Chimeric Virus-like Particles of Physalis Mottle Virus as Carriers of M2e Peptides of Influenza a Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Construction of Expression Vectors
2.2. Expression of Recombinant Proteins
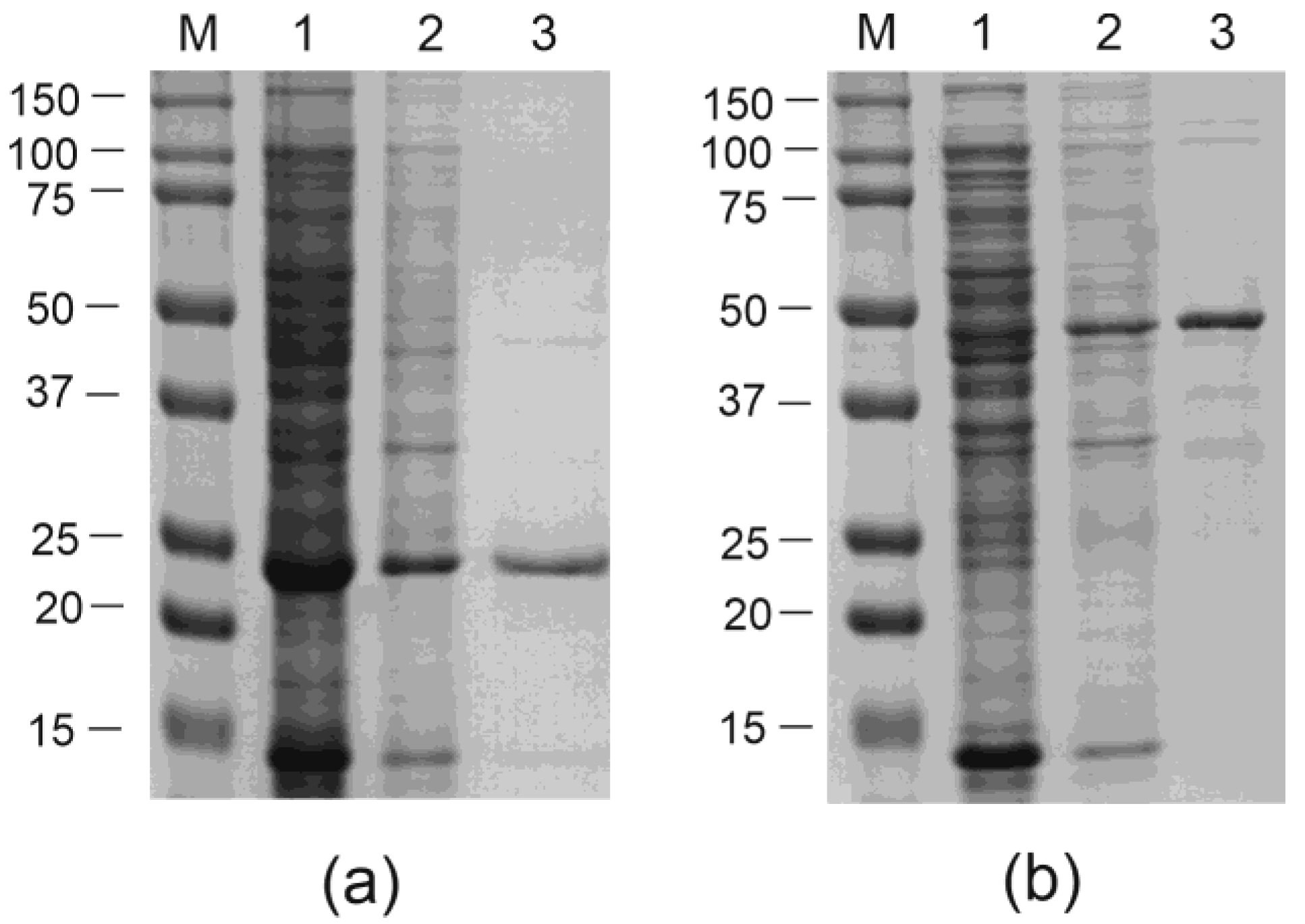
2.3. Purification of Virus-like Particles
2.4. Structural Analysis of VLPs
2.5. Recognition of M2e-Carrying VLPs by Anti-M2e Antibodies in ELISA
2.6. Immunization of Mice
2.7. Measurement of Antibody Titers in Sera by ELISA
2.8. Influenza Virus and Challenge Experiment
2.9. Statistical Analysis
2.10. Ethics Statement
3. Results
3.1. Design and Construction of Recombinant Proteins and Expression Vectors
3.2. Purification of VLPs
3.3. Antigenic Properties of 4M2e-Carrying Chimeric VLPs
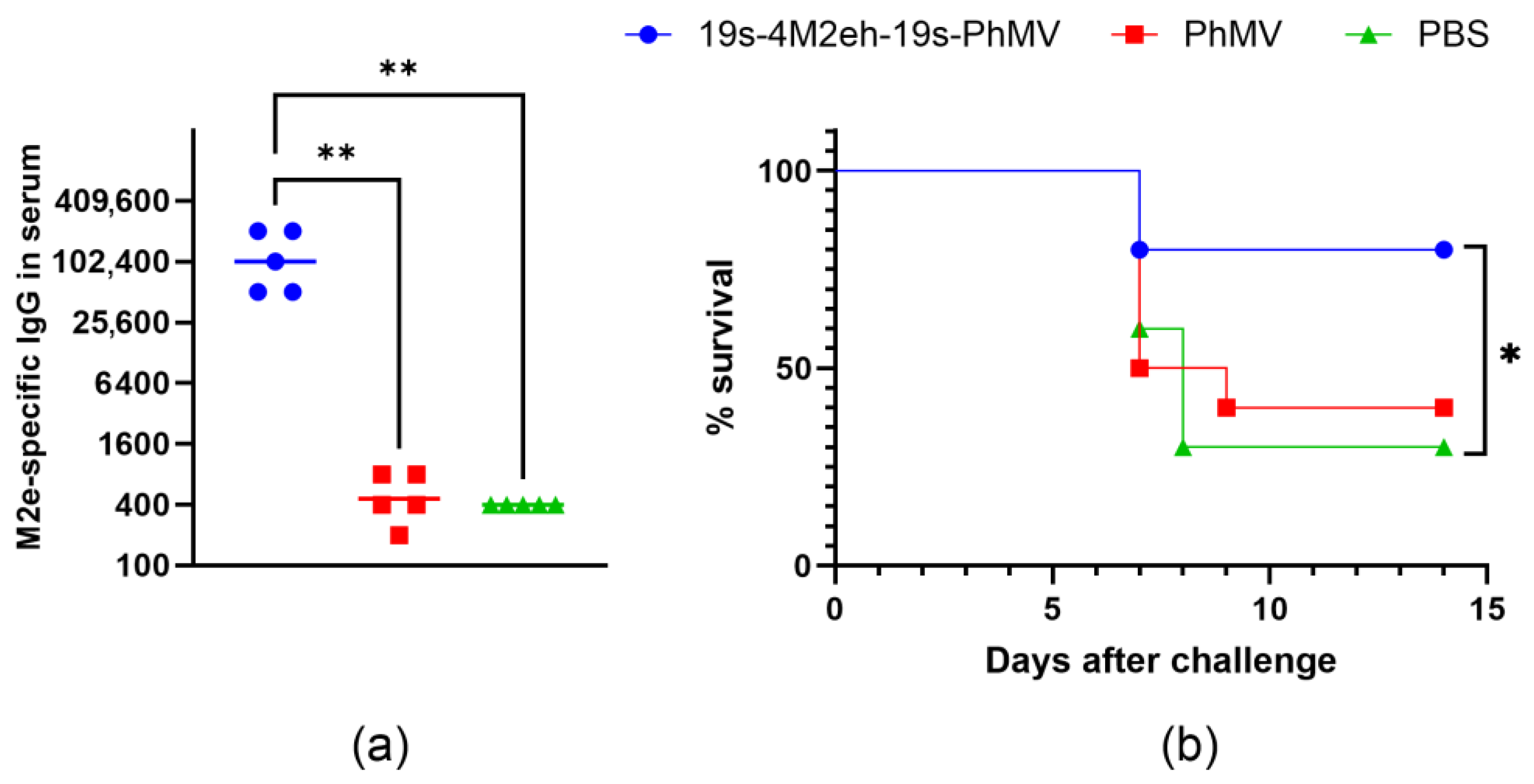
3.4. Immunogenicity and Protective Activity of 4M2e-Carrying Chimeric VLPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kheirvari, M.; Liu, H.; Tumban, E. Virus-like Particle Vaccines and Platforms for Vaccine Development. Viruses 2023, 15, 1109. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.D.; Harwood, N.E. The Who, How and Where of Antigen Presentation to B Cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef]
- Balke, I.; Zeltins, A. Use of Plant Viruses and Virus-like Particles for the Creation of Novel Vaccines. Adv. Drug Deliv. Rev. 2019, 145, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.R.; Cunningham, J.; von Seefried, A.; Lennick, M.; Garvin, R.T.; Shen, S.-H. Development of a Genetically–Engineered, Candidate Polio Vaccine Employing the Self–Assembling Properties of the Tobacco Mosaic Virus Coat Protein. Biotechnology 1986, 4, 637–641. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Han, S.S. Recombinant Helical Plant Virus-Based Nanoparticles for Vaccination and Immunotherapy. Virus Genes 2018, 54, 623–637. [Google Scholar] [CrossRef]
- Santoni, M.; Zampieri, R.; Avesani, L. Plant Virus Nanoparticles for Vaccine Applications. Curr. Protein Pept. Sci. 2020, 21, 344–356. [Google Scholar] [CrossRef]
