Identification of a Novel Antiviral Lectin against SARS-CoV-2 Omicron Variant from Shiitake-Mushroom-Derived Vesicle-like Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Purification of Vegetable VLNs
2.3. Pseudotyping Viruses
2.4. Pseudovirus Neutralization Assays
2.5. Cytotoxicity Assay of Vegetable VLNs
2.6. Inhibition Analysis of Infectious Viruses by Plaque Assay
2.7. Heat and Sonication of Shiitake Mushroom VLNs
2.8. VLNs Evaluation by Scanning Electron Microscopy (SEM)
2.9. Proteomics of Shiitake Mushroom VLNs
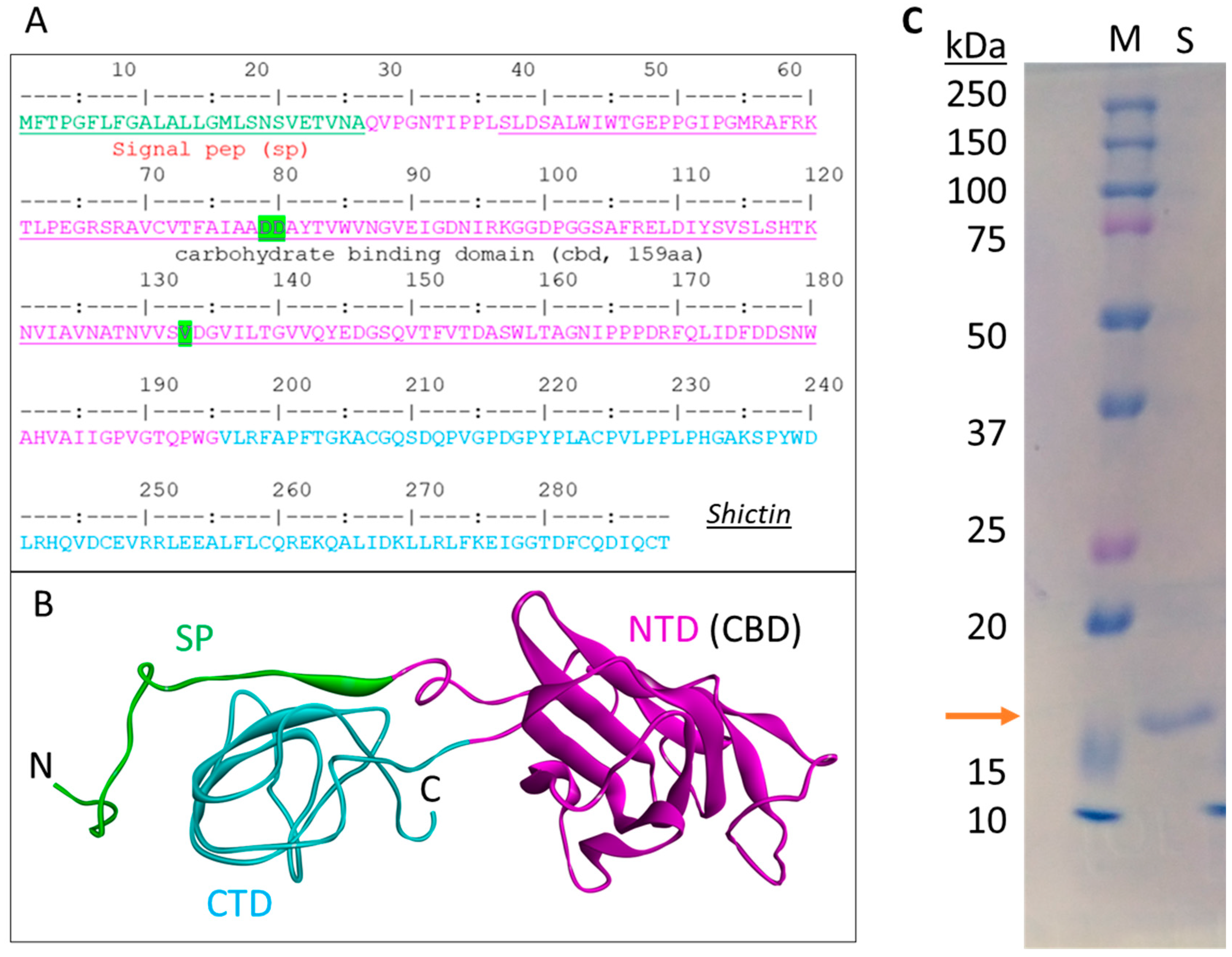
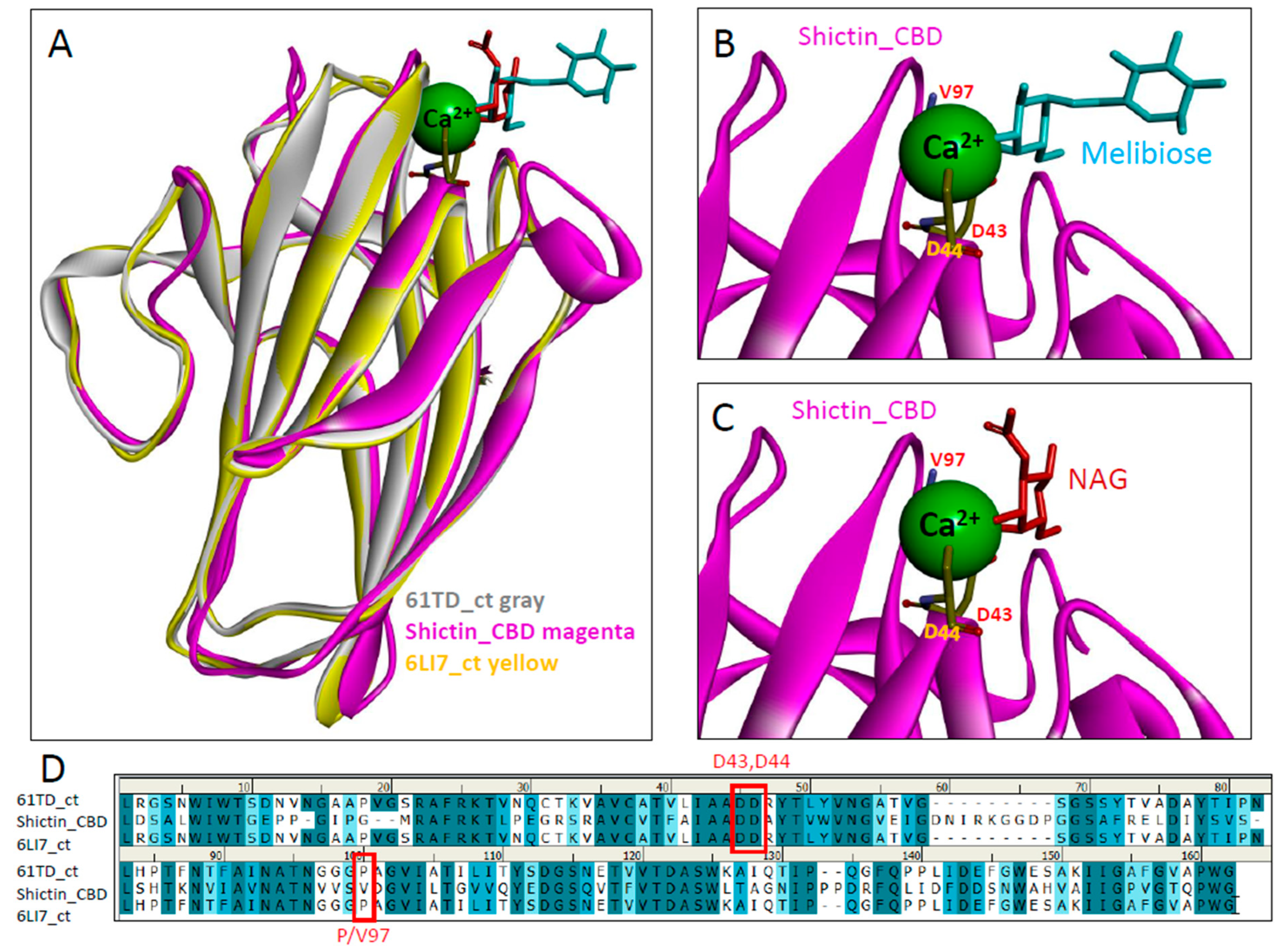
2.10. Molecular Modeling of Shictin
2.11. Recombinant Protein Production
2.12. Statistical Analysis
3. Results
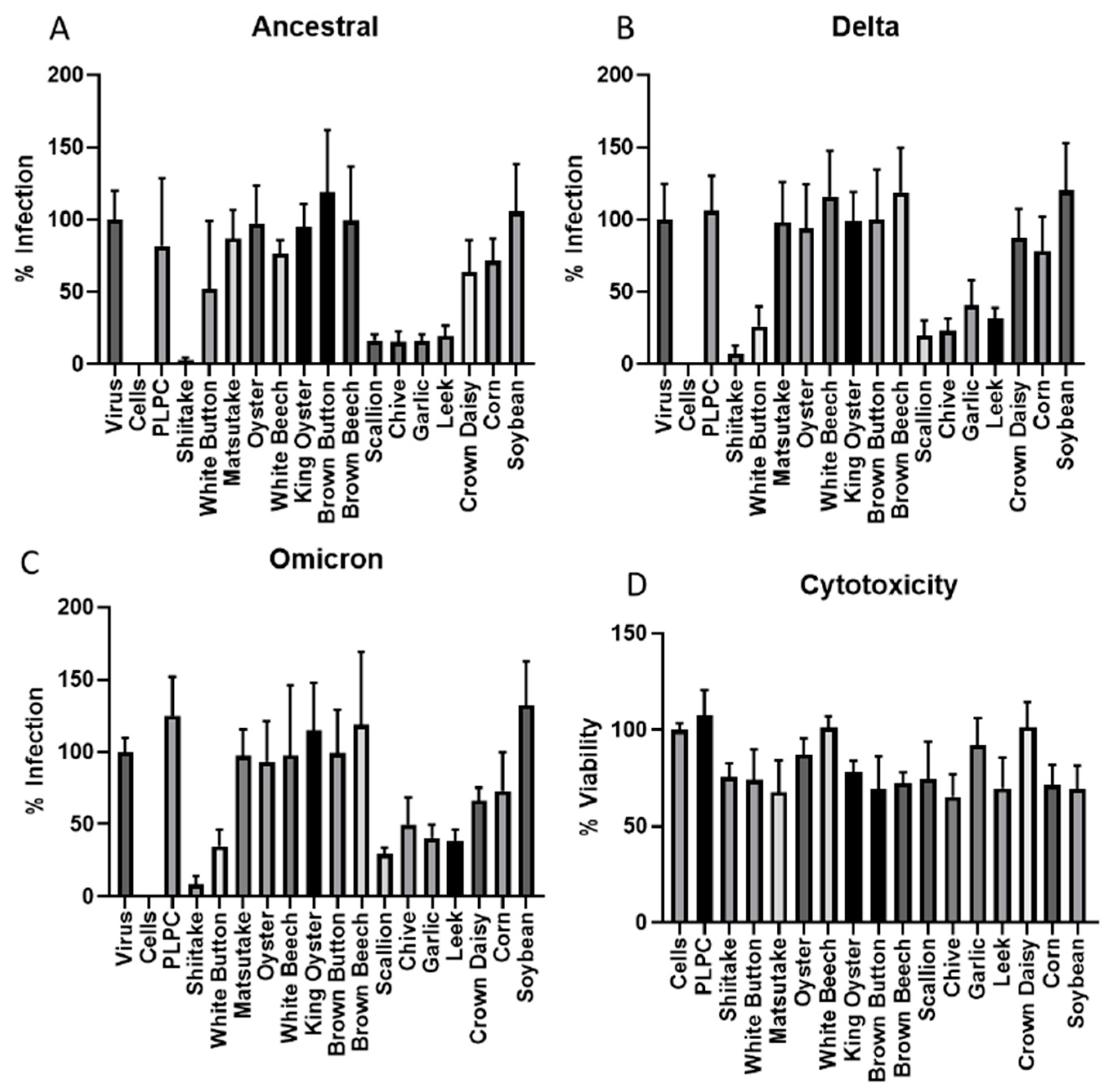
3.1. Screening of Vegetable VLNs Inhibiting SARS-CoV-2 Virus Infection
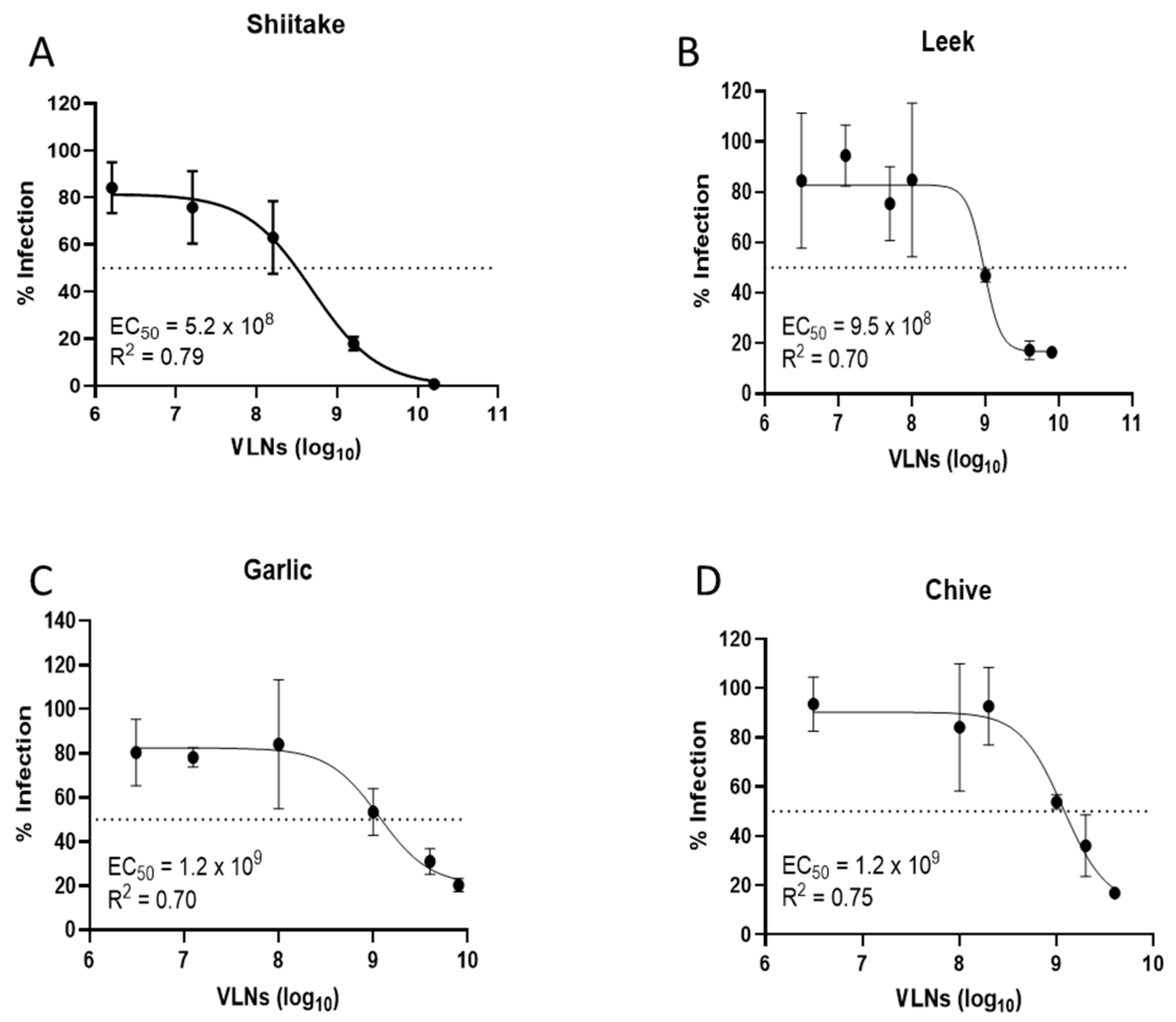
3.2. Proteins of Shiitake VLNs Contributed to the Antiviral Activities
3.3. Lectin (Shictin) of Shiitake VLNs Contributed to the Antiviral Activities
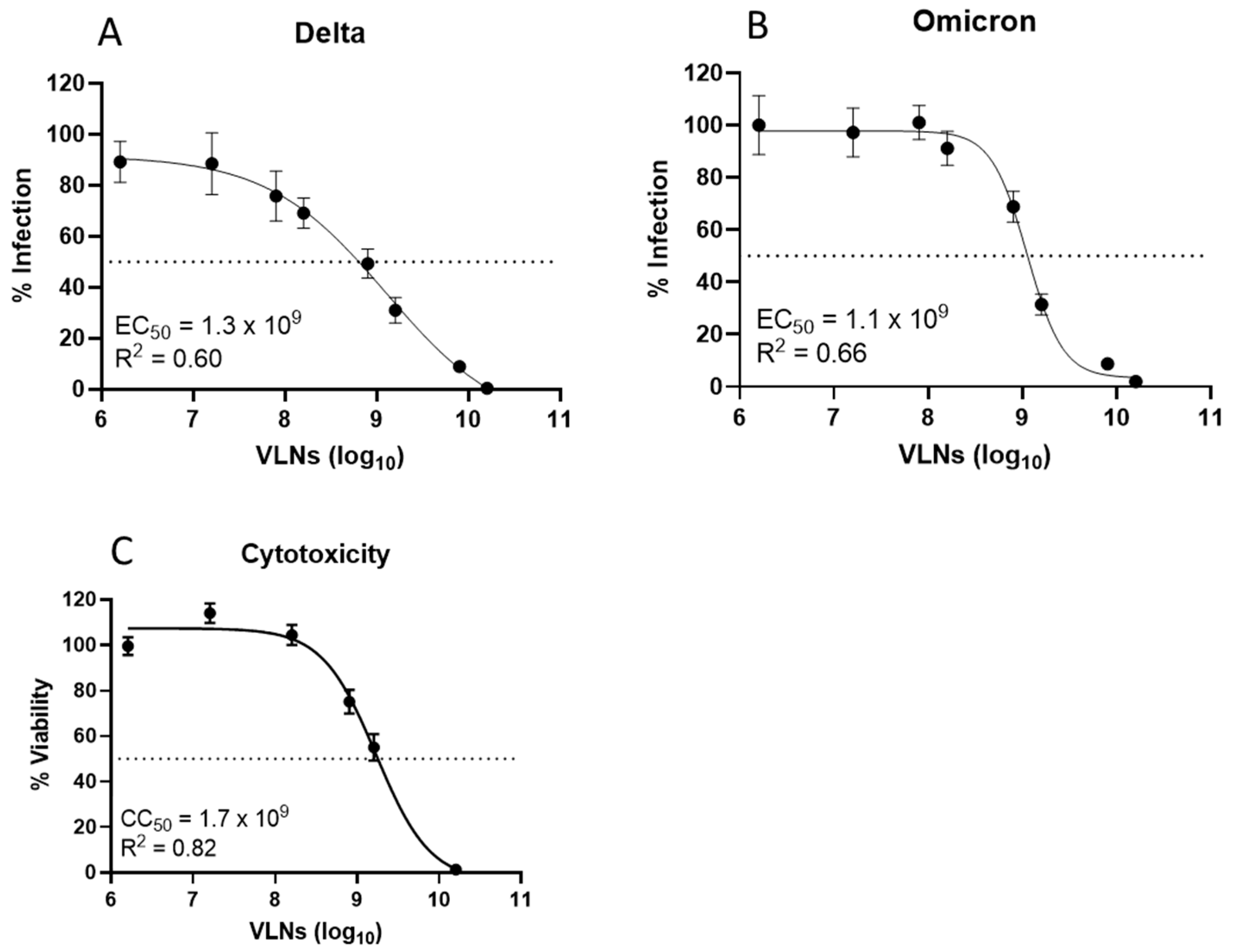
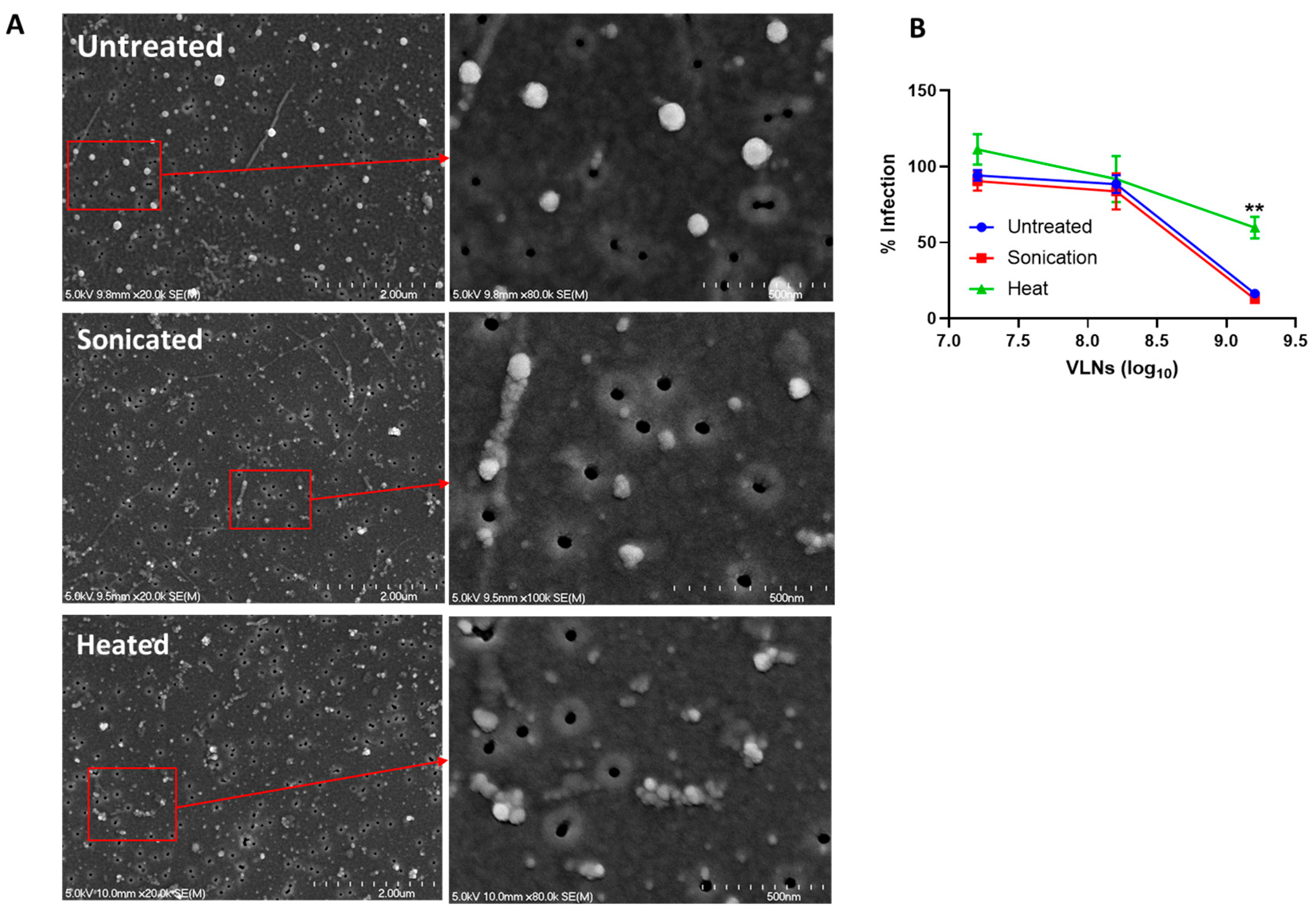
3.4. Shictin Inhibits SARS-CoV-2 Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilcher, M.; Werno, A.; Jennings, L.C. SARS-CoV-2: A Novel Deadly Virus in a Globalised World. N. Z. Med. J. 2020, 133, 6–11. [Google Scholar] [PubMed]
- Holmes, E.C. The Emergence and Evolution of SARS-CoV-2. Annu. Rev. Virol. 2024, 11, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Cai, Y.; Zhu, H.; Peng, H.; Voyer, J.; Rits-Volloch, S.; Cao, H.; Mayer, M.L.; Song, K.; Xu, C.; et al. Cryo-EM Structure of SARS-CoV-2 Postfusion Spike in Membrane. Nature 2023, 619, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, T.; Cai, Y.; Chen, B. Structure of SARS-CoV-2 Spike Protein. Curr. Opin. Virol. 2021, 50, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-Specific Glycan Analysis of the SARS-CoV-2 Spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baudys, J.; Osman, S.H.; Barr, J.R. Analysis of the N-Glycosylation Profiles of the Spike Proteins from the Alpha, Beta, Gamma, and Delta Variants of SARS-CoV-2. Anal. Bioanal. Chem. 2023, 415, 4779–4793. [Google Scholar] [CrossRef]
