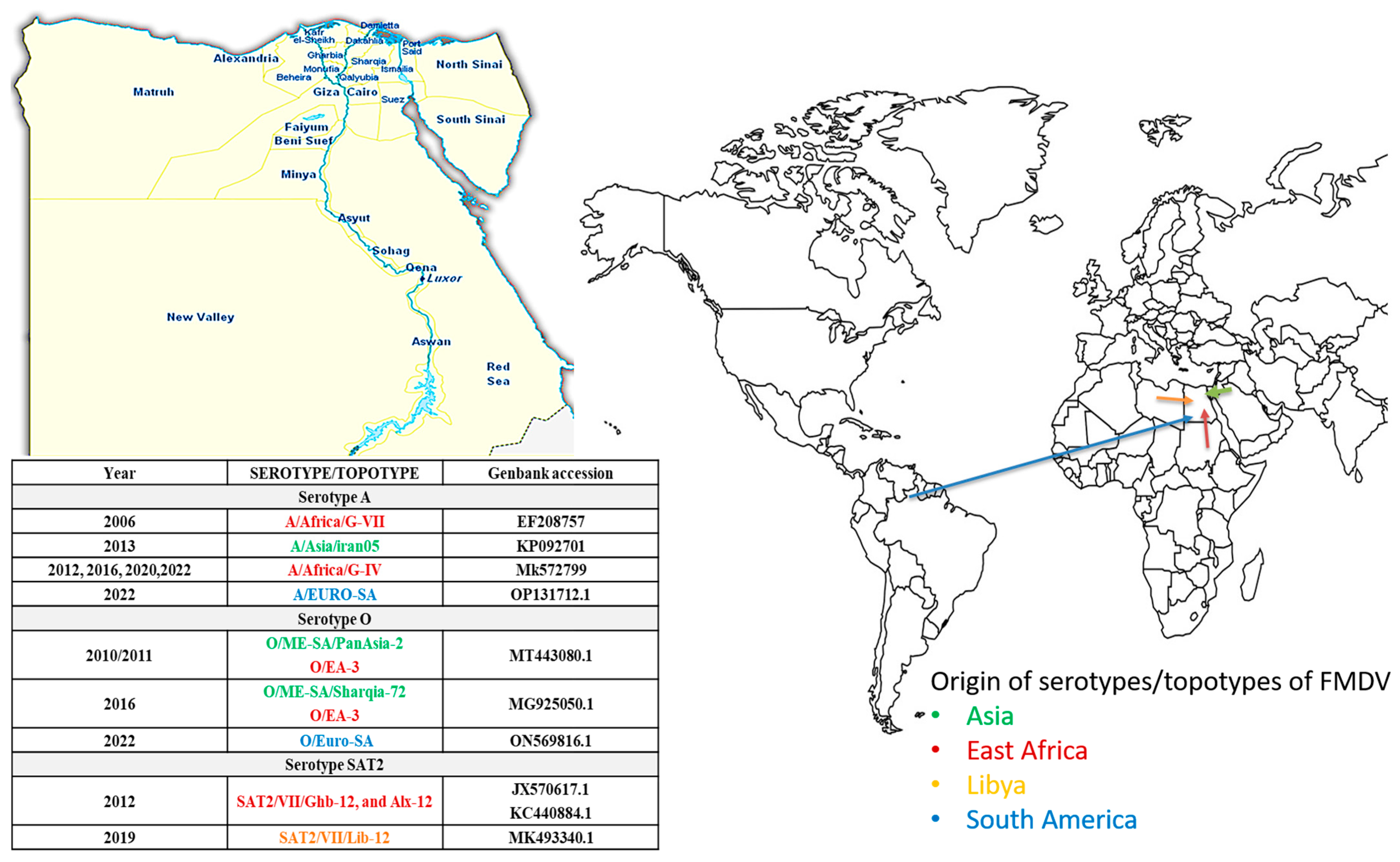
2.5. Egyptian Situation
| Year | Serotype/Topotype | Reference |
|---|---|---|
| 1950 | SAT2 | [16] |
| 1964–2005 | O | [14] |
| 1972 | A | [14] |
| 2006 | A/Africa/G-VII | [14] |
| 2010/2011 | A/Asia/Iran05 O/ME-SA/PanAsia-2 O/EA-3 | [3] |
| 2012 | SAT2/VII/Ghb-12 SAT2/VII/Alx-12 A/Africa/G-IV | [3,16] |
| 2016 | O/ME-SA/Sharqia-72 O/EA-3 A/Africa/G IV | [18] |
| 2019 | SAT2/VII, Lib12 | [18] |
| 2020,2022 | A/Africa/G IV O/Euro-SA topotype A, lineage EURO-SA | [23] [21,22] |
| 2022 | A-African topotype-G-IV | [24] |
2.6. Challenges in Control
3. Lumpy Skin Disease
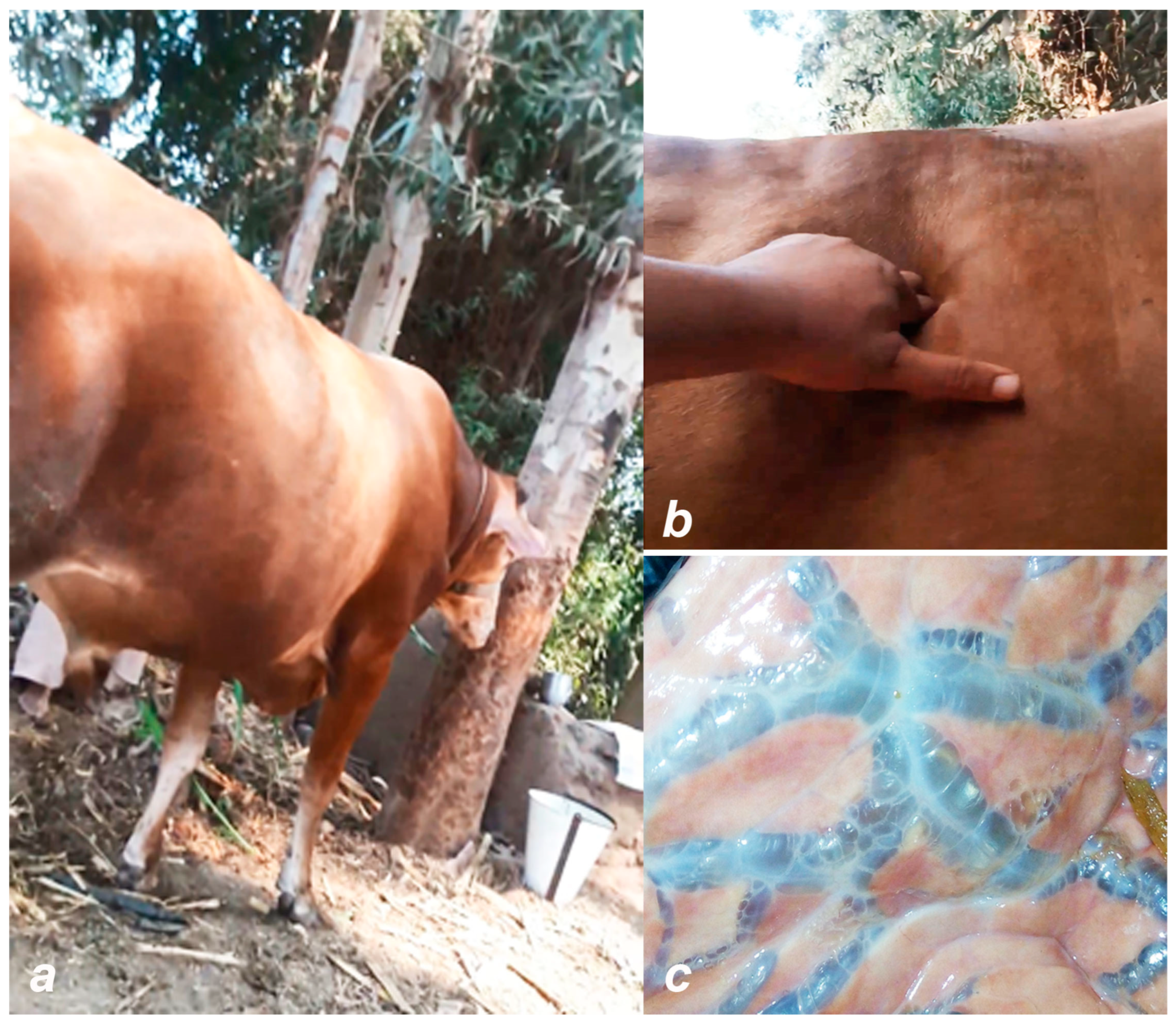
3.1. General Information
3.2. Economic Impact
3.3. The Virus
3.4. Global Burden
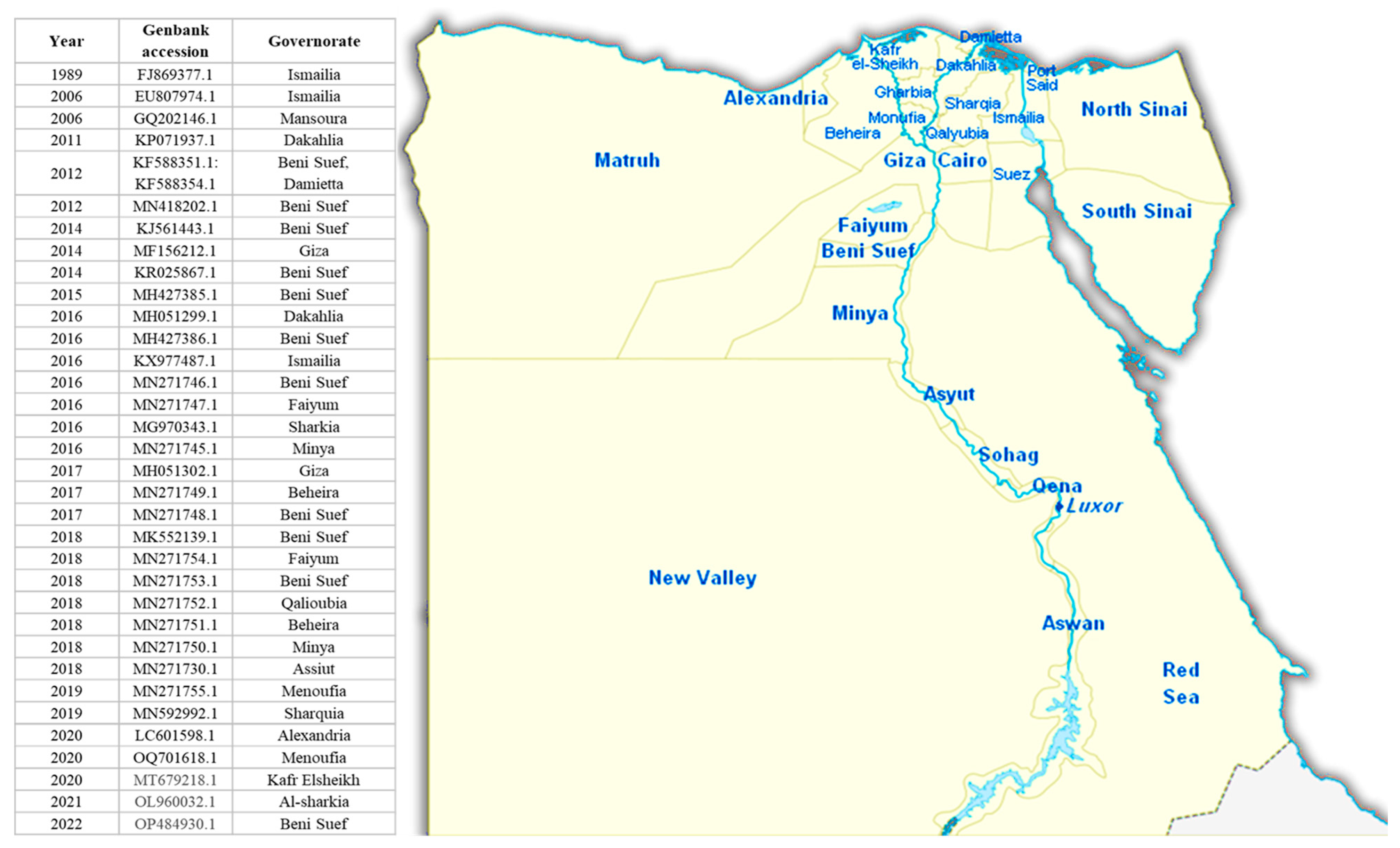
3.5. Egyptian Situation
3.6. Challenges in Control
| Year | Locality | References |
|---|---|---|
| June 1988 October 1988 | Farm near the quarantine station in Suez, the village of Tel El Kabir, Ismalia | [57] |
| May 1988 and May 1989 | Ismailia and Sharkia Governorates | [49] |
| 1988 | El-Menia Governorate | [59] |
| 1989 | Dakahlia | [71] |
| 1992 | Nag-Hamadi, New Valley, and Assiut | [58] |
| 2004–2007 | Lower Egypt | [72] |
| 2006 | EL Kanater-Kaluobia and El-Noubaria-Alexandria, Giza Governorate, and Damietta province | [44,73,74] |
| 2008 | Dakahlia Governorate | [75] |
| 2011 | El Menoufia and El Sadat city | [76] |
| 2012 | Behera and Alexandria Governorates | [61] |
| 2014 | Beni Suef | [62] |
| January 2014 to mid-2015 | Sharqia | [77] |
| May 2015 and August 2016 | Beni Suef Governorate | [78] |
| June 2015 to September 2016 | Ismailia and Beni-Suef | [33] |
| 2017 | Beni Suef, El-Fayoum El Giza, El-Menia, El-Gharbia, El-Qalyubia, and Sharkia | [43] |
| 2017 | Beni Suef | [32] |
| 2017–2018 | Beni Suef, Sohag and Aswan Governorates | [33] |
| 2019 | Sohag | [79] |
| 2019 | Elwasta, Beni Suef | [68] |
| 2020 | El-Wady El-Gedid Governorate | [66] |
| 2020–2021 | Alexandria, Beheira, Gharbia, Kafr El-Sheikh, Menofia, and Qalyubia | [36] |
| 2022 | Beni Suef | [69] |
| 2023 | Giza, Cairo, Fayoum, Beni Suef, Menia, Sohag, Assuit, Qena, Louxer, Aswan, Delta (Qaliobya, Monofya, Sharkya, Gharbya, Dakahlia, Domyat, Kaferelshikh, and Alexandria), Canal Suez, Portsaid, Ismailia, Red Sea Desert, New Valley, and Matrouh | [64] |
| 2023 | Dakahlia, El-Menia, and El-Fayoum | [67] |
4. Bovine Ephemeral Fever (Three-Day Stiff Sickness)
4.1. General Information
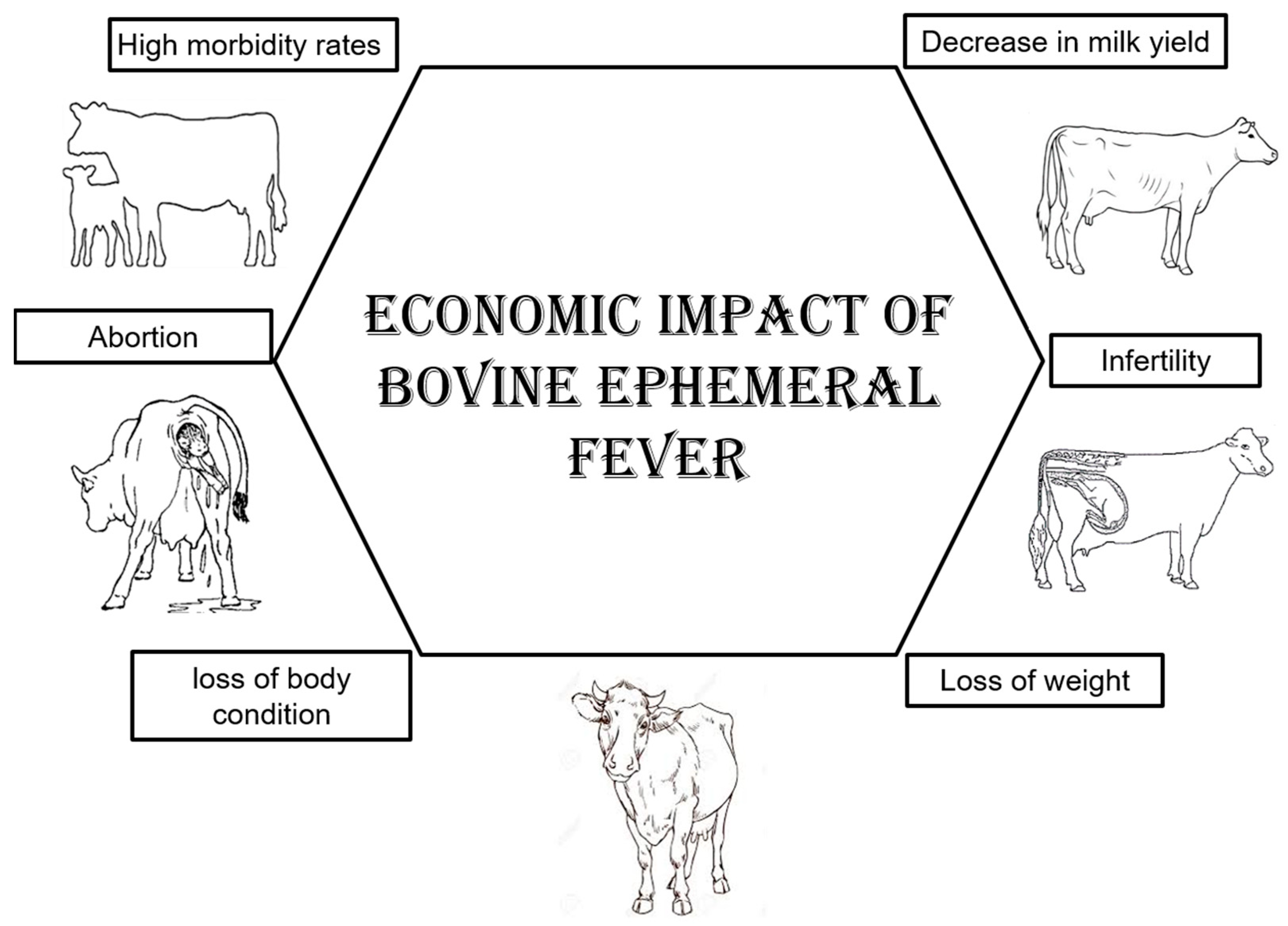
4.2. Economic Impact
4.3. The Virus
4.4. Global Burden
4.5. Egyptian Situation
4.6. Challenges in Control
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghonaim, A.H.; Hopo, M.G.; Ghonaim, N.H.; Jiang, Y.; He, Q.; Li, W. The Epidemiology of Circulating Rotavirus Associated with Diarrhea in Egyptian Kids and Calves: A Review. Zoonoses 2023, 3, 985. [Google Scholar] [CrossRef]