- Hassani-Mehraban, A.; Creutzburg, S.; van Heereveld, L.; Kormelink, R. Feasibility of Cowpea Chlorotic Mottle Virus-like Particles as Scaffold for Epitope Presentations. BMC Biotechnol. 2015, 15, 80. [Google Scholar] [CrossRef]
- Saini, M.; Vrati, S. A Japanese Encephalitis Virus Peptide Present on Johnson Grass Mosaic Virus-Like Particles Induces Virus-Neutralizing Antibodies and Protects Mice against Lethal Challenge. J. Virol. 2003, 77, 3487–3494. [Google Scholar] [CrossRef]
- Hu, H.; Steinmetz, N.F. Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine. Cancers 2021, 13, 2909. [Google Scholar] [CrossRef]
- Carignan, D.; Thérien, A.; Rioux, G.; Paquet, G.; Gagné, M.-È.L.; Bolduc, M.; Savard, P.; Leclerc, D. Engineering of the PapMV Vaccine Platform with a Shortened M2e Peptide Leads to an Effective One Dose Influenza Vaccine. Vaccine 2015, 33, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, S.; Gujar, R.; Pattabiraman, S.; Nesakumar, M.; Hanna, L.E.; Vadakkuppattu, R.D.; Usha, R. Expression and Immunological Characterization of Cardamom Mosaic Virus Coat Protein Displaying HIV Gp41 Epitopes. Microbiol. Immunol. 2013, 57, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Kalnciema, I.; Skrastina, D.; Ose, V.; Pumpens, P.; Zeltins, A. Potato Virus Y-Like Particles as a New Carrier for the Presentation of Foreign Protein Stretches. Mol. Biotechnol. 2012, 52, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Almendárez-Rodriguez, C.; Solis-Andrade, K.I.; Govea-Alonso, D.O.; Comas-Garcia, M.; Rosales-Mendoza, S. Production and characterization of chimeric SARS-CoV-2 antigens based on the capsid protein of cowpea chlorotic mottle virus. Int. J. Biol. Macromol. 2022, 213, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Hema, M.; Nagendrakumar, S.B.; Yamini, R.; Chandran, D.; Rajendra, L.; Thiagarajan, D.; Parida, S.; Paton, D.J.; Srinivasan, V.A. Chimeric Tymovirus-like Particles Displaying Foot-and-Mouth Disease Virus Non-Structural Protein Epitopes and Its Use for Detection of FMDV-NSP Antibodies. Vaccine 2007, 25, 4784–4794. [Google Scholar] [CrossRef] [PubMed]
