Dynamics of Low-Level Viremia and Immune Activation after Switching to a Darunavir-Based Regimen
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Data and Samples
2.3. HIV-RNA Load and Virological Response
2.4. Total and Intact/Defective HIV-1 DNA Quantification
2.5. msRNA Quantification
2.6. Immune Activation
2.7. DRV Drug Concentrations
2.8. Data Analysis and Statistics
3. Results
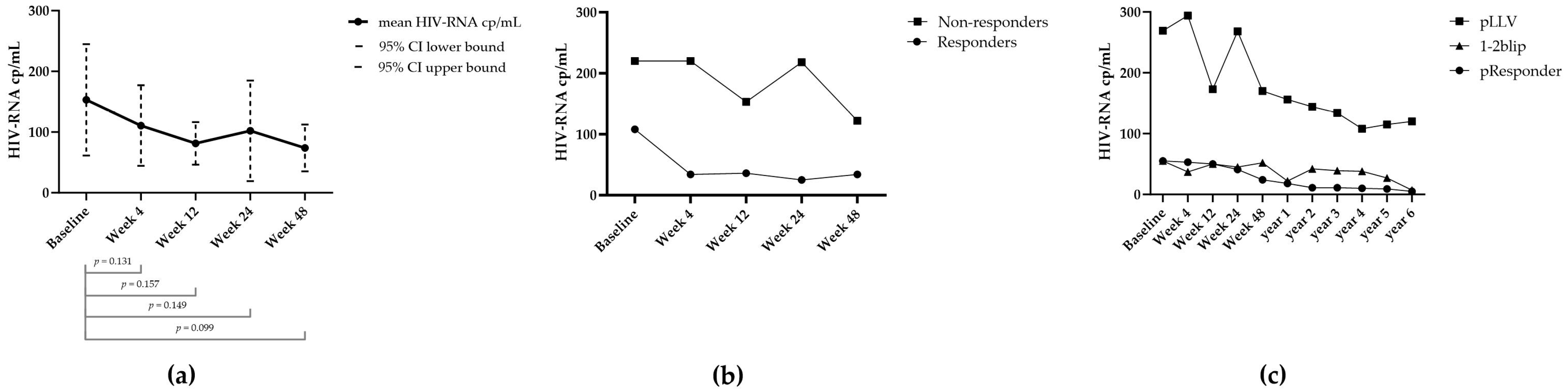
3.1. Virology
3.2. Quantification and Activity of HIV Viral Reservoir
3.3. Pharmacology and Therapy Adherence
3.4. Immune Activation
3.4.1. Soluble Markers
3.4.2. Cell-Bound Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taiwo, B.; Gallien, S.; Aga, E.; Ribaudo, H.; Haubrich, R.; Kuritzkes, D.R.; Eron, J.J. Antiretroviral Drug Resistance in HIV-1-Infected Patients Experiencing Persistent Low-Level Viremia during First-Line Therapy. J. Infect. Dis. 2011, 204, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, L.M.; Mudrikova, T.; Stam, A.J.; Otto, S.; Tesselaar, K.; Nijhuis, M.; Wensing, A.M. Residual Viremia Is Preceding Viral Blips and Persistent Low-Level Viremia in Treated HIV-1 Patients. PLoS ONE 2014, 9, e110749. [Google Scholar] [CrossRef]
- Bernal, E.; Gómez, J.M.; Jarrín, I.; Cano, A.; Muñoz, A.; Alcaraz, A.; Imaz, A.; Iribarren, J.A.; Rivero, M.; Arazo, P.; et al. Low-Level Viremia Is Associated With Clinical Progression in HIV-Infected Patients Receiving Antiretroviral Treatment. J. Acquir. Immune Defic. Syndr. 2018, 78, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Joya, C.; Won, S.H.; Schofield, C.; Lalani, T.; Maves, R.C.; Kronmann, K.; Deiss, R.; Okulicz, J.; Agan, B.K.; Ganesan, A. Persistent Low-Level Viremia While on Antiretroviral Therapy Is an Independent Risk Factor for Virologic Failure. Clin. Infect. Dis. 2019, 69, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Esber, A.; Polyak, C.; Kiweewa, F.; Maswai, J.; Owuoth, J.; Maganga, L.; Adamu, Y.; Hickey, P.W.; Ake, J.A.; Crowell, T.A. Persistent Low-Level Viremia Predicts Subsequent Virologic Failure: Is It Time to Change the Third 90? Clin. Infect. Dis. 2019, 69, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Hermans, L.E.; Moorhouse, M.; Carmona, S.; Grobbee, D.E.; Hofstra, L.M.; Richman, D.D.; Tempelman, H.A.; Venter, W.D.F.; Wensing, A.M.J. Effect of HIV-1 Low-Level Viraemia during Antiretroviral Therapy on Treatment Outcomes in WHO-Guided South African Treatment Programmes: A Multicentre Cohort Study. Lancet Infect. Dis. 2018, 18, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hanners, E.K.; Benitez-Burke, J.; Badowski, M.E. HIV: How to Manage Low-Level Viraemia in People Living with HIV. Drugs Context 2022, 11, 8–13. [Google Scholar] [CrossRef]