- Paton, D.J.; Gubbins, S.; King, D.P. Understanding the Transmission of Foot-and-Mouth Disease Virus at Different Scales. Curr. Opin. Virol. 2018, 28, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Woldemariyam, F.T.; Kariuki, C.K.; Kamau, J.; De Vleeschauwer, A.; De Clercq, K.; Lefebvre, D.J.; Paeshuyse, J. Epidemiological Dynamics of Foot-and-Mouth Disease in the Horn of Africa: The Role of Virus Diversity and Animal Movement. Viruses 2023, 15, 969. [Google Scholar] [CrossRef] [PubMed]
- Diab, E.; Bazid, A.-H.I.; Fawzy, M.; El-Ashmawy, W.R.; Fayed, A.A.; El-Sayed, M.M. Foot-and-Mouth Disease Outbreaks in Egypt during 2013-2014: Molecular Characterization of Serotypes A, O, and SAT2. Vet. World 2019, 12, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Teifke, J.P.; Breithaupt, A.; Haas, B. Foot-and-mouth disease and its differential diagnoses. Tierarztl. Praxis Ausg. G Grosstiere/Nutztiere 2012, 40, 225–237, quiz 238. [Google Scholar]
- Lyons, N.A.; Ludi, A.B.; Wilsden, G.; Hamblin, P.; Qasim, I.A.; Gubbins, S.; King, D.P. Evaluation of a Polyvalent Foot-and-Mouth Disease Virus Vaccine Containing A Saudi-95 against Field Challenge on Large-Scale Dairy Farms in Saudi Arabia with the Emerging A/ASIA/G-VII Viral Lineage. Vaccine 2017, 35, 6850–6857. [Google Scholar] [CrossRef]
- Bachrach, H.L. Foot-and-Mouth Disease. Annu. Rev. Microbiol. 1968, 22, 201–244. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, S.-Q.; Guo, H.-C. Biological Function of Foot-and-Mouth Disease Virus Non-Structural Proteins and Non-Coding Elements. Virol. J. 2016, 13, 107. [Google Scholar] [CrossRef]
- Haydon, D.T.; Samuel, A.R.; Knowles, N.J. The Generation and Persistence of Genetic Variation in Foot-and-Mouth Disease Virus. Prev. Vet. Med. 2001, 51, 111–124. [Google Scholar] [CrossRef]
- Mahy, B.W.J. Introduction and History of Foot-and-Mouth Disease Virus. Curr. Top. Microbiol. Immunol. 2005, 288, 1–8. [Google Scholar] [CrossRef]
- Mahapatra, M.; Parida, S. Foot and Mouth Disease Vaccine Strain Selection: Current Approaches and Future Perspectives. Expert Rev. Vaccines 2018, 17, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, S.; Mowat, N. Foot-and-Mouth Disease: Host Range and Pathogenesis BT—Foot-and-Mouth Disease Virus; Mahy, B.W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 9–42. ISBN 978-3-540-27109-3. [Google Scholar]
- Rweyemamu, M.; Roeder, P.; Mackay, D.; Sumption, K.; Brownlie, J.; Leforban, Y.; Valarcher, J.-F.; Knowles, N.J.; Saraiva, V. Epidemiological Patterns of Foot-and-Mouth Disease Worldwide. Transbound. Emerg. Dis. 2008, 55, 57–72. [Google Scholar] [CrossRef]
- Knowles, N.J.; Wadsworth, J.; Reid, S.M.; Swabey, K.G.; El-Kholy, A.A.; Abd El-Rahman, A.O.; Soliman, H.M.; Ebert, K.; Ferris, N.P.; Hutchings, G.H.; et al. Foot-and-Mouth Disease Virus Serotype A in Egypt. Emerg. Infect. Dis. 2007, 13, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, B.; Subramaniam, S.; Sanyal, A.; Mohapatra, J.; Dash, B.; Ranjan, R.; Rout, M. Foot-and-Mouth Disease: Global Status and Future Road Map for Control and Prevention in India. Agric. Res. 2012, 1, 132–147. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Salem, S.A.H.; Habashi, A.R.; Arafa, A.A.; Aggour, M.G.A.; Salem, G.H.; Gaber, A.S.; Selem, O.; Abdelkader, S.H.; Knowles, N.J.; et al. Emergence of Foot-and-Mouth Disease Virus SAT 2 in Egypt during 2012. Transbound. Emerg. Dis. 2012, 59, 476–481. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, S.; Wang, W.; Chen, W.; Wang, X.; Wu, K.; Li, X.; Fan, S.; Ding, H.; Yi, L.; et al. Development of Foot-and-Mouth Disease Vaccines in Recent Years. Vaccines 2022, 10, 1817. [Google Scholar] [CrossRef]