- Gong, Y.; Qin, S.; Dai, L.; Tian, Z. The Glycosylation in SARS-CoV-2 and Its Receptor ACE2. Sig. Transduct. Target. Ther. 2021, 6, 396. [Google Scholar] [CrossRef]
- Alvarez, C.; Félix, C.; Lemos, M.F.L. The Antiviral Potential of Algal Lectins. Mar. Drugs 2023, 21, 515. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Bourne, Y.; Van Damme, E.J.M.; Rougé, P. Overview of the Structure–Function Relationships of Mannose-Specific Lectins from Plants, Algae and Fungi. Int. J. Mol. Sci. 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K.; Khattar, J.S.; Singh, D.P.; Kennedy, J.F. Cyanobacterial Lectins Characteristics and Their Role as Antiviral Agents. Int. J. Biol. Macromol. 2017, 102, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.C.B.B.; Silva, P.M.D.S.; de Menezes Lima, V.L.; Pontual, E.V.; Paiva, P.M.G.; Napoleão, T.H.; Correia, M.T.D.S. Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications. Evid. Based Complement. Alternat. Med. 2017, 2017, 1594074. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Liu, W.; Ng, T.B. Development and Applications of Lectins as Biological Tools in Biomedical Research. Med. Res. Rev. 2016, 36, 221–247. [Google Scholar] [CrossRef]
- Singh, R.S.; Thakur, S.R.; Bansal, P. Algal Lectins as Promising Biomolecules for Biomedical Research. Crit. Rev. Microbiol. 2015, 41, 77–88. [Google Scholar] [CrossRef]
- Gupta, A.; Yadav, K.; Yadav, A.; Ahmad, R.; Srivastava, A.; Kumar, D.; Khan, M.A.; Dwivedi, U.N. Mannose-Specific Plant and Microbial Lectins as Antiviral Agents: A Review. Glycoconj. J. 2024, 41, 1–33. [Google Scholar] [CrossRef]
- Naik, S.; Kumar, S. Lectins from Plants and Algae Act as Anti-Viral against HIV, Influenza and Coronaviruses. Mol. Biol. Rep. 2022, 49, 12239–12246. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.; Dan, X.; Chan, Y.S.; Pan, W.; Cheung, R.C.F. Lectins with Anti-HIV Activity: A Review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef]
