Application of High-Throughput Sequencing for Comprehensive Virome Profiling in Grapevines Shows Yellows in Iran
Abstract
:1. Introduction
2. Materials and Methods
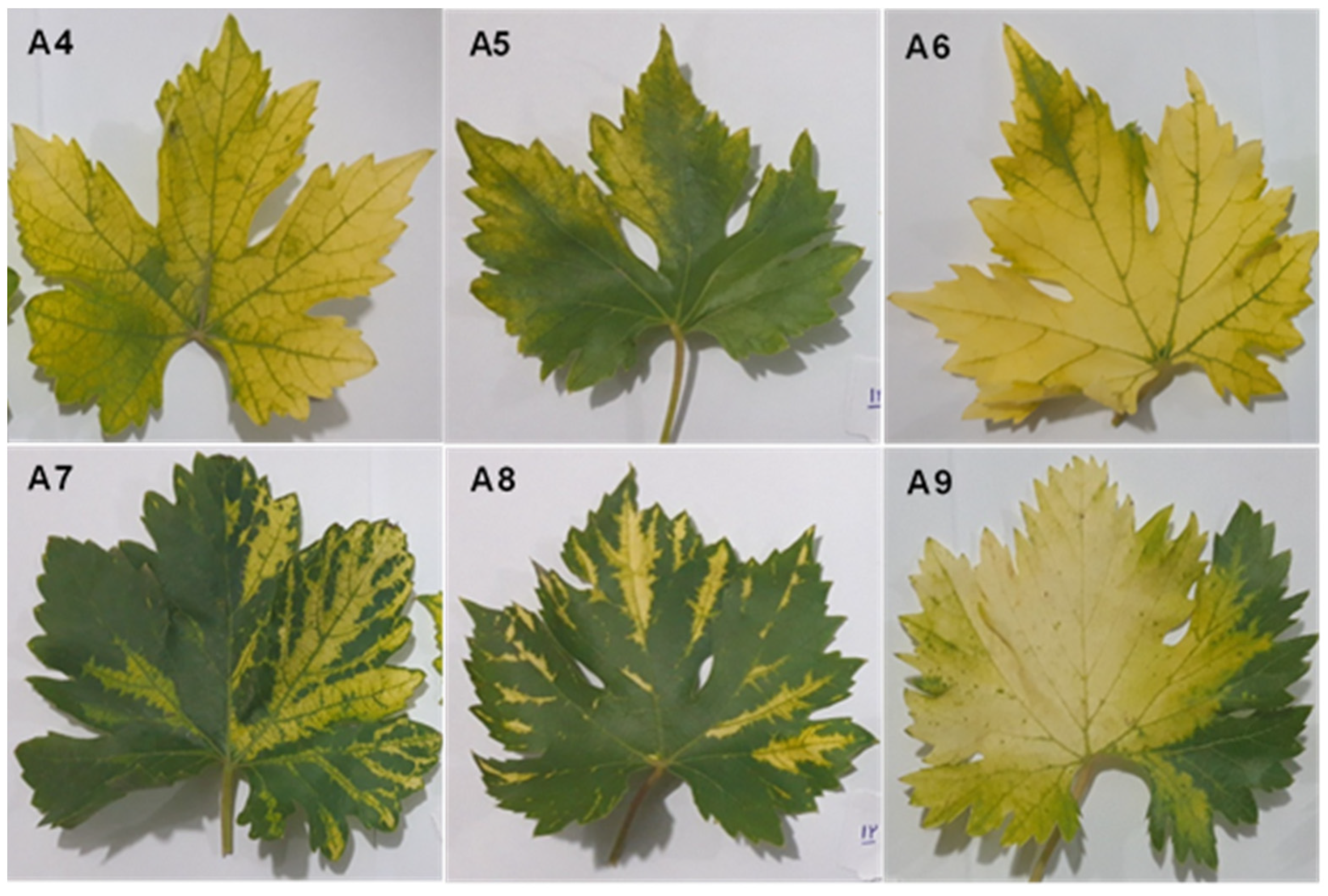
2.1. Plant Material
2.2. Library Preparation and Sequencing
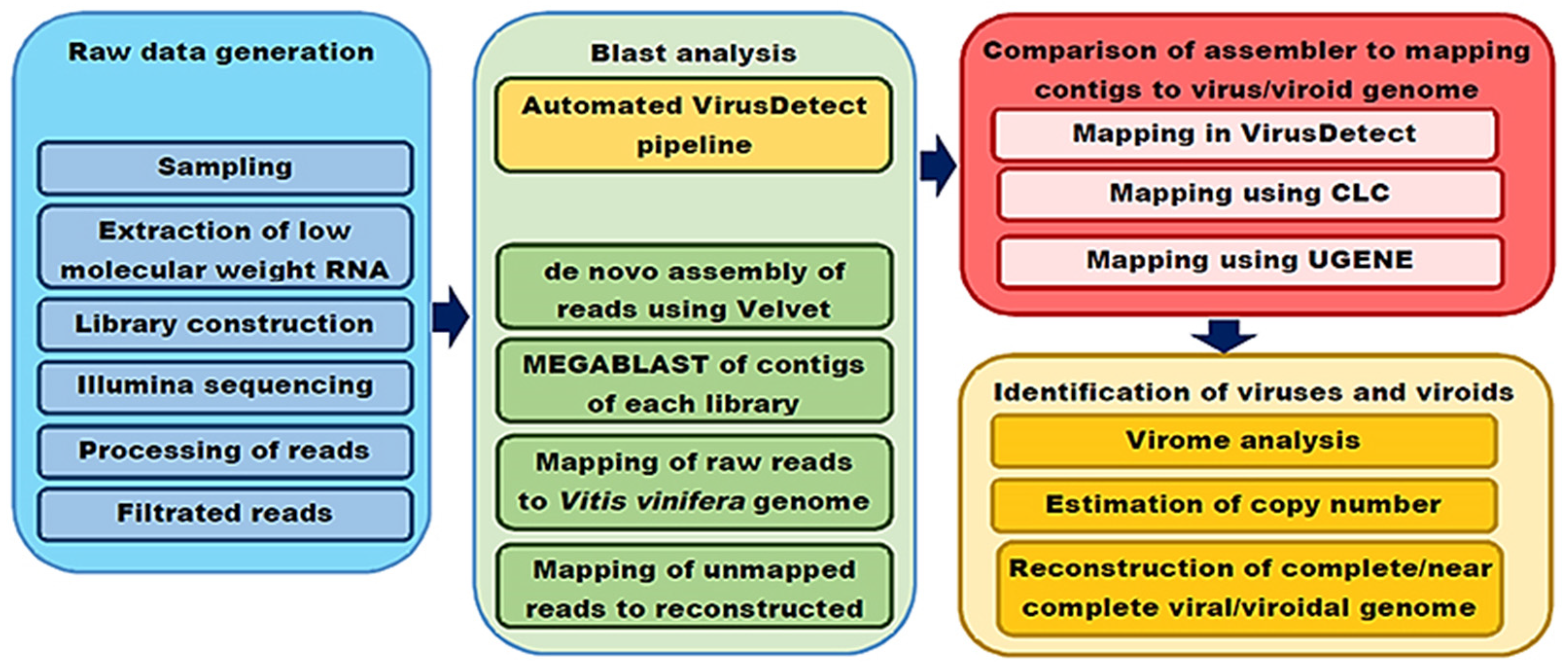
2.3. Analysis of Sequencing Data and Identification of Viruses
2.4. Confirmation of NGS Results by RT-PCR
2.5. Phylogenetic and Genetic Diversity Analysis
3. Results
3.1. Processing Raw Sequencing Data
3.2. Comparison of the Performance of Different Assemblers in the Detection and Reconstruction of Viral Genomes
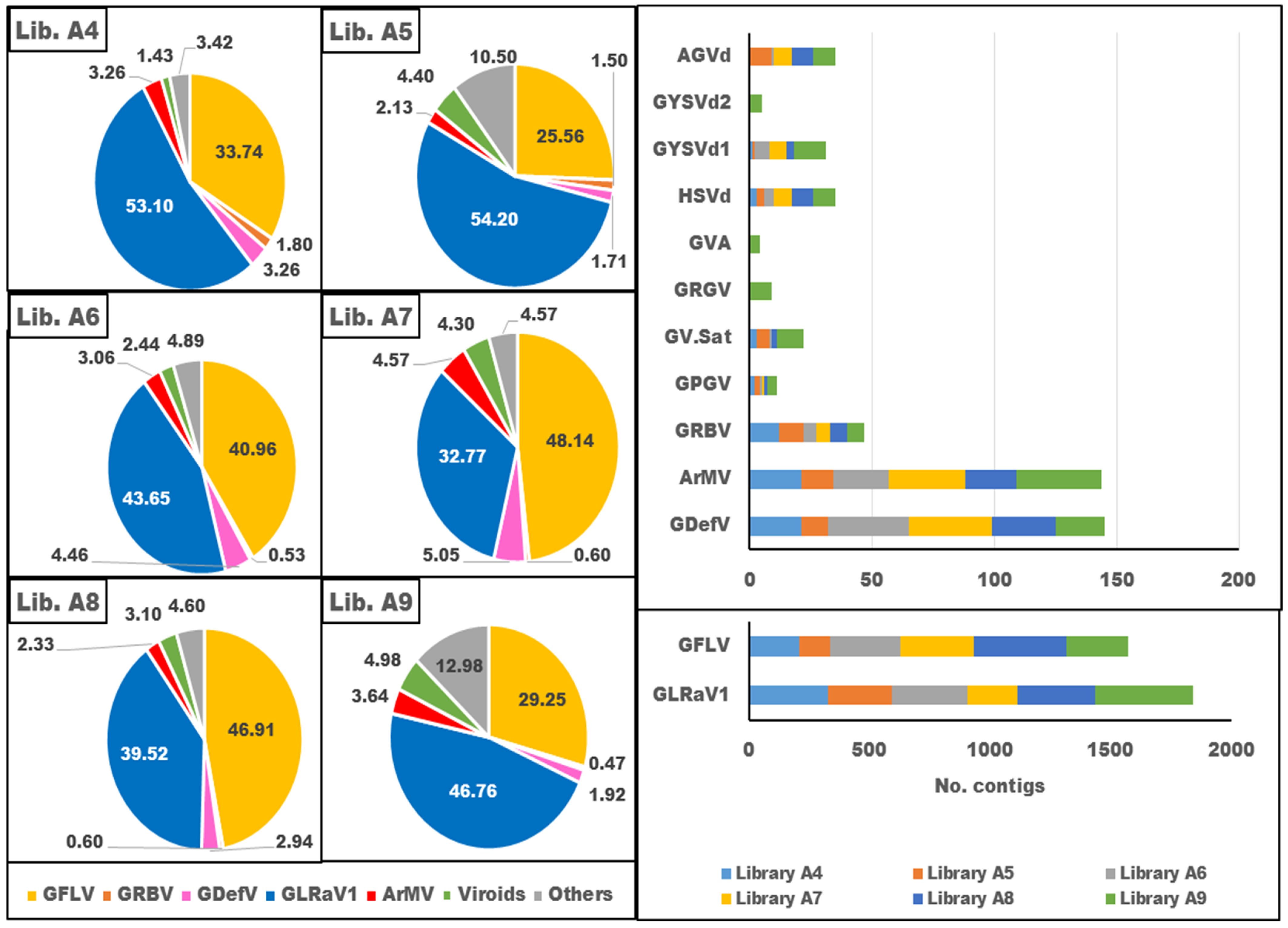
3.3. Diversity of Virome in Iranian Infected Grapevine Cultivars
3.4. Confirmation of Virus and Viroid Identified by HTS Data Analysis Using RT-PCR
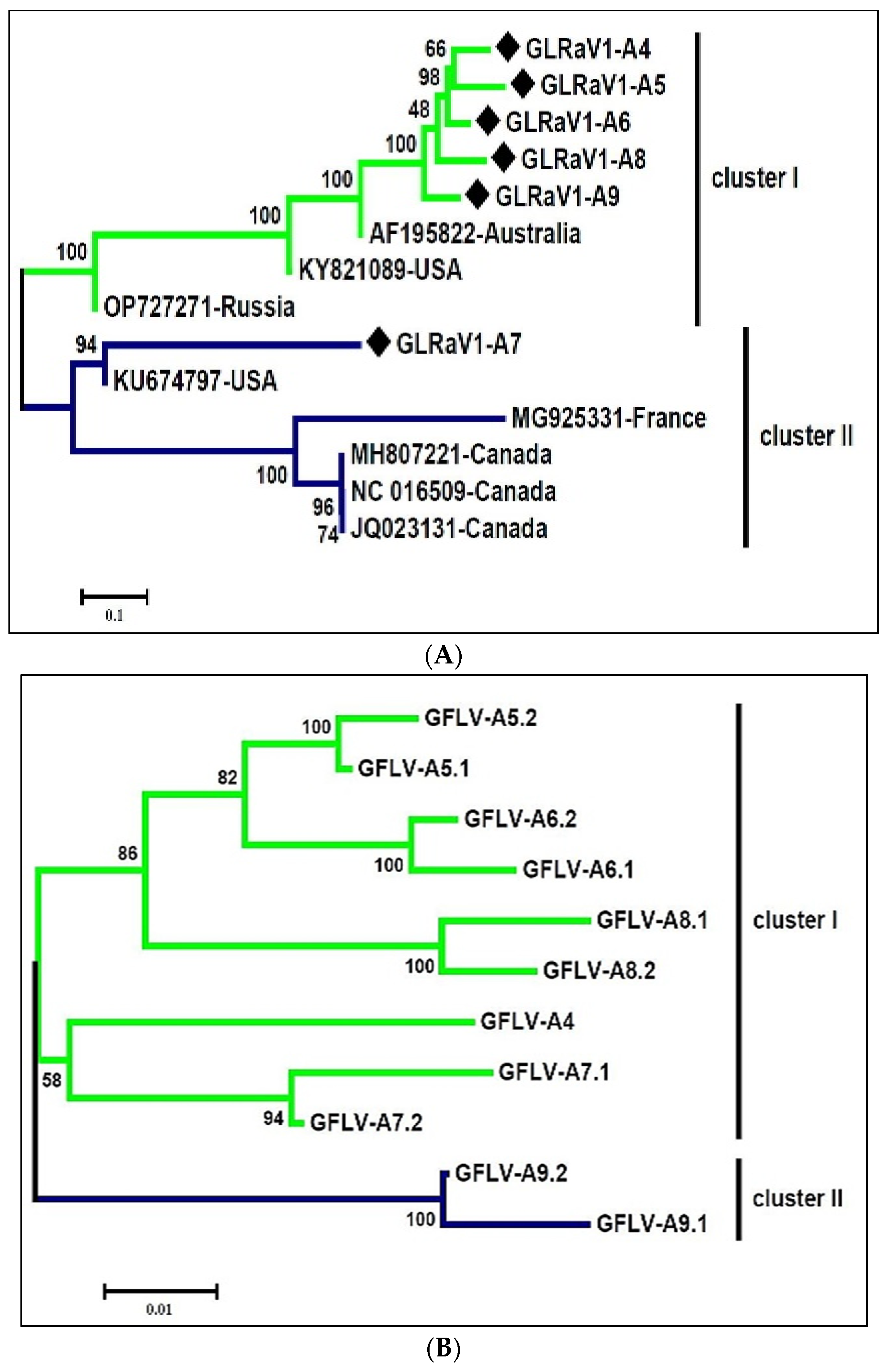
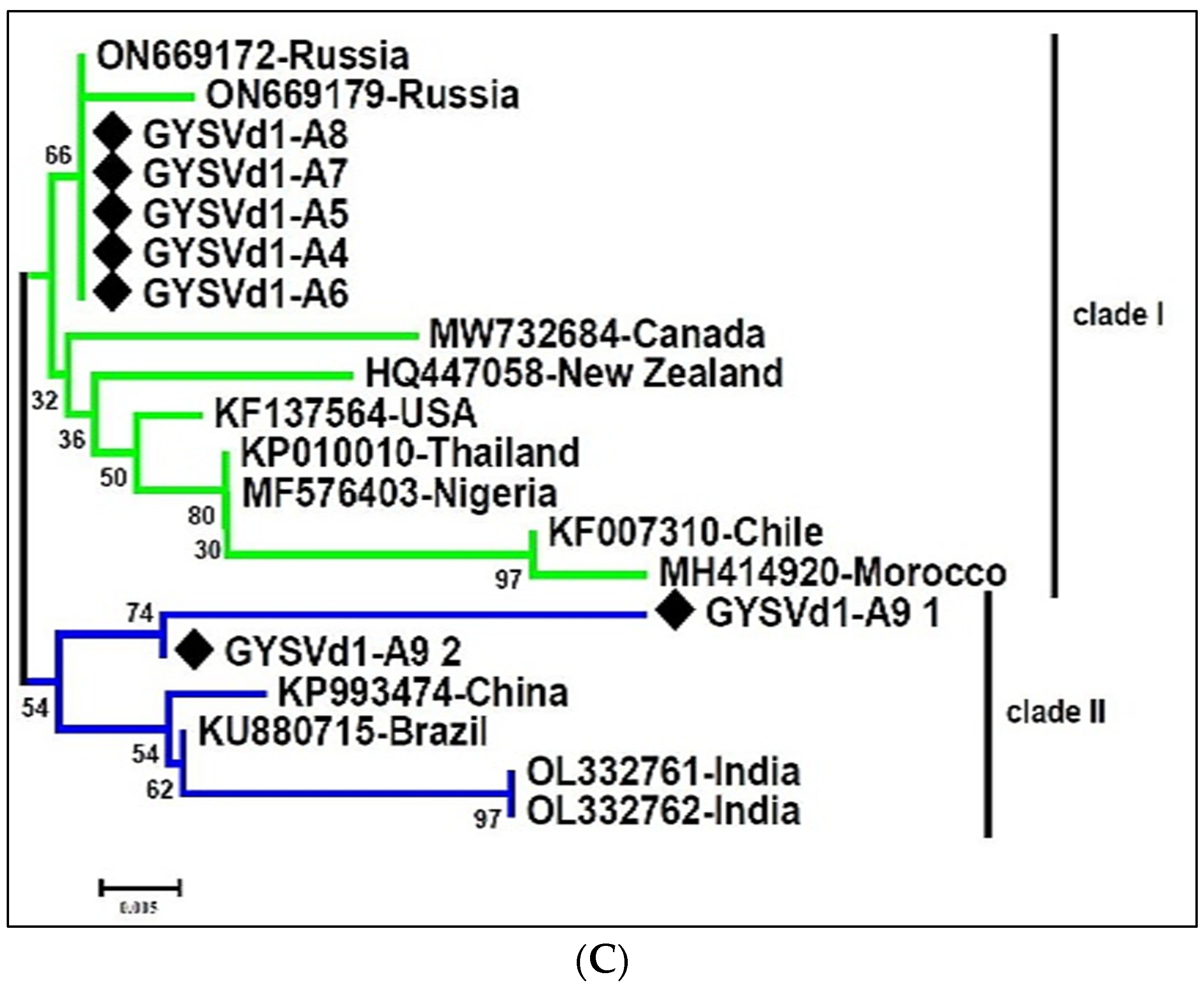
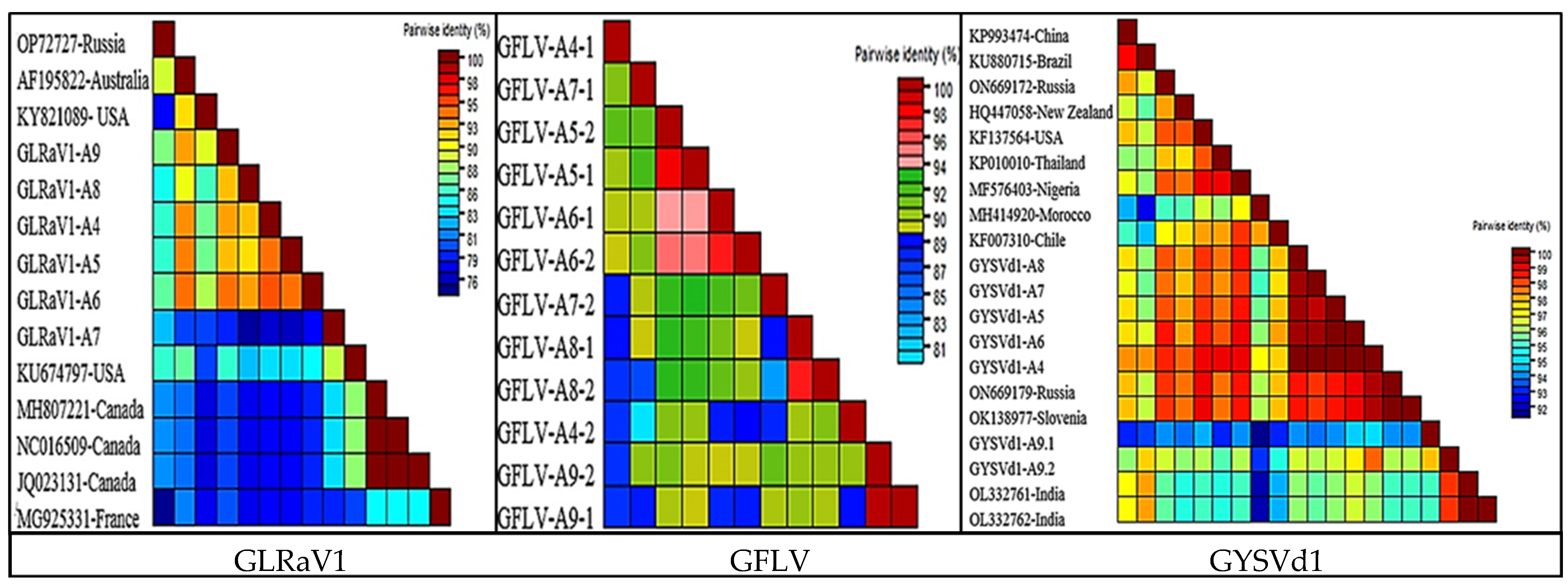
3.5. The Genetic Diversity of the Dominant Species in the Virome
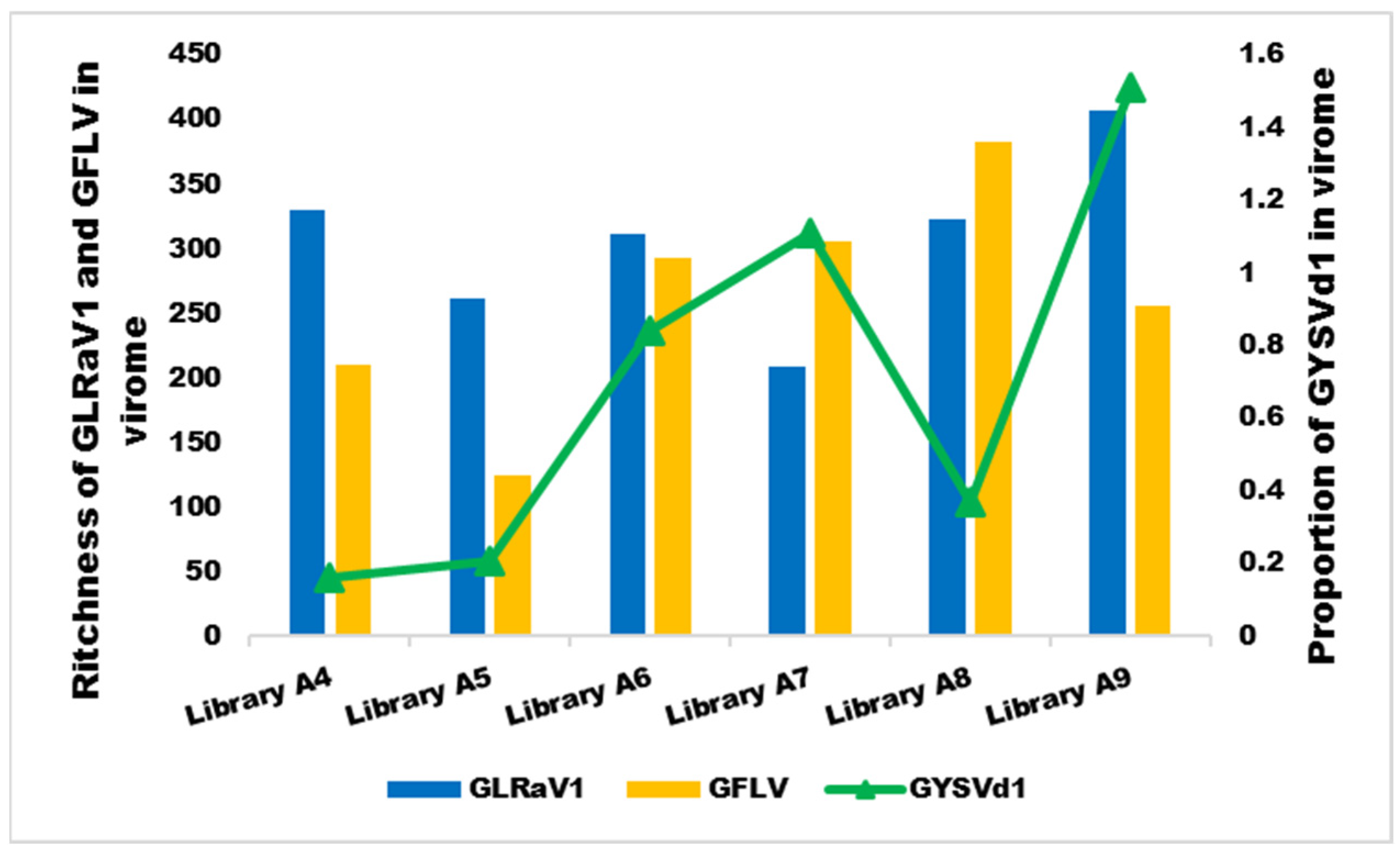
3.6. The Relationship between Symptoms and the Dominant Species in the Virome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGovern, P.E. Ancient Wine: The Search for the Origins of Viniculture; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Sidharthan, V.K.; Sevanthi, A.M.; Jaiswal, S.; Baranwal, V.K. Robust virome profiling and whole genome reconstruction of viruses and viroids enabled by use of available mRNA and sRNA-Seq datasets in grapevine (Vitis vinifera L.). Front. Microbiol. 2020, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Path. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Fall, M.L.; Xu, D.; Lemoyne, P.; Moussa, I.E.B.; Beaulieu, C.; Carisse, O. A diverse virome of leafroll-infected grapevine unveiled by dsRNA sequencing. Viruses 2020, 12, 1142. [Google Scholar] [CrossRef]
- Massart, S.; Candresse, T.; Gil, J.; Lacomme, C.; Predajna, L.; Ravnikar, M.; Reynard, J.S.; Rumbou, A.; Saldarelli, P.; Škorić, D.; et al. A framework for the evaluation of biosecurity, commercial, regulatory, and scientific impacts of plant viruses and viroids identified by NGS technologies. Front. Microbiol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Martelli, G.P. An overview on grapevine viruses, viroids, and the diseases they cause. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: New York, NY, USA, 2017; pp. 31–46. [Google Scholar]
- Eichmeier, A.; Komínková, M.; Komínek, P.; Baránek, M. Comprehensive virus detection using next generation sequencing in grapevine vascular tissues of plants obtained from the wine regions of Bohemia and Moravia (Czech Republic). PLoS ONE 2016, 11, e0167966. [Google Scholar] [CrossRef]
- Martínez, F.; Elena, S.F.; Daròs, J.A. Fate of artificial microRNA-mediated resistance to plant viruses in mixed infections. Phytopathology 2013, 103, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Syller, J.; Grupa, A. The effects of co-infection by different Potato virus Y (PVY) isolates on virus concentration in solanaceous hosts and efficiency of transmission. Plant Path. 2014, 63, 466–475. [Google Scholar] [CrossRef]