- Soltan, M.A.; Negmaldin, A.H.; El-Diasty, M.M.; Mansour, S.M.G.; Elbadry, M.A.; Wilkes, R.P. Molecular Characterization of Circulating Foot and Mouth Disease Virus (FMDV) Serotype O Topotype EA-3 and Serotype A (African Topotype) Genotype IV in Egypt, 2016. Vet. Microbiol. 2017, 208, 89–93. [Google Scholar] [CrossRef]
- Sobhy, N.M.; Mor, S.K.; Mohammed, M.E.M.; Bastawecy, I.M.; Fakhry, H.M.; Youssef, C.R.B.; Goyal, S.M. Phylogenetic Analysis of Egyptian Foot and Mouth Disease Virus Endemic Strains. Egypt. J. Virol. 2014, 11, 49–59. [Google Scholar]
- Soltan, M.A.; Dohreig, R.M.A.; Abbas, H.; Ellawa, M.; Yousif, I.; Aly, A.E.; Wasfy, M.; El-Sayed, M.M. Emergence of Foot and Mouth Disease Virus, Lib-12 Lineage of Topotype VII, Serotype SAT2 in Egypt, 2018. Transbound. Emerg. Dis. 2019, 66, 1105–1106. [Google Scholar] [CrossRef]
- Soltan, M.A.; Mahmoud, M.M.; Hegazy, Y.; Abd-Eldiam, M.M. Emergence of foot and mouth disease virus, serotype O, Europe-South America topotype in Egypt, 2022. Transbound Emerg Dis. 2022, 69, 2409–2411. [Google Scholar] [CrossRef]
- Hagag, N.M.; Hassan, A.M.; Zaher, M.R.; Elnomrosy, S.M.; Shemies, O.A.; Hussein, H.A.; Ahmed, E.S.; Ali, M.H.; Ateay, M.; Abdel-Hakim, M.A.; et al. Molecular Detection and Phylogenetic Analysis of Newly Emerging Foot-and-Mouth Disease Virus Type A, Lineage EURO-SA in Egypt in 2022. Virus Res. 2023, 323, 198960. [Google Scholar] [CrossRef]
- Shahein, M.; Ahmed Hussein, H.; Ali, M.; Ghoniem, S.; Shemies, O.; Afify, A.; Fuoad, A.; Muhammed Hassan, A.; Zaher, M.; Hussien, N.; et al. Circulating Foot-and-Mouth Disease Virus Serotype A African-Genotype IV in Egypt during 2022. Vet. World 2023, 16, 1429–1437. [Google Scholar] [CrossRef]
- El-Ansary, R.; Kasem, S.; El-Tabakh, M.; Badr, Y.; Abdel-Moneim, A. Isolation, Molecular Characterization, and Genetic Diversity of Recently Isolated Foot-and-Mouth Disease Virus Serotype A in Egypt. PLoS ONE 2023, 18, e0295319. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.D.; Rushton, J. The Economic Impacts of Foot and Mouth Disease—What Are They, How Big Are They and Where Do They Occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- Rady, A.; Khalil, S.; Torky, H. Molecular Epidemiology of FMDV in Northern Egypt (2012-214). Alexandria J. Vet. Sci. 2014, 41, 120. [Google Scholar] [CrossRef]
- Maree, F.F.; Kasanga, C.J.; Scott, K.A.; Opperman, P.A.; Melanie, C.; Sangula, A.K.; Raphael, S.; Yona, S.; Wambura, P.N.; King, D.P.; et al. Challenges and Prospects for the Control of Foot-and-Mouth Disease: An African Perspective. Vet. Med. Res. Rep. 2014, 5, 119–138. [Google Scholar] [CrossRef]
- ElAshmawy, W.R.; Aly, S.S.; Farouk, M.M. Decision Tree Risk Analysis for FMD Outbreak Prevention in Egyptian Feedlots. Prev. Vet. Med. 2023, 211, 105820. [Google Scholar] [CrossRef]