- Bolmstedt, A.J.; O’Keefe, B.R.; Shenoy, S.R.; McMahon, J.B.; Boyd, M.R. Cyanovirin-N Defines a New Class of Antiviral Agent Targeting N-Linked, High-Mannose Glycans in an Oligosaccharide-Specific Manner. Mol. Pharmacol. 2001, 59, 949–954. [Google Scholar] [CrossRef]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of Cyanovirin-N, a Novel Human Immunodeficiency Virus-Inactivating Protein That Binds Viral Surface Envelope Glycoprotein Gp120: Potential Applications to Microbicide Development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses 2016, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.H.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G.; et al. The Cyanobacterial Lectin Scytovirin Displays Potent in Vitro and in Vivo Activity against Zaire Ebola Virus. Antiviral Res. 2014, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McFeeters, R.L.; Xiong, C.; O’Keefe, B.R.; Bokesch, H.R.; McMahon, J.B.; Ratner, D.M.; Castelli, R.; Seeberger, P.H.; Byrd, R.A. The Novel Fold of Scytovirin Reveals a New Twist for Antiviral Entry Inhibitors. J. Mol. Biol. 2007, 369, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Botos, I.; Ziółkowska, N.E.; Bokesch, H.R.; Krumpe, L.R.; McKee, T.C.; O’Keefe, B.R.; Dauter, Z.; Wlodawer, A. Atomic-Resolution Crystal Structure of the Antiviral Lectin Scytovirin. Protein Sci. 2007, 16, 2756–2760. [Google Scholar] [CrossRef]
- Alexandre, K.B.; Gray, E.S.; Lambson, B.E.; Moore, P.L.; Choge, I.A.; Mlisana, K.; Abdool Karim, S.S.; McMahon, J.; O’Keefe, B.; Chikwamba, R.; et al. Mannose-Rich Glycosylation Patterns on HIV-1 Subtype C Gp120 and Sensitivity to the Lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology 2010, 402, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Q.; Wu, J.; Hu, Y.; Wu, G.; Yu, C.; Xu, K.; Liu, X.; Wang, Q.; Huang, W.; et al. Lentil Lectin Derived from Lens Culinaris Exhibit Broad Antiviral Activities against SARS-CoV-2 Variants. Emerg. Microbes Infect. 2021, 10, 1519–1529. [Google Scholar] [CrossRef]