- Roossinck, M.J.; Martin, D.P.; Roumagnac, P. Plant Virus Metagenomics: Advances in Virus Discovery. Phytopathology 2015, 105, 716–727. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, S.W.; Zhang, Y.; Zhu, S. Identification of viruses and viroids by next-generation sequencing and homology-dependent and homology-independent algorithms. Annu. Rev. Phytopath. 2015, 53, 425–444. [Google Scholar] [CrossRef]
- Mascia, T.; Gallitelli, D. Synergies and antagonisms in virus interactions. Plant Sci. 2016, 252, 176–192. [Google Scholar] [CrossRef]
- Vivek, A.T.; Zahra, S.; Kumar, S. From current knowledge to best practice: A primer on viral diagnostics using deep sequencing of virus-derived small interfering RNAs (vsiRNAs) in infected plants. Methods 2020, 183, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kreuze, J.F.; Perez, A.; Untiveros, M.; Quispe, D.; Fuentes, S.; Barker, I.; Simon, R. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 2009, 388, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pecman, A.; Kutnjak, D.; Gutiérrez-Aguirre, I.; Adams, I.; Fox, A.; Boonham, N.; Ravnikar, M. Next generation sequencing for detection and discovery of plant viruses and viroids: Comparison of two approaches. Fron. Microbiol. 2017, 8, 1998. [Google Scholar] [CrossRef] [PubMed]
- Kutnjak, D.; Rupar, M.; Gutierrez-Aguirre, I.; Curk, T.; Kreuze, J.F.; Ravnikar, M. Deep sequencing of virus-derived small interfering RNAs and RNA from viral particles shows highly similar mutational landscapes of a plant virus population. J. Virol. 2015, 89, 4760–4769. [Google Scholar] [CrossRef]
- Seguin, J.; Rajeswaran, R.; Malpica-Lopez, N.; Martin, R.R.; Kasschau, K.; Dolja, V.V.; Otten, P.; Farinelli, L.; Pooggin, M.M. De novo reconstruction of consensus master genomes of plant RNA and DNA viruses from siRNAs. PLoS ONE 2014, 9, e88513. [Google Scholar] [CrossRef]
- Kutnjak, D.; Tamisier, L.; Adams, I.; Boonham, N.; Candresse, T.; Chiumenti, M.; De Jonghe, K.; Kreuze, J.F.; Lefebvre, M.; Silva, G.; et al. A primer on the analysis of high-throughput sequencing data for detection of plant viruses. Microorganisms 2021, 9, 841. [Google Scholar] [CrossRef]
- Statistical Yearbook of Jihad-Agriculture. 2022. Available online: https://www.maj.ir/Dorsapax/userfiles/Sub65/amarbaghi1400.pdf (accessed on 31 May 2023). (In Persian).
- Moradi, R.; Koolivand, D.; Eini, O.; Hajizadeh, M. Molecular identification of four important nepovirus from vineyards of Zanjan province. Iranian J. Plant Prot. Sci. 2018, 49, 99–110. [Google Scholar] [CrossRef]
- Sokhandan-Bashir, N.; Nourinejhad-Zarghani, S.; Hajizadeh, M. Status of infection with Grapevine fanleaf virus in vineyards of Iran and molecular characteristics of the isolates. Res. Rev. Biosci. 2015, 10, 267–277. [Google Scholar]
- Khalili, M.; Zarghani, S.N.; Massart, S.; Dizadji, A.; Olmos, A.; Wetzel, T.; Ruiz-García, A.B. First report of Grapevine rupestris vein feathering virus in grapevine in Iran. J. Plant Pathol. 2020, 102, 1313. [Google Scholar] [CrossRef]
- Gholampour, Z.; Zakiaghl, M.; Jafarpour, B.; Mehrvar, M. Identification and prevalence of Grapevine fanleaf virus in Khorasan-Razavi vineyards. Iranian J. Plant Prot. Res. 2015, 29, 318–324. [Google Scholar] [CrossRef]