- Tuppurainen, E.; Alexandrov, T.; Beltrán-Alcrudo, D. Lupmy Skin Disease Field Manual—A Manual for Veterinarians; FAO Animal Production and Health Manual No.20. Rome; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017; ISBN 9789251097762. [Google Scholar]
- Davies, F.G. Lumpy Skin Disease, an African Capripox Virus Disease of Cattle. Br. Vet. J. 1991, 147, 489–503. [Google Scholar] [CrossRef]
- Babiuk, S.; Bowden, T.R.; Parkyn, G.; Dalman, B.; Manning, L.; Neufeld, J.; Embury-Hyatt, C.; Copps, J.; Boyle, D.B. Quantification of Lumpy Skin Disease Virus Following Experimental Infection in Cattle. Transbound. Emerg. Dis. 2008, 55, 299–307. [Google Scholar] [CrossRef]
- Rouby, S.; Abosria, K.; Aboelhadid, S.; Ahmed, E.S. Role of Rhipicephalus Annulatus Tick in Transmission of Lumpy Skin Disease Virus in Naturally Infected Cattle in Egypt. Adv. Anim. Vet. Sci. 2017, 5, 185–191. [Google Scholar] [CrossRef]
- Rouby, S.; Shehata, O.; Abdel-Moneim, A.; Abosria, K.; Ali, M. Lumpy Skin Disease in Calves: The Association Between Clinical Signs and Biochemical Alterations. Adv. Anim. Vet. Sci. 2021, 9, 1863–1868. [Google Scholar] [CrossRef]
- Coetzer, J.A.W.; Tustin, R.C. Infectious Diseases of Livestock, 2nd ed.; Oxford University Press: New York, NY, USA, 2004; Cape Town SE—3 volumes: Illustrations (some color), color maps; ISBN 0195761693; 9780195761696; 0195761707; 9780195761702; 0195761715; 9780195761719; 019578202X; 9780195782028. [Google Scholar]
- Ghonaim, A.H.; Yi, G.; Lei, M.; Xie, D.; Ma, H. Isolation, Characterization and Whole-Genome Analysis of G9 Group a Rotaviruses in China: Evidence for Possible Porcine—Human Interspecies Transmission. Virology 2024, 597, 110129. [Google Scholar] [CrossRef]
- Selim, A.; Manaa, E.; Khater, H. Seroprevalence and Risk Factors for Lumpy Skin Disease in Cattle in Northern Egypt. Trop. Anim. Health Prod. 2021, 53, 350. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Venter, E.H.; Coetzer, J.A.W. The Detection of Lumpy Skin Disease Virus in Samples of Experimentally Infected Cattle Using Different Diagnostic Techniques. Onderstepoort J. Vet. Res. 2005, 72, 153–164. [Google Scholar] [CrossRef]
- Akther, M.; Akter, S.H.; Sarker, S.; Aleri, J.W.; Annandale, H.; Abraham, S.; Uddin, J.M. Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses 2023, 15, 1861. [Google Scholar] [CrossRef]
- Bhanuprakash, V.; Indrani, B.K.; Hosamani, M.; Singh, R.K. The Current Status of Sheep Pox Disease. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 27–60. [Google Scholar] [CrossRef]
- Punyapornwithaya, V.; Seesupa, S.; Phuykhamsingha, S.; Arjkumpa, O.; Sansamur, C.; Jarassaeng, C. Spatio-Temporal Patterns of Lumpy Skin Disease Outbreaks in Dairy Farms in Northeastern Thailand. Front. Vet. Sci. 2022, 9, 957306. [Google Scholar] [CrossRef]
- Arjkumpa, O.; Suwannaboon, M.; Boonrod, M.; Punyawan, I.; Liangchaisiri, S.; Laobannue, P.; Lapchareonwong, C.; Sansri, C.; Kuatako, N.; Panyasomboonying, P.; et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front. Vet. Sci. 2021, 8, 799065. [Google Scholar] [CrossRef]