- Ahan, R.E.; Hanifehnezhad, A.; Kehribar, E.Ş.; Oguzoglu, T.C.; Földes, K.; Özçelik, C.E.; Filazi, N.; Öztop, S.; Palaz, F.; Önder, S.; et al. A Highly Potent SARS-CoV-2 Blocking Lectin Protein. ACS Infect. Dis. 2022, 8, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Klevanski, M.; Kim, H.; Heilemann, M.; Kuner, T.; Bartenschlager, R. Glycan-Directed SARS-CoV-2 Inhibition by Leek Extract and Lectins with Insights into the Mode-of-Action of Concanavalin A. Antivir. Res. 2024, 225, 105856. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Basagoiti, J.; Monteiro, F.L.L.; Krumpe, L.R.H.; Armario-Najera, V.; Shenoy, S.R.; Perez-Zsolt, D.; Westgarth, H.J.; Villorbina, G.; Bomfim, L.M.; Raïch-Regué, D.; et al. Cyanovirin-N Binds to Select SARS-CoV-2 Spike Oligosaccharides Outside of the Receptor Binding Domain and Blocks Infection by SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2023, 120, e2214561120. [Google Scholar] [CrossRef]
- Crispin, M.; Ward, A.B.; Wilson, I.A. Structure and Immune Recognition of the HIV Glycan Shield. Annu. Rev. Biophys. 2018, 47, 499–523. [Google Scholar] [CrossRef]
- Pritchard, L.K.; Spencer, D.I.R.; Royle, L.; Bonomelli, C.; Seabright, G.E.; Behrens, A.-J.; Kulp, D.W.; Menis, S.; Krumm, S.A.; Dunlop, D.C.; et al. Glycan Clustering Stabilizes the Mannose Patch of HIV-1 and Preserves Vulnerability to Broadly Neutralizing Antibodies. Nat. Commun. 2015, 6, 7479. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Tortorici, M.A.; Frenz, B.; Snijder, J.; Li, W.; Rey, F.A.; DiMaio, F.; Bosch, B.-J.; Veesler, D. Glycan Shield and Epitope Masking of a Coronavirus Spike Protein Observed by Cryo-Electron Microscopy. Nat. Struct. Mol. Biol. 2016, 23, 899–905. [Google Scholar] [CrossRef]
- Koharudin, L.M.I.; Gronenborn, A.M. Antiviral Lectins as Potential HIV Microbicides. Curr. Opin. Virol. 2014, 7, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Schols, D. Algal Lectins as Potential HIV Microbicide Candidates. Mar. Drugs 2012, 10, 1476–1497. [Google Scholar] [CrossRef] [PubMed]