- Shahana, P.V.; Das, D.; Gontu, A.; Chandran, D.; Maithal, K. Efficient Production of Tymovirus like Particles Displaying Immunodominant Epitopes of Japanese Encephalitis Virus Envelope Protein. Protein Expr. Purif. 2015, 113, 35–43. [Google Scholar] [CrossRef]
- Chandran, D.; Shahana, P.V.; Rani, G.S.; Sugumar, P.; Shankar, C.R.; Srinivasan, V.A. Display of Neutralizing Epitopes of Canine Parvovirus and a T-Cell Epitope of the Fusion Protein of Canine Distemper Virus on Chimeric Tymovirus-like Particles and Its Use as a Vaccine Candidate Both against Canine Parvo and Canine Distemper. Vaccine 2009, 28, 132–139. [Google Scholar] [CrossRef]
- Krishna, S.S.; Hiremath, C.N.; Munshi, S.K.; Prahadeeswaran, D.; Sastri, M.; Savithri, H.S.; Murthy, M.R.N. Three-Dimensional Structure of Physalis Mottle Virus: Implications for the Viral Assembly. J. Mol. Biol. 1999, 289, 919–934. [Google Scholar] [CrossRef]
- Sastri, M.; Kekuda, R.; Gopinath, K.; Kumar, C.T.R.; Jagath, J.R.; Savithri, H.S. Assembly of Physalis Mottle Virus Capsid Protein in Escherichia coli and the Role of Amino and Carboxy Termini in the Formation of the Icosahedral Particles. J. Mol. Biol. 1997, 272, 541–552. [Google Scholar] [CrossRef]
- Sahithi, K.D.; Nancy, P.A.; Vishnu Vardhan, G.P.; Kumanan, K.; Vijayarani, K.; Hema, M. Detection of Infectious Bursal Disease Virus (IBDV) Antibodies Using Chimeric Plant Virus-like Particles. Vet. Microbiol. 2019, 229, 20–27. [Google Scholar] [CrossRef]
- Masarapu, H.; Patel, B.K.; Chariou, P.L.; Hu, H.; Gulati, N.M.; Carpenter, B.L.; Ghiladi, R.A.; Shukla, S.; Steinmetz, N.F. Physalis Mottle Virus-Like Particles as Nanocarriers for Imaging Reagents and Drugs. Biomacromolecules 2017, 18, 4141–4153. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, K.J.; Wu, Z.; Zhao, Z.; Simms, A.; Chang, E.Y.; Steinmetz, N.F. Physalis Mottle Virus-Like Nanocarriers with Expanded Internal Loading Capacity. Bioconjug Chem. 2023, 34, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Neirynck, S.; Deroo, T.; Saelens, X.; Vanlandschoot, P.; Jou, W.M.; Fiers, W. A