- Gholampour, Z.; Kargar, M.; Zakiaghl, M.; Siampour, M.; Mehrvar, M.; Izadpanah, K. Dynamics of the population structure and genetic variability within Iranian isolates of Grapevine fanleaf virus, evidence for polyphyletic origin. Acta Virol. 2017, 61, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Uyemoto, J.K.; Martelli, G.P.; Rowhani, A. Grapevine viruses, virus-like diseases and other disorders. In Virus Diseases of Plants: Grape, Potato, and Wheat. Image Collection and Teaching Resource CD-Rom; Lipa, J.J., Ed.; APS Press: St. Paul, MN, USA, 2009. [Google Scholar]
- Carra, A.M.; Gambino, G.; Schubert, A. A cetyltrimethylammonium bromide-based method to extract low-molecular-weight RNA from polysaccharide-rich plant tissues. Anal. Biochem. 2007, 360, 318. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Meyers, B.C.; Green, P.J. Construction of small RNA cDNA libraries for deep sequencing. Methods 2007, 43, 110–117. [Google Scholar] [CrossRef] [PubMed]
- FastQC. A Quality Control Tool for High throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 25 May 2022).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, S.; Padmanabhan, C.; Li, R.; Galvez, M.; Gutierrez, D.; Fuentes, S.; Ling, K.S.; Kreuze, J.; Fei, Z. VirusDetect: An automated pipeline for efficient virus discovery using deep sequencing of small RNAs. Virology 2017, 500, 130–138. [Google Scholar] [CrossRef]
- Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11–17. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Ugene Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, B.; Freeborough, M.J.; Maree, H.J.; Celton, J.M.; Rees, D.J.G.; Burger, J.T. Deep sequencing analysis of viruses infecting grapevines: Virome of a vineyard. Virology 2010, 400, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Mackenzie, A. A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. J. Virol. Methods 1997, 63, 9–16. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Mascher, M.; Richmond, T.A.; Gerhardt, D.J.; Himmelbach, A.; Clissold, L.; Sampath, D.; Ayling, S.; Steuernagel, B.; Pfeifer, M.; D’Ascenzo, M.; et al. Barley whole exome capture: A tool for genomic research in the genus Hordeum and beyond. Plant J. 2013, 76, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Zakiaghl, M.; Izadpanah, K. Identification and partial molecular characterization of grapevine viroids in Fars Province. Iranian J. Plant Path. 2010, 46, 249–262. [Google Scholar]
- Staub, U.; Polivka, H.; Gross, H.J. Two rapid microscale procedures for isolation of total RNA from leaves rich in polyphe-nols and polysaccharides: Application for sensitive detection of grapevine viroids. J. Virol. Methods 1995, 52, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Roumi, V.; Afsharifar, A.; Izadpanah, K. Identification, distribution and prevalence of grapevine leafroll associated viruses and grapevine virus A in Iran and their rate of incidence in grapevine cultivars. Iranian J. Plant Path. 2006, 42, 223–240. [Google Scholar] [CrossRef]
- Digiaro, M.; Elbeaino, T.; Martelli, G.P. Development of degenerate and species-specific primers for the differential and simultaneous RT-PCR detection of grapevine-infecting nepoviruses of subgroups A, B and C. J. Virol. Methods 2007, 141, 34–40. [Google Scholar] [CrossRef]
- Hossein-alizadeh, S.; Pourrahim, R. Molecular characterization of Arabis mosaic virus from grapevines in Iran. J. Plant Pathol. 2020, 102, 179–181. [Google Scholar] [CrossRef]