- Altıntaş, Ö.; Saylan, Y. Exploring the Versatility of Exosomes: A Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal. Chem. 2023, 95, 16029–16048. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, Y.; Ha, S.; Sheller-Miller, S.; Yoo, J.; Choi, C.; Park, C.H. The Emerging Role of Exosomes as Novel Therapeutics: Biology, Technologies, Clinical Applications, and the Next. Am. J. Reprod. Immunol. 2021, 85, e13329. [Google Scholar] [CrossRef]
- Mutai, E.; Ngu, A.K.H.; Zempleni, J. Preliminary Evidence That Lectins in Infant Soy Formula Apparently Bind Bovine Milk Exosomes and Prevent Their Absorption in Healthy Adults. BMC Nutr. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal Therapy—A New Frontier in Regenerative Medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Gajos-Michniewicz, A.; Duechler, M.; Czyz, M. MiRNA in Melanoma-Derived Exosomes. Cancer Lett. 2014, 347, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The Biology, Function, and Applications of Exosomes in Cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yan, Z.; Yuan, Y.; Xing, C.; Jiang, N. The Role of Exosomes in Viral Hepatitis and Its Associated Liver Diseases. Front. Med. 2021, 8, 782485. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-X.; Cai, Q. Plant Exosome-like Nanovesicles and Their Role in the Innovative Delivery of RNA Therapeutics. Biomedicines 2023, 11, 1806. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Yu, H.; Shi, X.; Zhou, Y.; Alvarez, S.; Naldrett, M.J.; Kachman, S.D.; Ro, S.-H.; Sun, X.; et al. Therapeutic Potential of Garlic Chive-Derived Vesicle-like Nanoparticles in NLRP3 Inflammasome-Mediated Inflammatory Diseases. Theranostics 2021, 11, 9311–9330. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Tang, T.; Nathan, L.; Jaimes, J.A.; Hsu, H.-L.; Daniel, S.; Whittaker, G.R. Production of Pseudotyped Particles to Study Highly Pathogenic Coronaviruses in a Biosafety Level 2 Setting. J. Vis. Exp. 2019, 145, e59010. [Google Scholar] [CrossRef]