Universal Influenza A Vaccine Based on the Extracellular Domain of the M2 Protein. Nat. Med. 1999, 5, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Cho, K.; Fiers, W.; Saelens, X. M2e-Based Universal Influenza A Vaccines. Vaccines 2015, 3, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, A.; Schmitz, N.; Storni, T.; Bachmann, M.F. Influenza A Vaccine Based on the Extracellular Domain of M2: Weak Protection Mediated via Antibody-Dependent NK Cell Activity. J. Immunol. 2004, 172, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- El Bakkouri, K.; Descamps, F.; De Filette, M.; Smet, A.; Festjens, E.; Birkett, A.; Van Rooijen, N.; Verbeek, S.; Fiers, W.; Saelens, X. Universal Vaccine Based on Ectodomain of Matrix Protein 2 of Influenza A: Fc Receptors and Alveolar Macrophages Mediate Protection. J. Immunol. 2011, 186, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, E.A.; Kuprianov, V.V.; Stepanova, L.A.; Tsybalova, L.M.; Kiselev, O.I.; Ravin, N.V.; Skryabin, K.G. A Molecular Assembly System for Presentation of Antigens on the Surface of HBc Virus-like Particles. Virology 2013, 435, 293–300. [Google Scholar] [CrossRef]
- Kolpe, A.; Schepens, B.; Fiers, W.; Saelens, X. M2-Based Influenza Vaccines: Recent Advances and Clinical Potential. Expert Rev. Vaccines 2017, 16, 123–136. [Google Scholar] [CrossRef]
- Lim, C.M.L.; Komarasamy, T.V.; Adnan, N.A.A.B.; Radhakrishnan, A.K.; Balasubramaniam, V.R.M.T. Recent Advances, Approaches and Challenges in the Development of Universal Influenza Vaccines. Influenza Other Respir. Viruses 2024, 18, e13276. [Google Scholar] [CrossRef]
- Feranmi, F. Universal Flu Vaccine Protects against Influenza A and B. Lancet Microbe 2022, 3, e902. [Google Scholar] [CrossRef]