- Lathouwers, E.; Wong, E.Y.; Luo, D.; Seyedkazemi, S.; De Meyer, S.; Brown, K. HIV-1 Resistance Rarely Observed in Patients Using Darunavir Once-Daily Regimens across Clinical Studies. HIV Clin. Trials 2017, 18, 196–204. [Google Scholar] [CrossRef]
- Ferretti, F.; Mackie, N.E.; Singh, G.K.J.; Fox, J.; Kaye, S.; McClure, M.O.; Taylor, G.; Boffito, M. Characterization of Low Level Viraemia in HIV-Infected Patients Receiving Boosted Protease Inhibitor-Based Antiretroviral Regimens. HIV Res. Clin. Pract. 2019, 20, 107–110. [Google Scholar] [CrossRef]
- Vancoillie, L.; Hebberecht, L.; Dauwe, K.; Demecheleer, E.; Dinakis, S.; Vaneechoutte, D.; Mortier, V.; Verhofstede, C. Longitudinal Sequencing of HIV-1 Infected Patients with Low-Level Viremia for Years While on ART Shows No Indications for Genetic Evolution of the Virus. Virology 2017, 510, 185–193. [Google Scholar] [CrossRef]
- Vardhanabhuti, S.; Taiwo, B.; Kuritzkes, D.R.; Eron, J.J.; Bosch, R.J. Phylogenetic Evidence of HIV-1 Sequence Evolution in Subjects with Persistent Low-Level Viraemia. Antivir. Ther. 2015, 20, 73–76. [Google Scholar] [CrossRef]
- Mzingwane, M.L.; Tiemessen, C.T. Mechanisms of HIV Persistence in HIV Reservoirs. Rev. Med. Virol. 2017, 27, e1924. [Google Scholar] [CrossRef]
- Podsadecki, T.J.; Vrijens, B.C.; Tousset, E.P.; Rode, R.A.; Hanna, G.J. Decreased Adherence to Antiretroviral Therapy Observed Prior to Transient Human Immunodeficiency Virus Type 1 Viremia. J. Infect. Dis. 2007, 196, 1773–1778. [Google Scholar] [CrossRef]
- Konstantopoulos, C.; Ribaudo, H.; Ragland, K.; Bangsberg, D.R.; Li, J.Z. Antiretroviral Regimen and Suboptimal Medication Adherence Are Associated with Low-Level Human Immunodeficiency Virus Viremia. Open Forum. Infect. Dis. 2015, 2, ofu119. [Google Scholar] [CrossRef]
- Swenson, L.C.; Min, J.E.; Woods, C.K.; Cai, E.; Li, J.Z.; Montaner, J.S.G.; Harrigan, P.R.; Gonzalez-Serna, A. HIV Drug Resistance Detected During Low-Level Viremia Is Associated with Subsequent Virologic Failure. AIDS 2014, 28, 1125–1134. [Google Scholar] [CrossRef]
- Palich, R.; Wirden, M.; Peytavin, G.; Le, M.P.; Seang, S.; Abdi, B.; Schneider, L.; Tubiana, R.; Valantin, M.A.; Paccoud, O.; et al. Persistent Low-Level Viraemia in Antiretroviral Treatment-Experienced Patients Is Not Linked to Viral Resistance or Inadequate Drug Concentrations. J. Antimicrob. Chemother. 2020, 75, 2981–2985. [Google Scholar] [CrossRef]
- Grennan, J.T.; Loutfy, M.R.; Su, D.; Harrigan, P.R.; Cooper, C.; Klein, M.; MacHouf, N.; Montaner, J.S.G.; Rourke, S.; Tsoukas, C.; et al. Magnitude of Virologic Blips Is Associated with a Higher Risk for Virologic Rebound in HIV-Infected Individuals: A Recurrent Events Analysis. J. Infect. Dis. 2012, 205, 1230–1238. [Google Scholar] [CrossRef]