- Conforti, A.; Marra, E.; Palombo, F.; Roscilli, G.; Ravà, M.; Fumagalli, V.; Muzi, A.; Maffei, M.; Luberto, L.; Lione, L.; et al. COVID-eVax, an Electroporated DNA Vaccine Candidate Encoding the SARS-CoV-2 RBD, Elicits Protective Responses in Animal Models. Mol. Ther. 2022, 30, 311–326. [Google Scholar] [CrossRef]
- Cho, H.; Gonzales-Wartz, K.K.; Huang, D.; Yuan, M.; Peterson, M.; Liang, J.; Beutler, N.; Torres, J.L.; Cong, Y.; Postnikova, E.; et al. Bispecific Antibodies Targeting Distinct Regions of the Spike Protein Potently Neutralize SARS-CoV-2 Variants of Concern. Sci. Transl. Med. 2021, 13, eabj5413. [Google Scholar] [CrossRef]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.-C.D.; Lin, T.-H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly Neutralizing Antibodies Target the Coronavirus Fusion Peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef]
- Wiggins, J.; Nguyen, N.; Wei, W.; Wang, L.L.; Hollingsead Olson, H.; Xiang, S.-H. Lactic Acid Bacterial Surface Display of Scytovirin Inhibitors for Anti-Ebolavirus Infection. Front. Microbiol. 2023, 14, 1269869. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Klinke, D.J. Exosomes: Improved Methods to Characterize Their Morphology, RNA Content, and Surface Protein Biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Z.; Jackson, A.; Michikawa, M.; Maehara, T.; Momma, M.; Henrissat, B.; Gilbert, H.J.; Kaneko, S. The Structure of a Streptomyces Avermitilis α-l-Rhamnosidase Reveals a Novel Carbohydrate-Binding Module CBM67 within the Six-Domain Arrangement. J. Biol. Chem. 2013, 288, 12376–12385. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Bowder, D.; Wei, W.; Thompson, J.; Wilson, M.A.; Xiang, S.-H. Tryptophan 375 Stabilizes the Outer-Domain Core of Gp120 for HIV Vaccine Immunogen Design. Vaccine 2017, 35, 3067–3075. [Google Scholar] [CrossRef]
- Vajravijayan, S.; Pletnev, S.; Luo, Z.; Pletnev, V.Z.; Nandhagopal, N.; Gunasekaran, K. Crystallographic and Calorimetric Analysis on Pleurotus Ostreatus Lectin and Its Sugar Complexes—Promiscuous Binding Driven by Geometry. Int. J. Biol. Macromol. 2020, 152, 862–872. [Google Scholar] [CrossRef]