- Petukhova, N.; Gasanova, T.; Stepanova, L.; Rusova, O.; Potapchuk, M.; Korotkov, A.; Skurat, E.; Tsybalova, L.; Kiselev, O.; Ivanov, P.; et al. Immunogenicity and Protective Efficacy of Candidate Universal Influenza A Nanovaccines Produced in Plants by Tobacco Mosaic Virus-Based Vectors. Curr. Pharm. Des. 2013, 19, 5587–5600. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, N.; Gasanova, T.; Ivanov, P.; Atabekov, J. High-Level Systemic Expression of Conserved Influenza Epitope in Plants on the Surface of Rod-Shaped Chimeric Particles. Viruses 2014, 6, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Gasanova, T.V.; Koroleva, A.A.; Skurat, E.V.; Ivanov, P.A. Complexes Formed via Bioconjugation of Genetically Modified TMV Particles with Conserved Influenza Antigen: Synthesis and Characterization. Biochemistry 2020, 85, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Tyulkina, L.G.; Skurat, E.V.; Frolova, O.Y.; Komarova, T.V.; Karger, E.M.; Atabekov, I.G. New Viral Vector for Superproduction of Epitopes of Vaccine Proteins in Plants. Acta Naturae 2011, 3, 73–82. [Google Scholar] [CrossRef]
- Denis, J.; Acosta-Ramirez, E.; Zhao, Y.; Hamelin, M.-E.; Koukavica, I.; Baz, M.; Abed, Y.; Savard, C.; Pare, C.; Lopez Macias, C.; et al. Development of a Universal Influenza A Vaccine Based on the M2e Peptide Fused to the Papaya Mosaic Virus (PapMV) Vaccine Platform. Vaccine 2008, 26, 3395–3403. [Google Scholar] [CrossRef]
- Watkins, H.C.; Pagan, C.L.; Childs, H.R.; Posada, S.; Chau, A.; Rios, J.; Guarino, C.; DeLisa, M.P.; Whittaker, G.R.; Putnam, D. A Single Dose and Long Lasting Vaccine against Pandemic Influenza through the Controlled Release of a Heterospecies Tandem M2 Sequence Embedded within Detoxified Bacterial Outer Membrane Vesicles. Vaccine 2017, 35, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Thérien, A.; Bédard, M.; Carignan, D.; Rioux, G.; Gauthier-Landry, L.; Laliberté-Gagné, M.-È.; Bolduc, M.; Savard, P.; Leclerc, D. A Versatile Papaya Mosaic Virus (PapMV) Vaccine Platform Based on Sortase-Mediated Antigen Coupling. J. Nanobiotechnol. 2017, 15, 54. [Google Scholar] [CrossRef]
- Meshcheriakova, I.A.; El’darov, M.A.; Migunov, A.I.; Stepanova, L.A.; Repko, I.A.; Kiselev, O.I.; Lomonosov, D.P.; Skriabin, K.G. [Cowpea Mosaic Virus Chimeric Particles Bearing Ectodomain of Matrix Protein 2 (M2E) of Influenza A Virus: Production and Characteristics]. Mol. Boil. 2009, 43, 741–750. [Google Scholar] [CrossRef]