- Gasperin-Bulbarela, J.; Licea-Navarro, A.F.; Pino-Villar, C.; Hernández-Martínez, R.; Carrillo-Tripp, J. First report of Grapevine red blotch virus in Mexico. Plant Dis. 2019, 103, 381. [Google Scholar] [CrossRef]
- MacKenzie, D.J.; McLean, M.A.; Mukerji, S.; Green, M. Improved RNA Extraction from Woody Plants for the Detection of Viral Pathogens by Reverse Transcription-Polymerase Chain Reaction. Plant Dis. 1997, 81, 222–226. [Google Scholar] [CrossRef]
- Xu, H.; Nie, J. Identification, characterization, and molecular detection of Alfalfa mosaic virus in potato. Phytopathology 2006, 96, 1237–1242. [Google Scholar] [CrossRef]
- Gawande, S.J.; Gurav, V.S.; Ingle, A.A.; Gopal, J. First Report of Garlic virus A in Garlic from India. Plant Disease 2015, 99, 1288. [Google Scholar]
- Maliogka, V.I.; Minafra, A.; Saldarelli, P.; Ruiz-García, A.B.; Glasa, M.; Katis, N.; Olmos, A. Recent advances on detection and characterization of fruit tree viruses using high-throughput sequencing technologies. Viruses 2018, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Miljanić, V.; Jakše, J.; Kunej, U.; Rusjan, D.; Škvarč, A.; Štajner, N. Virome status of preclonal candidates of grapevine varieties (Vitis vinifera L.) from the Slovenian wine-growing region primorska as determined by high-throughput sequencing. Front. Microbiol. 2022, 13, 830866. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Wang, L.; Feng, H.; He, X.; Chang, S.; Wang, D.; Wang, L.; Yang, J.; An, G.; et al. Whole-plant microbiome profiling reveals a novel geminivirus associated with soybean stay-green disease. Plant Biotechnol. J. 2022, 20, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Buko, D.H.; Spetz, C.; Hvoslef-Eide, A.K. Next generation sequencing as a method to verify virus elimination using heat treatment and meristem tip culture in the five most widely used sweet potato varieties in Ethiopia. Afri. J. Biot. 2020, 19, 458–463. [Google Scholar] [CrossRef]
- Tahzima, R.; Foucart, Y.; Peusens, G.; Beliën, T.; Massart, S.; De Jonghe, K. High-throughput sequencing assists studies in genomic variability and epidemiology of little cherry virus 1 and 2 infecting Prunus spp. in Belgium. Viruses 2019, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Vicente, A.; Donaire, L.; Torre, C.; Gómez-Aix, C.; Sánchez-Pina, M.A.; Juarez, M.; Hernando, Y.; Aranda, M.A. Small RNA-seq to characterize viruses responsible of Lettuce big vein disease in Spain. Front. Microbiol. 2018, 9, 3188. [Google Scholar] [CrossRef]
- Eichmeier, A.; Kominkova, M.; Pecenka, J.; Kominek, P. High-throughput small RNA sequencing for evaluation of grapevine sanitation efficacy. J. Virol. Methods 2019, 267, 66–70. [Google Scholar] [CrossRef]
- Velasco, L.; Padilla, C.V. High-Throughput Sequencing of Small RNAs for the Sanitary Certification of Viruses in Grapevine. Front. Plant Sci. 2021, 12, 682879. [Google Scholar] [CrossRef]
- Massart, S.; Chiumenti, M.; De Jonghe, K.; Glover, R.; Haegeman, A.; Koloniuk, I.; Kominek, P.; Kreuze, J.; Kutnjak, D.; Lotos, L.; et al. Virus detection by high-throughput sequencing of small RNAs: Large-scale performance testing of sequence analysis strategies. Phytopathology 2019, 109, 488–497. [Google Scholar] [CrossRef]
- Jo, Y.; Lian, S.; Chu, H.; Cho, J.K.; Yoo, S.H.; Choi, H.; Yoon, J.Y.; Choi, S.K.; Lee, B.C.; Cho, W.K. Peach RNA viromes in six different peach cultivars. Sci. Rep. 2018, 8, 1844. [Google Scholar] [CrossRef]