- Le Goff, C.; Lamien, C.E.; Fakhfakh, E.; Chadeyras, A.; Aba-Adulugba, E.; Libeau, G.; Tuppurainen, E.; Wallace, D.B.; Adam, T.; Silber, R.; et al. Capripoxvirus G-Protein-Coupled Chemokine Receptor: A Host-Range Gene Suitable for Virus Animal Origin Discrimination. J. Gen. Virol. 2009, 90, 1967–1977. [Google Scholar] [CrossRef]
- Zeedan, G.S.G.; Mahmoud, A.H.; Abdalhamed, A.M.; El-Razik, K.A.E.-H.A.; Khafagi, M.H.; Zeina, H.A.A.A. Detection of Lumpy Skin Disease Virus in Cattle Using Real-Time Polymerase Chain Reaction and Serological Diagnostic Assays in Different Governorates in Egypt in 2017. Vet. World 2019, 12, 1093–1100. [Google Scholar] [CrossRef]
- Salib, F.; Osman, A.H. Incidence of Lumpy Skin Disease among Egyptian Cattle in Giza Governorate, Egypt. Vet. World 2011, 4, 162–167. [Google Scholar] [CrossRef]
- MacDonald, R.A.S. Annual Report for 1930–1931; Department of Animal Health, Government of Northern Rhodesia: Lusaka, Zambia, 1931; Pseudo-urticaria of cattle; pp. 20–21. [Google Scholar]
- Thomas, A.M. Knopvelsiekte. J. S. Afr. Vet. Assoc. 1945, 16, 36–43. [Google Scholar]
- Nawathe, D.R.; Gibbs, E.P.; Asagba, M.O.; Lawman, M.J. Lumpyskin Disease in Nigeria. Trop. Anim. Health Prod. 1978, 10, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.; Wallace, D. Lumpy Skin Disease in Southern Africa: A Review of the Disease and Aspects of Control. J. S. Afr. Vet. Assoc. 2001, 72, 68–71. [Google Scholar] [CrossRef]
- Ali, A.A.; Esmat, M.; Attia, H.; Selim, A.; Abdel-Hamid, Y.M. Clinical and Pathological Studies on Lumpy Skin Disease in Egypt. Vet. Rec. 1990, 127, 549–550. [Google Scholar]
- Brenner, J.; Bellaiche, M.; Gross, E.; Elad, D.; Oved, Z.; Haimovitz, M.; Wasserman, A.; Friedgut, O.; Stram, Y.; Bumbarov, V.; et al. Appearance of Skin Lesions in Cattle Populations Vaccinated against Lumpy Skin Disease: Statutory Challenge. Vaccine 2009, 27, 1500–1503. [Google Scholar] [CrossRef]
- Greth, A.; Gourreau, J.M.; Vassart, M.; Nguyen-Ba-Vy; Wyers, M.; Lefevre, P.C. Capripoxvirus Disease in an Arabian Oryx (Oryx Leucoryx) from Saudi Arabia. J. Wildl. Dis. 1992, 28, 295–300. [Google Scholar] [CrossRef]
- Tageldin, M.H.; Wallace, D.B.; Gerdes, G.H.; Putterill, J.F.; Greyling, R.R.; Phosiwa, M.N.; Al Busaidy, R.M.; Al Ismaaily, S.I. Lumpy Skin Disease of Cattle: An Emerging Problem in the Sultanate of Oman. Trop. Anim. Health Prod. 2014, 46, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Namazi, F.; Khodakaram Tafti, A. Lumpy Skin Disease, an Emerging Transboundary Viral Disease: A Review. Vet. Med. Sci. 2021, 7, 888–896. [Google Scholar] [CrossRef]
- Al Salihi, K. Lumpy Skin Disease: Review of Literature. Mirror Res. Vet. Sci. Anim. 2014, 3, 6–23. [Google Scholar]
- Calistri, P.; DeClercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Abrahantes, J.; Antoniou, S.-E.; Broglia, A.; Gogin, A. Lumpy Skin Disease: III. Data Collection and Analysis. EFSA J. 2019, 17, e05638. [Google Scholar] [CrossRef] [PubMed]
- Mulatu, E.; Feyisa, A. Review: Lumpy Skin Disease. J. Vet. Sci. Technol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- House, J.A.; Wilson, T.M.; el Nakashly, S.; Karim, I.A.; Ismail, I.; el Danaf, N.; Moussa, A.M.; Ayoub, N.N. The Isolation of Lumpy Skin Disease Virus and Bovine Herpesvirus-4 from Cattle in Egypt. J. Vet. Diagn. Investig. 1990, 2, 111–115. [Google Scholar] [CrossRef] [PubMed]