- Hunt, P.W.; Brenchley, J.; Sinclair, E.; Mccune, J.M.; Page-shafer, K.; Hsue, P.; Emu, B.; Krone, M.; Douek, D.; Martin, J.N.; et al. Relationship between T Cell Activation and CD4+ T Cell Count in HIV-Seropositive Individuals with Undetectable Plasma HIV RNA Levels in the Absence of Therapy. JID 2008, 197, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Martin, J.N.; Sinclair, E.; Bredt, B.; Hagos, E.; Lampiris, H.; Deeks, S.G. T Cell Activation Is Associated with Lower CD4+ T Cell Gains in Human Immunodeficiency Vires-Infected Patients with Sustained Viral Suppression during Antiretroviral Therapy. J. Infect. Dis. 2003, 187, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Bui, J.K.; Mellors, J.W.; Cillo, A.R. HIV-1 Virion Production from Single Inducible Proviruses Following T-Cell Activation Ex Vivo. J. Virol. 2016, 90, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Bruner, K.M.; Wang, Z.; Simonetti, F.R.; Bender, A.M.; Kwon, K.J.; Sengupta, S.; Fray, E.J.; Beg, S.A.; Jenike, K.M.; Bertagnolli, L.N.; et al. A Novel Quantitative Approach for Measuring the Reservoir of Latent HIV-1 Proviruses. Nature 2019, 566, 120–125. [Google Scholar] [CrossRef]
- Sarmati, L.; D’Ettorre, G.; Parisi, S.; Andreoni, M. HIV Replication at Low Copy Number and Its Correlation with the HIV Reservoir: A Clinical Perspective. Curr. HIV Res. 2015, 13, 250–257. [Google Scholar] [CrossRef]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2022 Update of the Drug Resistance Mutations in HIV-1. Top. Antivir. Med. 2022, 30, 559–574. [Google Scholar]
- Bosman, K.J.; Wensing, A.M.; Pijning, A.E.; van Snippenberg, W.J.; van Ham, P.M.; de Jong, D.M.; Hoepelman, A.I.; Nijhuis, M. Development of Sensitive DdPCR Assays to Reliably Quantify the Proviral DNA Reservoir in All Common Circulating HIV Subtypes and Recombinant Forms. J. Int. AIDS Soc. 2018, 21, e25185. [Google Scholar] [CrossRef] [PubMed]
- Buchholtz, N.V.E.J.; Nühn, M.M.; de Jong, T.C.M.; Stienstra, T.A.T.; Reddy, K.; Ndung’u, T.; Ndhlovu, Z.M.; Fisher, K.; Palmer, S.; Wensing, A.M.J.; et al. Development of a Highly Sensitive and Specific Intact Proviral DNA Assay for HIV-1 Subtype B and C. Virol. J. 2024; accepted. [Google Scholar]
- Kinloch, N.N.; Ren, Y.; Conce Alberto, W.D.; Dong, W.; Khadka, P.; Huang, S.H.; Mota, T.M.; Wilson, A.; Shahid, A.; Kirkby, D.; et al. HIV-1 Diversity Considerations in the Application of the Intact Proviral DNA Assay (IPDA). Nat. Commun. 2021, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- De Jager, W.; Prakken, B.J.; Bijlsma, J.W.J.; Kuis, W.; Rijkers, G.T. Improved Multiplex Immunoassay Performance in Human Plasma and Synovial Fluid Following Removal of Interfering Heterophilic Antibodies. J. Immunol. Methods 2005, 300, 124–135. [Google Scholar] [CrossRef] [PubMed]