- Pourrahim, R.; Ahoonmanesh, A.; Farzadfar, S.; Golnaraghi, A.R. Occurrence of Arabis mosaic virus and Grapevine leaf roll associated virus-3 on grapevines in Iran. Plant Dis. 2004, 88, 424. [Google Scholar] [CrossRef]
- Hajizadeh, M.; Sokhandan-Bashir, N.; Elbeaino, T. First report of Grapevine deformation virus and Grapevine Anatolian ring spot virus in Iran. J. Plant Pathol. 2012, 94, S96. [Google Scholar]
- Izadpanah, K. An Annotated List of Virus and Virus-Like Diseases of Plants in Fars; College of Agriculture, Shiraz University: Shiraz, Iran, 1983; p. 188. [Google Scholar]
- Tokhmechi, K.; Koolivand, D. First report of Grapevine Pinot gris virus infecting grapevine in Iran. J. Plant Path. 2020, 102, 549. [Google Scholar] [CrossRef]
- Nourinejhad Zarghani, S.; Khalili, M.; Dizadji, A.; Wetzel, T. First report of grapevine red globe virus in grapevine in Iran. J. Plant Pathol. 2021, 103, 661. [Google Scholar]
- Jo, Y.; Choi, H.; Kim, S.M.; Kim, S.L.; Lee, B.C.; Cho, W.K. The pepper virome: Natural co-infection of diverse viruses and their quasispecies. BMC Genom. 2017, 18, 453. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, V.K.; Jain, P.; Saritha, R.K.; Jain, R.K.; Gautam, N.K. Detection and partial characterization of Cowpea mild mottle virus in mungbean and urdbean by deep sequencing and RT-PCR. Crop. Prot. 2015, 75, 77–79. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, H.; Kim, S.M.; Kim, S.L.; Lee, B.C.; Cho, W.K. Integrated analyses using RNA-Seq data reveal viral genomes, single nucleotide variations, the phylogenetic relationship, and recombination for Apple stem grooving virus. BMC Genom. 2016, 17, 579. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Cazelles, K.; Gravel, D. Co-occurrence is not evidence of ecological interactions. Ecol. Lett. 2020, 23, 1050–1063. [Google Scholar] [CrossRef]
- Monis, J.; Bestwick, R.K. Detection and localization of grapevine leafroll associated closteroviruses in greenhouse and tissue culture grown plants. Am. J. Enol Vitic. 1996, 47, 199–205. [Google Scholar] [CrossRef]
- Thompson, B.D.; Dahan, J.; Lee, J.; Martin, R.R.; Karasev, A.V. A novel genetic variant of Grapevine leafroll-associated virus-3 (GLRaV-3) from Idaho grapevines. Plant Dis. 2019, 103, 509–518. [Google Scholar] [CrossRef]
- Nourinejhad-Zarghani, S.; Shams-Bakhsh, M.; Sokhandan Bashir, N.; Wetzel, T. Molecular characterization of whole genomic RNA2 from Iranian isolates of Grapevine fanleaf virus. J. Phytopath. 2013, 161, 419–425. [Google Scholar] [CrossRef]
- Sokhandan-Bashir, N.; Hooshmand, A.; Delpasand-Khabazi, A. Molecular Characterization of phylogenetically distinct iso-lates of Grapevine fanleaf virus from Iran Based on 2A (HP) Gene. Indian J. Virol. 2012, 23, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, Z. Identification and genetic variability of the coat protein gene of Grapevine fanleaf virus in Khorasan Razavi. Master’s Thesis, Ferdowsi University of Mashhad, Mashhad, Iran, 2014. [Google Scholar]
- Saldarelli, P.; Giampetruzzi, A.; Maree, H.J.; Rwahnih, M.A. High-throughput sequencing: Advantages beyond virus identi-fication. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: New York, NY, USA, 2017; pp. 625–642. [Google Scholar] [CrossRef]