- El-Allawy, T.A.A.; Mahmoud, F.S.; Malek, S. EPIDEMIOLOGICAL STUDY ON BOVINE EPHEMERAL FEVER VIRUS (BEFV) INFECTION IN CATTLE AND BUFFALOES IN EGYPT. Assiut Vet. Med. J. 2021, 67, 120–132. [Google Scholar] [CrossRef]
- Abd El-Rahim, I.; Elballal, S.; Hussein, M. An outbreak of lumpy skin disease among cattle in Upper Egypt (El-Menia governorate). Minufyia Vet. J. 2002, 2, 185–200. [Google Scholar]
- El-Kholy, A.; Soliman, H.; Abdelrahman, K. Polymerase Chain Reaction for Rapid Diagnosis of a Recent Lumpy Skin Disease Virus Incursion to Egypt. Arab J. Biotechnol. 2008, 11, 293–302. [Google Scholar]
- El-Neweshy, M.; El-Shemey, T.M.; Youssef, S. Pathologic and Immunohistochemical Findings of Natural Lumpy Skin Disease in Egyptian Cattle. Pak. Vet. J. 2013, 33, 60–64. [Google Scholar]
- Rouby, S.; Aboulsoud, E. Evidence of Intrauterine Transmission of Lumpy Skin Disease Virus. Vet. J. 2016, 209, 193–195. [Google Scholar] [CrossRef]
- El-Tholoth, M.; El-Kenawy, A.A. G-Protein-Coupled Chemokine Receptor Gene in Lumpy Skin Disease Virus Isolates from Cattle and Water Buffalo (Bubalus Bubalis) in Egypt. Transbound. Emerg. Dis. 2016, 63, e288–e295. [Google Scholar] [CrossRef] [PubMed]
- Ezzeldin, A.M.; Bashandy, E.Y.; Ahmed, Z.A.M.; Ismail, T.F. Epidemiology of Lumpy Skin Disease in Egypt between 2006 and 2018. J. Appl. Vet. Sci. 2023, 8, 90–96. [Google Scholar] [CrossRef]
- Ali, A.A.; Neamat-Allah, A.N.F.; Sheire, H.A.E.-M.; Mohamed, R.I. Prevalence, Intensity, and Impacts of Non-Cutaneous Lesions of Lumpy Skin Disease among Some Infected Cattle Flocks in Nile Delta Governorates, Egypt. Comp. Clin. Path. 2021, 30, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Current Situation of Lumpy Skin Disease Virus in Some Areas in El-Wady El-Gedid Governorate During 2020. Egypt. J. Anim. Health 2021, 1, 9–20. [CrossRef]
- El-Hamady, M.G.; El-Bagoury, G.F.; El-Toukhy, E.I. Molecular Characterization of Lumpy Skin Disease Virus in Specific Egyptian Governorates Using ORF 103 Sequencing during Early 2023. Benha Vet. Med. J. 2023, 45, 163–167. [Google Scholar] [CrossRef]
- Rouby, S.R.; Safwat, N.M.; Hussein, K.H.; Abdel-Ra’ouf, A.M.; Madkour, B.S.; Abdel-Moneim, A.S.; Hosein, H.I. Lumpy Skin Disease Outbreaks in Egypt during 2017-2018 among Sheeppox Vaccinated Cattle: Epidemiological, Pathological, and Molecular Findings. PLoS ONE 2021, 16, e0258755. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Rouby, S.; Elsaied, A.; El-Sherif, A. Assessment of Heterologous Lumpy Skin Disease Vaccine-Induced Immunity in Pregnant Cattle Vaccinated at Different Times of Gestation Period and Their Influence on Maternally Derived Antibodies. Vet. Immunol. Immunopathol. 2022, 244, 110380. [Google Scholar] [CrossRef]
- Bazid, A.-H.; Wasfy, M.; Fawzy, M.; Nayel, M.; Abdelmegeid, M.; Thabet, R.Y.; Yong, H.S.; El-Sayed, M.M.; Magouz, A.; Badr, Y. Emergency Vaccination of Cattle against Lumpy Skin Disease: Evaluation of Safety, Efficacy, and Potency of MEVAC(®) LSD Vaccine Containing Neethling Strain. Vet. Res. Commun. 2023, 47, 767–777. [Google Scholar] [CrossRef]