- De Jager, W.; Hoppenreijs, E.P.A.H.; Wulffraat, N.M.; Wedderburn, L.R.; Kuis, W.; Prakken, B.J. Blood and Synovial Fluid Cytokine Signatures in Patients with Juvenile Idiopathic Arthritis: A Cross-Sectional Study. Ann. Rheum. Dis. 2007, 66, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sayer, J.M.; Liu, F.; Ishima, R.; Weber, I.T.; Louis, J.M. Effect of the Active Site D25N Mutation on the Structure, Stability, and Ligand Binding of the Mature HIV-1 Protease. J. Biol. Chem. 2008, 283, 13459–13470. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Verhofstede, C.; D’Avolio, A.; Watson, V.; Hagberg, L.; Fuchs, D.; Svennerholm, B.; Gisslén, M. Treatment Intensification Has No Effect on the HIV-1 Central Nervous System Infection in Patients on Suppressive Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2010, 55, 590–596. [Google Scholar] [CrossRef]
- Havlir, D.V.; Strain, M.C.; Clerici, M.; Ignacio, C.; Trabattoni, D.; Ferrante, P.; Wong, J.K. Productive Infection Maintains a Dynamic Steady State of Residual Viremia in Human Immunodeficiency Virus Type 1-Infected Persons Treated with Suppressive Antiretroviral Therapy for Five Years. J. Virol. 2003, 77, 11212–11219. [Google Scholar] [CrossRef]
- Hatano, H.; Hayes, T.L.; Dahl, V.; Sinclair, E.; Lee, T.H.; Hoh, R.; Lampiris, H.; Hunt, P.W.; Palmer, S.; McCune, J.M.; et al. A Randomized, Controlled Trial of Raltegravir Intensification in Antiretroviral-Treated, HIV-Infected Patients with a Suboptimal CD4+ T Cell Response. J. Infect. Dis. 2011, 203, 960–968. [Google Scholar] [CrossRef]
- Dinoso, J.B.; Kim, S.Y.; Wiegand, A.M.; Palmer, S.E.; Gange, S.J.; Cranmer, L.; O’Shea, A.; Callender, M.; Spivak, A.; Brennan, T.; et al. Treatment Intensification Does Not Reduce Residual HIV-1 Viremia in Patients on Highly Active Antiretroviral Therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 9403–9408. [Google Scholar] [CrossRef]
- Sayana, S.; Hamwi, G.; DenOuden, P.; Easley, A.; Khanlou, H. The Use of Darunavir/Ritonavir as Intensification in Low Viremic HIV-Infected Patients Treated with Boosted Protease Inhibitor-Containing Regimens. J. Int. Assoc. Physicians AIDS Care 2009, 8, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, M.C.; Collins, K.L. The Impact of Cellular Proliferation on the Hiv-1 Reservoir. Viruses 2020, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Qian Wang, X.; Lee, A.; Morcilla, V.; de Vries, A.; Lee, E.; Eden, J.-S.; Deeks, S.G.; Kelleher, A.D.; Palmer, S. Plasma-Derived HIV-1 Virions Contain Considerable Levels of Defective Genomes. J. Virol. 2022, 96, e0201121. [Google Scholar] [CrossRef] [PubMed]