- Perduca, M.; Destefanis, L.; Bovi, M.; Galliano, M.; Munari, F.; Assfalg, M.; Ferrari, F.; Monaco, H.L.; Capaldi, S. Structure and Properties of the Oyster Mushroom (Pleurotus ostreatus) Lectin. Glycobiology 2020, 30, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.; Ng, T.B. A New Lectin with Highly Potent Antihepatoma and Antisarcoma Activities from the Oyster Mushroom Pleurotus Ostreatus. Biochip. Biophys. Res. Commun. 2000, 275, 810–816. [Google Scholar] [CrossRef]
- Shajahan, A.; Pepi, L.E.; Kumar, B.; Murray, N.B.; Azadi, P. Site Specific N- and O-Glycosylation Mapping of the Spike Proteins of SARS-CoV-2 Variants of Concern. Sci. Rep. 2023, 13, 10053. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.G.; Onigbinde, S.; Sanni, A.; Bennett, A.I.; Jiang, P.; Daramola, O.; Ahmadi, P.; Fowowe, M.; Atashi, M.; Sandilya, V.; et al. N-Glycome Profile of the Spike Protein S1: Systemic and Comparative Analysis from Eleven Variants of SARS-CoV-2. Biomolecules 2023, 13, 1421. [Google Scholar] [CrossRef]
- Aloor, A.; Aradhya, R.; Venugopal, P.; Gopalakrishnan Nair, B.; Suravajhala, R. Glycosylation in SARS-CoV-2 Variants: A Path to Infection and Recovery. Biochem. Pharmacol. 2022, 206, 115335. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wu, L.-A.; Wang, Q.; Qi, J.; Gao, G.F. Cell Entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Okada, S.; Ozeki, T.; Yoshikawa, R.; Kinoshita, T.; Yasuda, J. SARS-CoV-2 Omicron Subvariants Progressively Adapt to Human Cells with Altered Host Cell Entry. mSphere 2024, 9, e0033824. [Google Scholar] [CrossRef] [PubMed]
- Pires De Souza, G.A.; Le Bideau, M.; Boschi, C.; Wurtz, N.; Colson, P.; Aherfi, S.; Devaux, C.; La Scola, B. Choosing a Cellular Model to Study SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022, 12, 1003608. [Google Scholar] [CrossRef] [PubMed]
- Eghianruwa, Q.; Odekanyin, O.; Kuku, A. Physicochemical Properties and Acute Toxicity Studies of a Lectin from the Saline Extract of the Fruiting Bodies of the Shiitake Mushroom, Lentinula Edodes (Berk). Int. J. Biochem. Mol. Biol. 2011, 2, 309–317. [Google Scholar]