- Kovacs, L.G.; Hanami, H.; Fortenberry, M.; Kaps, M.L. Latent infection by leafroll agent GLRaV-3 is linked to lower fruit quality in French-American hybrid grapevines Vidal blanc and St. Vincent. Am. J. Enol. Vitic. 2001, 52, 254–259. [Google Scholar] [CrossRef]
- Beuve, M.; Hily, J.M.; Alliaume, A.; Reinbold, C.; Le Maguet, J.; Candresse, T.; Herrbach, E.; Lemaire, O. A complex virome unveiled by deep sequencing analysis of RNAs from a French Pinot Noir grapevine exhibiting strong leafroll symptoms. Arch. Virol. 2018, 163, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Maree, H.J.; Almeida, R.P.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef]
- Mokhtarian, A. Identification and study of dominant grapevine varieties in Kashmar. Master’s Thesis, University of Tehran, Tehran, Iran, 1995. [Google Scholar]
- Mirchenari, S.M.; Massah, A.; Zirak, L. Bois noir: New phytoplasma disease of grapevine in Iran. J. Plant Prot. Res. 2015, 55, 88–93. [Google Scholar] [CrossRef]
- Almeida, R.P.; Daane, K.M.; Bell, V.A.; Blaisdell, G.K.; Cooper, M.L.; Herrbach, E.; Pietersen, G. Ecology and management of grapevine leafroll disease. Front. Microbiol. 2013, 4, 94. [Google Scholar] [CrossRef]
- Andret-Link, P.; Laporte, C.; Valat, L.; Ritzenthaler, C.; Demangeat, G.; Vigne, E.; Laval, V.; Pfeiffer, P.; Stussi-Garaud, C.; Fuchs, M. Grapevine fanleaf virus: Still a major threat to the grapevine industry. J. Plant Pathol. 2004, 86, 183–195. [Google Scholar]
- Gucek, T.; Trdan, S.; Jakse, J.; Javornik, B.; Matousek, J.; Radisek, S. Diagnostic techniques for viroids. Plant Pathol. 2017, 66, 339–358. [Google Scholar] [CrossRef]
- Zakiaghl, M.; Izadpanah, K.; Niazi, A.; Behjatnia, S.A.A.; Afsharifar, A.R. Molecular and biological characterization of the Iranian isolate of the Australian grapevine viroid. J. Agri. Sci. Tech. 2013, 15, 855–865. [Google Scholar]
- Zhong, X.; Archual, A.J.; Amin, A.A.; Ding, B. A genomic map of viroid RNA motifs critical for replication and systemic trafficking. Plant Cell. 2008, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, J.A.; McKenry, M.V.; Walker, M.A.; Wolpert, J.A.; Credi, R.; Semancik, J.S. The vein-banding disease syndrome: A synergistic reaction between grapevine viroids and fanleaf virus. Vitis 1995, 34, 229–232. [Google Scholar] [CrossRef]
- Hily, J.M.; Candresse, T.; Garcia, S.; Vigne, E.; Tannière, M.; Komar, V.; Barnabé, G.; Alliaume, A.; Gilg, S.; Hommay, G.; et al. High-throughput sequencing and the viromic study of grapevine leaves: From the detection of grape-vine-infecting viruses to the description of a new environmental Tymovirales member. Front. Microbiol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Xia, C.; Li, S.; Hou, W.; Fan, Z.; Xiao, H.; Lu, M.; Sano, T.; Zhang, Z. Global transcriptomic changes induced by infection of cucumber (Cucumis sativus L.) with mild and severe variants of hop stunt viroid. Front. Microbiol. 2017, 8, 2427. [Google Scholar] [CrossRef]

| Library | Grapevine Cultivar | Raw Reads | Number of Unique Reads | No. Contigs | No. Identified Viruses | No. Identified Viroids | Viral/Viroidal Contigs | % Viral Reads/Unmapped Reads |
|---|---|---|---|---|---|---|---|---|
| A4 | Vitis vinifera cv. Peykani | 15,781,885 | 14,520,017 | 12,373 | 11 | 3 | 620 | 5.01 |