- Svicher, V.; Ceccherini-Silberstein, F.; Antinori, A.; Aquaro, S.; Perno, C.F. Understanding HIV Compartments and Reservoirs. Curr. HIV/AIDS Rep. 2014, 11, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Siliciano, J.D.; Siliciano, R.F. Nonsuppressible HIV-1 Viremia: A Reflection of How the Reservoir Persists. J. Clin. Investig. 2020, 130, 5665–5667. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Wu, F.; Yasin, S. Clonally Expanded HIV-1 Proviruses with 5′-Leader Defects Can Give Rise to Nonsuppressible Residual Viremia. J. Clin. Investig. 2023, 133, e165245. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.L.; Halvas, E.K.; Tosiano, M.A.; Mellors, J.W. Persistent HIV-1 Viremia on Antiretroviral Therapy: Measurement and Mechanisms. Front Microbiol. 2019, 10, 2383. [Google Scholar] [CrossRef]
- de Armas, L.R.; Pallikkuth, S.; George, V.; Rinaldi, S.; Pahwa, R.; Arheart, K.L.; Pahwa, S. Reevaluation of Immune Activation in the Era of CART and an Aging HIV-Infected Population. JCI Insight 2017, 2, e95726. [Google Scholar] [CrossRef] [PubMed]
- Falasca, F.; Di Carlo, D.; De Vito, C.; Bon, I.; d’Ettorre, G.; Fantauzzi, A.; Mezzaroma, I.; Fimiani, C.; Re, M.C.; Vullo, V.; et al. Evaluation of HIV-DNA and Inflammatory Markers in HIV-Infected Individuals with Different Viral Load Patterns. BMC Infect. Dis. 2017, 17, 581. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mancilla, J.R.; Morrow, M.; Coyle, R.P.; Coleman, S.S.; Zheng, J.H.; Ellison, L.; Bushman, L.R.; Kiser, J.J.; Anderson, P.L.; Mawhinney, S. Low-Level Viremia Is Associated with Cumulative Adherence to Antiretroviral Therapy in Persons with HIV. Open Forum Infect. Dis. 2021, 8, ofab463. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Di Filippo, E.; Comi, L.; Callegaro, A.; Colombo, G.; Di Matteo, S.; Valsecchi, D.; Rizzi, M. Reduced Adherence to Antiretroviral Therapy Is Associated with Residual Low-Level Viremia. Pragmat. Obs. Res. 2017, 8, 91–97. [Google Scholar] [CrossRef]


| Baseline Characteristics | N = 30 |
|---|---|
| Mean age (σ) | 49.4 (8.4) |
| Sex (male) | 86.7% |
| Mean viral load (σ) | 153 cp/mL (246) |
| Mean CD4 count (σ) | 667 cells/mm3 (364) |
| HIV subtype | B:20; C:2; CRF02_AG:2; F:2; A:1; unknown: 3 |
| Mean intact proviral DNA (σ) | 172 cp/106 cells (293) |
| Mean msRNA (σ) | 11.9 cp/µg RNA (26.1) |
| Mean LTR-DNA (σ) | 1754 cp/106 cells (1333) |
| ART at switch | TDF, FTC, EFV: 6 TDF, FTC, DTG: 3 TAF, FTC, DTG: 1 TAF, FTC, EVG/c: 3 TDF, FTC, ATV/r: 2 TDF, FTC, NVP: 2 ABC, 3TC, NVP: 2 ABC, 3TC, DTG: 2 TDF, FTC, RAL: 1 TDF, FTC, RIL: 1 TAF, FTC, RIL:1 TDF, 3TC, EFV: 1 ABC, 3TC, LPV/r: 1 ABC, 3TC, EFV: 1 TDF, DTG, ATV/r: 1 TDF, FTC, DTG, MVC, LPV/r: 1 |