- ABD El-Hamed, H.A.; Ali, W. CLINICOPATHOLOGICAL AND VIROLOGICAL STUDIES ON CATTLE INFECTED WITH LUMPY SKIN DISEASE. Assiut Vet. Med. J. 2017, 63, 119–127. [Google Scholar] [CrossRef]
- Ahmed, W.; Zaher, K. Observations on Lumpy Skin Disease in Local Egyptian Cows with Emphasis on Its Impact on Ovarian Function. African J. Microbiol. Res. 2008, 2, 252–257. [Google Scholar]
- Sohair, I.; Ibrahim, E.; Shahein, M. Virological and Pathological Studies on Lumpy Skin Disease in Naturally Infected Cattle. Egypt J. Comp. Path Clin. Path 2008, 21, 446–465. [Google Scholar]
- Awadin, W.; Hussein, H.; Elseady, Y.; Babiuk, S.; Furuoka, H. Detection of Lumpy Skin Disease Virus Antigen and Genomic DNA in Formalin-Fixed Paraffin-Embedded Tissues from an Egyptian Outbreak in 2006. Transbound. Emerg. Dis. 2011, 58, 451–457. [Google Scholar] [CrossRef]
- El-Kenawy, A.A.; El-Tholoth, M.S. Sequence Analysis of Attachment Gene of Lumpy Skin Disease and Sheep Poxviruses. Virol. Sin. 2010, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Elkerdasy, A. Immunobiochemical Profile in Cattle Infected with Lumpy Skin Disease. J. Basic Appl. Chem. 2010, 1, 21–25. [Google Scholar]
- Elhaig, M.M.; Selim, A.; Mahmoud, M. Lumpy Skin Disease in Cattle: Frequency of Occurrence in a Dairy Farm and a Preliminary Assessment of Its Possible Impact on Egyptian Buffaloes. Onderstepoort J. Vet. Res. 2017, 84, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Rouby, S. RPO30 Gene Based PCR for Detection and Differentiation of Lumpy Skin Disease Virus and Sheep Poxvirus Field and Vaccinal Strains. Vet. Sci. Res. Rev. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Diab, H.; Ahmed, A.; Batiha, G.; Alkazmi, L.; El-Zamkan, M. Molecular Surveillance of Lumpy Skin Disease Outbreak, 2019 in Sohag, Egypt: Enzootic Potential, Phylogenetic Assessment and Implications on Cattle Herds Health. J. Anim. Health Prod. 2021, 9, 406–416. [Google Scholar] [CrossRef]
- Zaghawa, A.A.; Housawi, F.; Al-Naeem, A.; Elsify, A.; Hegazy, Y.M. Bovine Ephemeral Fever Epidemics in Kingdom Saudi Arabia: Clinical, Epidemiological and Molecular Investigation. J. Infect. Dev. Ctries. 2017, 11, 854–860. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, P.; He, H.; Wang, H. Cell Apoptosis Regulated by Interaction between Viral Gene Alpha 3 and Host Heterogeneous Nuclear Ribonucleoprotein K Facilitates Bovine Ephemeral Fever Virus Replication. Vet. Microbiol. 2020, 240, 108510. [Google Scholar] [CrossRef]
- Walker, P.J.; Klement, E. Epidemiology and Control of Bovine Ephemeral Fever. Vet. Res. 2015, 46, 124. [Google Scholar] [CrossRef]
- Bakhshesh, M.; Abdollahi, D. Bovine Ephemeral Fever in Iran: Diagnosis, Isolation and Molecular Characterization. J. Arthropod. Borne. Dis. 2015, 9, 195–203. [Google Scholar]
- Walker, P.J. Bovine Ephemeral Fever in Australia and the World. Curr. Top. Microbiol. Immunol. 2005, 292, 57–80. [Google Scholar] [CrossRef]