| A5 | Vitis vinifera cv. Askari-bidaneh | 16,038,160 | 15,566,856 | 19,875 | 9 | 4 | 478 | 2.41 |
| A6 | Vitis vinifera cv. Peykani | 15,775,003 | 15,686,878 | 7152 | 11 | 4 | 712 | 9.96 |
| A7 | Vitis vinifera cv. Rezghi | 16,754,257 | 16,468,660 | 7586 | 8 | 3 | 631 | 8.32 |
| A8 | Vitis vinifera cv. Sahebi | 15,952,556 | 15,766,196 | 9209 | 9 | 3 | 812 | 8.82 |
| A9 | Vitis vinifera cv. Fakhri | 15,452,253 | 15,232,125 | 8595 | 13 | 5 | 863 | 10.04 |
| Library | A4 | A5 | A6 | A7 | A8 | A9 | |
|---|---|---|---|---|---|---|---|
| unique reads | 14,520,017 | 15,566,856 | 15,686,878 | 16,468,660 | 15,766,196 | 15,452,253 | |
| hash length | |||||||
| No. contigs | K-mer: 13 | 16,767 | 17,482 | 10,436 | 11,484 | 11,796 | 11,049 |
| K-mer: 15 | 12,373 | 19,875 | 7152 | 7586 | 9209 | 8595 | |
| K-mer: 17 | 7157 | 11,637 | 3676 | 4203 | 4744 | 4800 | |
| K-mer: 19 | 4110 | 5859 | 1934 | 2101 | 2136 | 2752 | |
| K-mer: 21 | 2574 | 3520 | 663 | 1175 | 931 | 977 | |
| K-mer: 23 | 1530 | 2048 | 223 | 608 | 416 | 582 | |
| K-mer: 25 | 1024 | 1287 | 126 | 368 | 246 | 304 | |
| Max contig length | k-mer: 13 | 87 | 53 | 77 | 71 | 55 | 66 |
| K-mer: 15 | 360 | 314 | 260 | 382 | 327 | 364 | |
| K-mer: 17 | 469 | 578 | 294 | 410 | 372 | 351 | |
| K-mer: 19 | 519 | 643 | 325 | 597 | 397 | 294 | |
| K-mer: 21 | 678 | 1225 | 524 | 514 | 550 | 555 | |
| K-mer: 23 | 502 | 827 | 1162 | 568 | 913 | 809 | |
| K-mer: 25 | 514 | 1092 | 1464 | 1165 | 1172 | 882 | |
| No. detected virus/viroid | K-mer: 13 | 8 | 6 | 10 | 6 | 5 | 13 |
| K-mer: 15 | 11 | 13 | 12 | 10 | 11 | 15 | |
| K-mer: 17 | 8 | 12 | 10 | 9 | 8 | 13 | |
| K-mer: 19 | 8 | 12 | 9 | 9 | 7 | 12 | |
| K-mer: 21 | 8 | 10 | 8 | 7 | 6 | 7 | |
| K-mer: 23 | 8 | 9 | 6 | 6 | 5 | 6 | |
| K-mer: 25 | 8 | 8 | 2 | 4 | 4 | 4 | |
| VirusDetect pipeline | No. detected virus/viroid | 9 | 10 | 8 | 7 | 10 | 11 |
| Virus/Viroid | Reference GenBank Accession No. | Reference Genome Size (nt) | CLC Workbench | UGENE Package | VirusDetect Pipeline | |||
|---|---|---|---|---|---|---|---|---|
| Genome Recovery (nt) | Genome Coverage (%) | Genome Recovery (nt) | Genome Coverage (%) | Genome Recovery (nt) | Genome Coverage (%) | |||
| Australian grapevine viroid | NC003553 | 370 | 370 | 100.00 | 370 | 100.00 | 370 | 100 |
| Hop stunt viroid | NC001351 | 302 | 299 | 99.01 | 302 | 100.00 | 297 | 98.34 |
| Grapevine yellow speckle viroid 2 | KJ489020 | 363 | 355 | 97.80 | 363 | 100.00 | nf | 0 |
| Grapevine satellite virus | NC021480 | 1060 | 932 | 87.92 | 1043 | 98.40 | 877 | 82.74 |
| Grapevine yellow speckle viroid 1 | NC001920 | 366 | 311 | 84.97 | 366 | 100.00 | 366 | 1 |
| Grapevine leafroll associated virus 1 | NC016509 | 18,659 | 13,177 | 70.62 | 18,596 | 99.66 | 8501 | 45.56 |
| Grapevine fanleaf virus-RNA2 | KU522585 | 3788 | 2306 | 60.88 | 3756 | 99.16 | 2868 | 75.71 |
| Grapevine deformation virus-RNA1 | NC017939 | 7386 | 3746 | 50.72 | 7332 | 99.27 | 3485 | 47.18 |
| Grapevine Red Globe virus | NC030693 | 6863 | 3355 | 48.89 | 6858 | 99.93 | 614 | 8.95 |
| Grapevine fanleaf virus-RNA1 | KU522584 | 7367 | 3561 | 48.34 | 7341 | 99.65 | 4316 | 58.59 |
| Grapevine deformation virus-RNA2 | NC017938 | 3753 | 1392 | 37.09 | 3728 | 99.33 | nf | 0 |
| Arabis mosaic virus-RNA2 | NC006056 | 3820 | 1083 | 28.35 | 3762 | 98.48 | nf | 0 |