| Substance use | Nicotine 13 (43.3%) Alcohol > 2 per day: 2 (6.7%) Cannabis: 7 (23.3%) |
| Co-morbidities | DM: 0% CVD: 3 (10%) |
| COPD: 3 (10%) |
| HIV-DNA | IPDA (Intact) | msRNA | HIV-RNA | Short Term (WK24) | Follow-Up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | WK24 | BL | WK24 | BL | WK24 | SCR | BL | WK4 | WK12 | WK24 | WK48 | Responder (R) Non-Responder (NR) | % Measurements >50 cp/mL | Time (yrs) | |||
| L-1 | 1767 | 1317 | 1.96 | 1.96 | 0 | 0 | 80 | 0 | 40 | 40 | 20 | 0 | R | persistent responder (0%) | = | 6.3 | |
| L-7 | 3083 | 1903 | 1171 | 630 | 0 | 0 | 46 | 46 | <40 | <40 | <40 | 65 | R | persistent responder (0%) | = | 4.6 | |
| L-12 | 1522 | 1968 | - | 1.96 | - | 32 | 77 | <40 | 80 | <40 | <40 | <40 | R | persistent responder (0%) | = | 5.2 | |
| L-16 | 388 | 400 | 1.96 | 10 | 0 | 0 | 75 | 0 | 48 | 40 | 48 | <40 | R | persistent responder (0%) | = | 5.9 | |
| L-17 | 538 | 234 | 13 | 1.96 | 0 | 0 | 44 | 0 | 0 | 0 | 0 | 0 | R | persistent responder (0%) | = | 5.3 | |
| L-19 | 1473 | 1353 | - | - | - | - | 50 | 50 | <20 | 37 | <20 | <20 | R | persistent responder (0%) | = | 5.5 | |
| L-21 | 1189 | 571 | - | - | - | - | 83 | 112 | 50 | <40 | <40 | <40 | R | persistent responder (0%) | = | 4.9 | |
| L-29 | 266 | 553 | 1.96 | 1.96 | - | 0 | 53 | 53 | 0 | <40 | 0 | <40 | R | persistent responder (0%) | = | 3.5 | |
| L-10 | 1459 | 821 | 450 | 190 | 0 | 0 | 52 | 76 | 62 | <40 | 40 | 53 | R | 2 blip (<25%) | O | 5.3 | |
| L-15 | 5811 | 5397 | 188 | 387 | 64 | - | 75 | <40 | <40 | <40 | <40 | <40 | R | 1 blip (<25%) | O | 5.9 | |
| L-30 | 1028 | 896 | 1.96 | 1.96 | 0 | 0 | 41 | 41 | 0 | <40 | 0 | <40 | R | 2 blip (<25%) | O | 3.2 | |
| L-27 | 2783 | 4806 | 1.96 | 1.96 | 14 | - | 56 | 56 | 17 | <40 | <40 | 100 | R | pLLV 53% | O | 3.4 | |
| L-6 | 2794 | 2665 | - | - | - | - | 66 | 0 | 55 | 40 | 0 | - | R | no longitudinal follow-up | . | - | |
| L-9 | 924 | 3836 | - | 35 | - | 0 | 125 | 1010 | 0 | <40 | 40 | - | R | no longitudinal follow-up | . | - | |
| L-23 | 731 | 1410 | 77 | 1.96 | 0 | - | 151 | 146 | 57 | 18 | 0 | 0 | R | no longitudinal follow-up | . | - | |
| L-25 | 2617 | - | 715 | - | 0 | - | 51 | 51 | <40 | - | - | - | R | no longitudinal follow-up | . | - | |
| L-2 | 3077 | 2722 | 13 | - | 0 | 0 | 189 | 219 | 176 | 62 | 144 | 64 | NR | pLLV 100% | = | 5.3 | |
| L-3 | 3179 | 3935 | 281 | 28 | 23 | 21 | 189 | 1020 | 487 | 413 | 1010 | 348 | NR | pLLV 82% | = | 7.0 | |
| L-18 | 1226 | 2051 | 1.96 | 1.96 | 0 | 0 | 168 | 168 | 842 | 202 | 83 | 231 | NR | pLLV 75% | = | 4.2 | |
| L-22 | 1211 | 914 | 490 | 66 | 0 | 0 | 200 | 93 | 270 | 128 | 167 | 88 | NR | pLLV 100% | = | 4.2 | |