- Trinidad, L.; Blasdell, K.R.; Joubert, D.A.; Davis, S.S.; Melville, L.; Kirkland, P.D.; Coulibaly, F.; Holmes, E.C.; Walker, P.J. Evolution of Bovine Ephemeral Fever Virus in the Australian Episystem. J. Virol. 2014, 88, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Kun, J.; Rongrong, J.; Xiangbin, W.; Yan, Z.; Yiping, D.; Gang, L.; Pei, Z.; Shoujun, L. Genetic Characterization of Bovine Ephemeral Fever Virus in Southern China, 2013–2017. Virus Genes 2020, 56, 390–395. [Google Scholar] [CrossRef]
- Aziz-Boaron, O.; Klausner, Z.; Hasoksuz, M.; Shenkar, J.; Gafni, O.; Gelman, B.; David, D.; Klement, E. Circulation of Bovine Ephemeral Fever in the Middle East--Strong Evidence for Transmission by Winds and Animal Transport. Vet. Microbiol. 2012, 158, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Akakpo, A.J. Three-Day Fever. Rev. Sci. Tech. 2015, 34, 533–538. [Google Scholar] [PubMed]
- Lavon, Y.; Ezra, E.; Friedgut, O.; Behar, A. Economic Aspects of Bovine Ephemeral Fever (BEF) Outbreaks in Dairy Cattle Herds. Vet. Sci. 2023, 10, 645. [Google Scholar] [CrossRef]
- Zaher, K.; Ahmed, W. Investigations on Bovine Ephemeral Fever Virus in Egyptian Cows and Buffaloes with Emphasis on Isolation and Identification of a Field Strain. Glob. Vet. 2011, 6, 447–452. [Google Scholar]
- El-Bagoury, G.F.; El-Nahas, E.M.; El-Habbaa, A.S. Serological and Molecular Identification of Bovine Ephemeral Fever Virus Isolates from Cattle and Buffaloes in Qaluobia Province, Egypt 2014. Egypt. J. Virol. 2014, 11, 176–182. [Google Scholar]
- Albehwa, A.M.; Ismaeil, W.M.; Fakhry, H.M.; El-Emam, H.S. Molecular Characterization of Recent Isolates of Bef Virus in Egypt. Benha J. Appl. Sci. 2017, 2, 137–144. [Google Scholar] [CrossRef]
- Della-Porta, A.J.; Snowdon, W.A. An Experimental Inactivated Virus Vaccine against Bovine Ephemeral Fever 2. Do Neutralizing Antibodies Protect against Infection? Vet. Microbiol. 1979, 4, 197–208. [Google Scholar] [CrossRef]
- Inaba, Y.; Kurogi, H.; Takahashi, A.; Sato, K.; Omori, T.; Goto, Y.; Hanaki, T.; Yamamoto, M.; Kishi, S.; Kodama, K. Vaccination of Cattle against Bovine Ephemeral Fever with Live Attenuated Virus Followed by Killed Virus. Arch. Gesamte Virusforsch. 1974, 44, 121–132. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Zieny, A.; Mikhael, C.; YA, S. Further Evaluation of Locally Prepared Live Attenuated Bovine Ephemeral Fever Vaccine in Cattle. J. Vet. Med. Res. 2016, 23, 174–183. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouby, S.R.; Ghonaim, A.H.; Chen, X.; Li, W. The Current Epizootiological Situation of Three Major Viral Infections Affecting Cattle in Egypt. Viruses 2024, 16, 1536. https://doi.org/10.3390/v16101536
Rouby SR, Ghonaim AH, Chen X, Li W. The Current Epizootiological Situation of Three Major Viral Infections Affecting Cattle in Egypt. Viruses. 2024; 16(10):1536. https://doi.org/10.3390/v16101536
Chicago/Turabian StyleRouby, Sherin R., Ahmed H. Ghonaim, Xingxiang Chen, and Wentao Li. 2024. "The Current Epizootiological Situation of Three Major Viral Infections Affecting Cattle in Egypt" Viruses 16, no. 10: 1536. https://doi.org/10.3390/v16101536