- Xu, Y.; Chen, S.; Liu, Q. Lectin from the Late Oyster Mushroom, Hohenbuehelia Serotina (Agaricomycetes), and Its Novel Effect as an Adjuvant of the HBV DNA Vaccine. Int. J. Med. Mushrooms 2017, 19, 1123–1133. [Google Scholar] [CrossRef] [PubMed]


| No | Name | Concentration (/mL) |
|---|---|---|
| 1 | Shiitake mushroom (Lentinula edodes) | 1.05 × 1013 |
| 2 | White button mushroom (Agaricus bisporus) | 3.15 × 1012 |
| 3 | Matsutake mushroom (Tricholoma matsutake) | 2.3 × 1012 |
| 4 | Oyster mushroom (Pleurotus ostreatus) | 5.0 × 1012 |
| 5 | White beech mushroom (Hypsizygus tessellatus) | 7.0 × 1012 |
| 6 | King Oyster mushroom (Pleurotus eryngii) | 1.3 × 1013 |
| 7 | Brown button mushroom (Agaricus bisporus) | 2.65 × 1012 |
| 8 | Brown beech mushroom (Hypsizygus tessellatus) | 6.5 × 1012 |
| 9 | Scallion (Allium fistulosum) | 2.2 × 1012 |
| 10 | Chive (Allium tuberosum) | 8.0 × 1012 |
| 11 | Garlic (Allium sativum) | 2.6 × 1012 |
| 12 | Leek (Allium ampeloprasum) | 2.6 × 1012 |
| 13 | Crown daisy (Glebionis coronaria) | 3.6 × 1012 |
| 14 | Sweet corn (Zea mays) | 1.45 × 1012 |
| 15 | Soybean (Glycine max) | 6.0 × 1012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiggins, J.; Karim, S.-U.; Liu, B.; Li, X.; Zhou, Y.; Bai, F.; Yu, J.; Xiang, S.-H. Identification of a Novel Antiviral Lectin against SARS-CoV-2 Omicron Variant from Shiitake-Mushroom-Derived Vesicle-like Nanoparticles. Viruses 2024, 16, 1546. https://doi.org/10.3390/v16101546
Wiggins J, Karim S-U, Liu B, Li X, Zhou Y, Bai F, Yu J, Xiang S-H. Identification of a Novel Antiviral Lectin against SARS-CoV-2 Omicron Variant from Shiitake-Mushroom-Derived Vesicle-like Nanoparticles. Viruses. 2024; 16(10):1546. https://doi.org/10.3390/v16101546
Chicago/Turabian StyleWiggins, Joshua, Shazeed-Ul Karim, Baolong Liu, Xingzhi Li, You Zhou, Fengwei Bai, Jiujiu Yu, and Shi-Hua Xiang. 2024. "Identification of a Novel Antiviral Lectin against SARS-CoV-2 Omicron Variant from Shiitake-Mushroom-Derived Vesicle-like Nanoparticles" Viruses 16, no. 10: 1546. https://doi.org/10.3390/v16101546