| L-26 | 1715 | 1560 | - | - | - | - | 268 | 268 | 228 | 205 | 300 | 266 | NR | pLLV 100% | = | 3.3 | |
| L-28 | 744 | 1302 | 25 | 39 | 30 | - | 70 | 58 | <40 | 162 | 135 | 90 | NR | pLLV 63% | = | 3.6 | |
| L-14 | 261 | 106 | 16 | 1.96 | - | - | 60 | 63 | 44 | 80 | 100 | 73 | NR | 1 blip (<25%) | O | 6.5 | |
| L-4 | 1129 | 1242 | 1.96 | 1.96 | 6 | 15 | 46 | 42 | 100 | 100 | 100 | 40 | NR | persistent responder (0%) | O | 2.1 | |
| L-5 | - | - | 1.96 | 1.96 | 0 | 0 | 172 | 172 | 170 | 138 | 92 | <20 | NR | persistent responder (0%) | O | 6.3 | |
| L-11 | 4474 | 2623 | 1.96 | 1.96 | 100 | 0 | 133 | 93 | 40 | <40 | 52 | 0 | NR | persistent responder (0%) | O | 5.1 | |
| L-8 | 1265 | - | 133 | - | 0 | - | 272 | 272 | 75 | - | - | - | NR | no longitudinal follow-up | . | - | |
| L-20 | 149 | 289 | 56 | 1.96 | 0 | - | 110 | 170 | 170 | - | - | - | NR | no longitudinal follow-up | . | - | |
| L-13 | - | 10080 | - | - | - | - | 166 | 166 | 20 | 50 | 228,000 | - | - | no longitudinal follow-up | . | - | |
| L-24 | 2314 | - | 313 | - | - | - | 69 | 69 | - | - | - | - | - | no longitudinal follow-up | . | - | |
| N | 30 | 30 | 29 | 26 | 26 | 23 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stam, A.J.; Buchholtz, N.V.E.J.; Bierman, W.F.W.; van Crevel, R.; Hoepelman, A.I.M.; Claassen, M.A.A.; Ammerlaan, H.S.M.; van Welzen, B.J.; van Kasteren, M.E.E.; van Lelyveld, S.F.L.; et al. Dynamics of Low-Level Viremia and Immune Activation after Switching to a Darunavir-Based Regimen. Viruses 2024, 16, 182. https://doi.org/10.3390/v16020182
Stam AJ, Buchholtz NVEJ, Bierman WFW, van Crevel R, Hoepelman AIM, Claassen MAA, Ammerlaan HSM, van Welzen BJ, van Kasteren MEE, van Lelyveld SFL, et al. Dynamics of Low-Level Viremia and Immune Activation after Switching to a Darunavir-Based Regimen. Viruses. 2024; 16(2):182. https://doi.org/10.3390/v16020182
Chicago/Turabian StyleStam, Arjen J., Ninée V. E. J. Buchholtz, Wouter F. W. Bierman, Reinout van Crevel, Andy I. M. Hoepelman, Mark A. A. Claassen, Heidi S. M. Ammerlaan, Berend J. van Welzen, Marjo E. E. van Kasteren, Steven F. L. van Lelyveld, and et al. 2024. "Dynamics of Low-Level Viremia and Immune Activation after Switching to a Darunavir-Based Regimen" Viruses 16, no. 2: 182. https://doi.org/10.3390/v16020182
APA StyleStam, A. J., Buchholtz, N. V. E. J., Bierman, W. F. W., van Crevel, R., Hoepelman, A. I. M., Claassen, M. A. A., Ammerlaan, H. S. M., van Welzen, B. J., van Kasteren, M. E. E., van Lelyveld, S. F. L., de Jong, D., Tesselaar, K., van Luin, M., Nijhuis, M., Wensing, A. M. J., & LOWERIT Study Team. (2024). Dynamics of Low-Level Viremia and Immune Activation after Switching to a Darunavir-Based Regimen. Viruses, 16(2), 182. https://doi.org/10.3390/v16020182