| Arabis mosaic virus-RNA1 | NC006057 | 7334 | 1891 | 25.78 | 7263 | 99.03 | 815 | 11.11 |
| Grapevine Pinot gris virus | NC015782 | 7259 | 1614 | 22.23 | 7175 | 98.84 | nf | 0 |
| Grapevine red blotch virus | NC022002 | 3206 | 513 | 16.00 | 3142 | 98.00 | 0 | 0 |
| Grapevine virus A | DQ855088 | 7342 | 1013 | 13.80 | 7296 | 99.37 | 0 | 0 |
| Family | Genus | Virus /Viroid Species | Cultivar | Peykani | Askari-Bidaneh | Peykani | Rezghi | Sahebi | Fakhri | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Library | A4 | A5 | A6 | A7 | A8 | A9 | |||||||||
| Abbreviation | NGS | PCR | NGS | PCR | NGS | PCR | NGS | PCR | NGS | PCR | NGS | PCR | |||
| Alphaflexiviridae | Allexivirus | Garlic virus A * | 0.31 | - | nf | nt | 0.17 | - | nf | nt | nf | nt | 0.28 | - | |
| Betaflexiviridae | Trichovirus | Grapevine Pinot gris virus | 0.62 | nt | 1.91 | nt | 0.17 | nt | 0.14 | nt | 0.64 | nt | 1.05 | + | |
| Vitivirus | Grapevine virus A | nf | nt | nf | nt | nf | nt | nf | nt | nf | nt | 0.53 | + | ||
| Bromoviridae | Alfamovirus | Alfalfa mosaic virus * | 0.31 | - | nf | - | nf | - | nf | - | nf | - | 1.05 | - | |
| Closteroviridae | Ampelovirus | Grapevine leafroll associated virus 1 | 53.1 | + | 54.2 | + | 43.65 | + | 32.77 | + | 39.52 | + | 46.76 | + | |
| Geminiviridae | Grablovirus | Grapevine red blotch virus | 1.8 | + | 1.5 | + | 0.53 | + | 0.6 | + | 0.6 | + | 0.47 | + | |
| Secoviridae | Nepovirus | Arabis mosaic virus | 3.26 | + | 1.71 | + | 4.46 | + | 50.5 | + | 2.94 | + | 1.92 | + | |
| Grapevine deformation virus | 3.26 | + | 1.71 | + | 4.46 | + | 5.05 | + | 2.94 | + | 1.92 | + | |||
| Grapevine fanleaf virus | 33.74 | + | 25.56 | + | 40.96 | + | 48.14 | + | 46.91 | + | 29.25 | + | |||
| Tymoviridae | Maculavirus | Grapevine Red Globe virus | nf | nt | nf | nt | nf | nt | nf | nt | nf | nt | 2.39 | + | |
| Virtovirus | Grapevine satellite virus | nf | nt | 4.77 | + | 0.17 | nt | nf | nt | 1.34 | + | 2.91 | + | ||
| Pospiviroidae | Apscaviroid | Grapevine yellow speckle viroid 1 | 0.36 | + | 0.34 | + | 1.05 | + | 2.15 | + | 0.78 | + | 1.92 | + | |
| Grapevine yellow speckle viroid 2 | nf | nt | nf | nt | nf | nt | nf | nt | nf | nt | 0.73 | + | |||
| Australian grapevine viroid | nf | - | 3.04 | + | 0.69 | + | nf | nt | nf | - | 1.02 | + | |||
| Hostuviroid | Hop stunt viroid | 1.07 | + | 1.02 | + | 0.7 | + | 2.15 | + | 2.33 | + | 1.31 | + | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholampour, Z.; Zakiaghl, M.; Asquini, E.; Moser, M.; Gualandri, V.; Mehrvar, M.; Si-Ammour, A. Application of High-Throughput Sequencing for Comprehensive Virome Profiling in Grapevines Shows Yellows in Iran. Viruses 2024, 16, 204. https://doi.org/10.3390/v16020204
Gholampour Z, Zakiaghl M, Asquini E, Moser M, Gualandri V, Mehrvar M, Si-Ammour A. Application of High-Throughput Sequencing for Comprehensive Virome Profiling in Grapevines Shows Yellows in Iran. Viruses. 2024; 16(2):204. https://doi.org/10.3390/v16020204
Chicago/Turabian StyleGholampour, Zahra, Mohammad Zakiaghl, Elisa Asquini, Mirko Moser, Valeria Gualandri, Mohsen Mehrvar, and Azeddine Si-Ammour. 2024. "Application of High-Throughput Sequencing for Comprehensive Virome Profiling in Grapevines Shows Yellows in Iran" Viruses 16, no. 2: 204. https://doi.org/10.3390/v16020204