- Jacob, A.N.K.; Murthy, M.R.N.; Savithri, H.S. Nucleotide Sequence of the 3? Terminal Region of Belladonna Mottle Virus-Iowa (Renamed Physalis Mottle Virus) RNA and an Analysis of the Relationships of Tymoviral Coat Proteins. Arch. Virol. 1992, 123, 367–377. [Google Scholar] [CrossRef]
- De Filette, M.; Min Jou, W.; Birkett, A.; Lyons, K.; Schultz, B.; Tonkyro, A.; Resch, S.; Fiers, W. Universal Influenza A Vaccine: Optimization of M2-Based Constructs. Virology 2005, 337, 149–161. [Google Scholar] [CrossRef]
- Robinson, C.R.; Sauer, R.T. Optimizing the Stability of Single-Chain Proteins by Linker Length and Composition Mutagenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 5929–5934. [Google Scholar] [CrossRef] [PubMed]
- Ravin, N.V.; Blokhina, E.A.; Kuprianov, V.V.; Stepanova, L.A.; Shaldjan, A.A.; Kovaleva, A.A.; Tsybalova, L.M.; Skryabin, K.G. Development of a Candidate Influenza Vaccine Based on Virus-like Particles Displaying Influenza M2e Peptide into the Immunodominant Loop Region of Hepatitis B Core Antigen: Insertion of Multiple Copies of M2e Increases Immunogenicity and Protective Efficiency. Vaccine 2015, 33, 3392–3397. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-Microparticles as Immune Adjuvants: Correlating Particle Sizes and the Resultant Immune Responses. Expert. Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef]
- Wibowo, N.; Chuan, Y.P.; Lua, L.H.L.; Middelberg, A.P.J. Modular Engineering of a Microbially-Produced Viral Capsomere Vaccine for Influenza. Chem. Eng. Sci. 2013, 103, 12–20. [Google Scholar] [CrossRef]
- Huleatt, J.W.; Nakaar, V.; Desai, P.; Huang, Y.; Hewitt, D.; Jacobs, A.; Tang, J.; McDonald, W.; Song, L.; Evans, R.K.; et al. Potent Immunogenicity and Efficacy of a Universal Influenza Vaccine Candidate Comprising a Recombinant Fusion Protein Linking Influenza M2e to the TLR5 Ligand Flagellin. Vaccine 2008, 26, 201–214. [Google Scholar] [CrossRef]
- Vasyagin, E.A.; Zykova, A.A.; Mardanova, E.S.; Nikitin, N.A.; Shuklina, M.A.; Ozhereleva, O.O.; Stepanova, L.A.; Tsybalova, L.M.; Blokhina, E.A.; Ravin, N.V. Influenza A Vaccine Candidates Based on Virus-like Particles Formed by Coat Proteins of Single-Stranded RNA Phages Beihai32 and PQ465. Vaccines 2024, 12, 1033. [Google Scholar] [CrossRef]
- Zykova, A.A.; Blokhina, E.A.; Stepanova, L.A.; Shuklina, M.A.; Tsybalova, L.M.; Kuprianov, V.V.; Ravin, N.V. Nanoparticles based on artificial self-assembling peptide and displaying M2e peptide and stalk HA epitopes of influenza A virus induce potent humoral and T-cell responses and protect against the viral infection. Nanomedicine 2022, 39, 102463. [Google Scholar] [CrossRef]


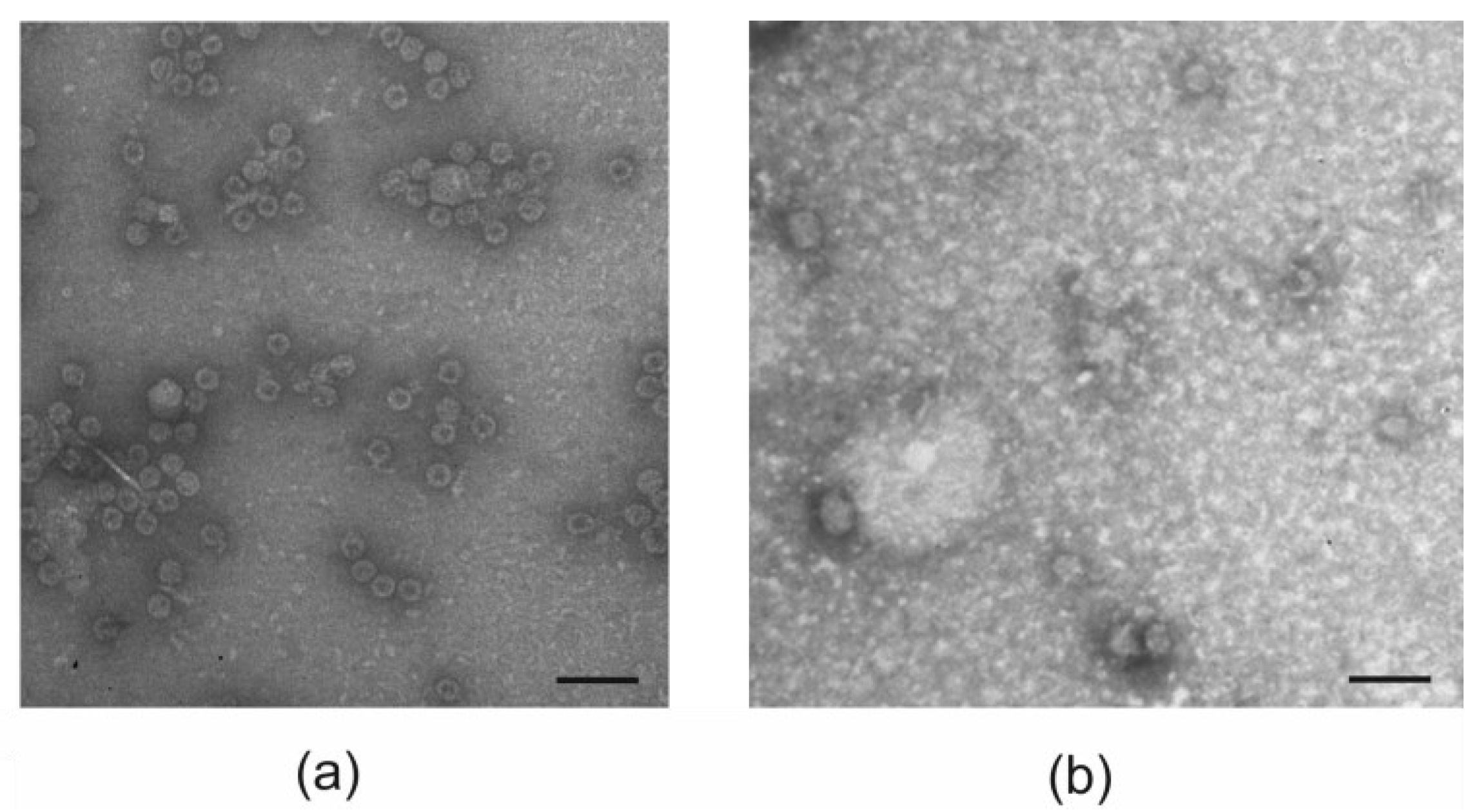

| Parameter | PhMV | 19s-4M2eh-19s-PhMV |
|---|---|---|
| Size (nm) * | 42.1 ± 3.4 | 37.5 ± 1.8 |
| PDI | 0.24 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blokhina, E.A.; Mardanova, E.S.; Zykova, A.A.; Shuklina, M.A.; Stepanova, L.A.; Tsybalova, L.M.; Ravin, N.V. Chimeric Virus-like Particles of Physalis Mottle Virus as Carriers of M2e Peptides of Influenza a Virus. Viruses 2024, 16, 1802. https://doi.org/10.3390/v16111802
Blokhina EA, Mardanova ES, Zykova AA, Shuklina MA, Stepanova LA, Tsybalova LM, Ravin NV. Chimeric Virus-like Particles of Physalis Mottle Virus as Carriers of M2e Peptides of Influenza a Virus. Viruses. 2024; 16(11):1802. https://doi.org/10.3390/v16111802
Chicago/Turabian StyleBlokhina, Elena A., Eugenia S. Mardanova, Anna A. Zykova, Marina A. Shuklina, Liudmila A. Stepanova, Liudmila M. Tsybalova, and Nikolai V. Ravin. 2024. "Chimeric Virus-like Particles of Physalis Mottle Virus as Carriers of M2e Peptides of Influenza a Virus" Viruses 16, no. 11: 1802. https://doi.org/10.3390/v16111802
APA StyleBlokhina, E. A., Mardanova, E. S., Zykova, A. A., Shuklina, M. A., Stepanova, L. A., Tsybalova, L. M., & Ravin, N. V. (2024). Chimeric Virus-like Particles of Physalis Mottle Virus as Carriers of M2e Peptides of Influenza a Virus. Viruses, 16(11), 1802. https://doi.org/10.3390/v16111802










