Abstract
Human type A rotavirus (RV-A) is world-recognized as the major pathogen causing viral gastroenteritis in children under 5 years of age. The literature indicates a substantial increase in the diversity of rotavirus strains across continents, especially in Africa, which can pose significant challenges including an increase of disease burden and a reduction of vaccines’ effectiveness. However, few studies have mapped the variety of circulating virus strains in different regions, which may hamper decisions on epidemiological surveillance and preventive public health measures. Thus, our aim was to compile the most updated available evidence on the genetic profile of RV-A among children in Africa and determine the prevalence of different genotypes according to the geographical regions by means of a broad systematic review. Systematic searches were performed in PubMed, Scopus, Web of Science, and Scielo without language, time limits, or geographical restrictions within the African continent. We selected full-text peer-reviewed articles assessing the genetic profile (i.e., genotyping) of RV-A in children up to 5 years old in Africa. Overall, 682 records were retrieved, resulting in 75 studies included for evidence synthesis. These studies were published between 1999 and 2022, were conducted in 28 countries from the five African regions, and 48% of the studies were carried out for 24 months or more. Most studies (n = 55; 73.3%) evaluated RV-A cases before the introduction of the vaccines, while around 20% of studies (n = 13) presented data after the vaccine approval in each country. Only seven (9.3%) studies compared evidence from both periods (pre- and post-vaccine introduction). Genotyping methods to assess RV-A varied between RT-PCR, nested or multiplex RT-PCR, testing only the most common P and G-types. We observed G1 and P[8] to be the most prevalent strains in Africa, with values around 31% and 43%, respectively. Yet if all the genotypes with the following highest prevalence were added ((G1 + G2, G3, G9) and (P[8] + P[6], P[4])), these figures would represent 80% and 99% of the total prevalence. The combination G1P[8] was the most reported in the studies (around 22%). This review study demonstrated an increased strain diversity in the past two decades, which could represent a challenge to the efficacy of the current vaccine.
1. Introduction
Severe dehydrating diarrhea caused by rotavirus remains one of the major causes of morbidity and mortality among children under 5 years old worldwide, despite some decreasing trends in these figures in the last decade [1]. In 2019, rotavirus infections were responsible for an estimated two million hospitalizations and over 25 million outpatient visits globally. In this same year, from the over five million accounted deaths (95% CI 4.92–5.68) in children younger than 5 years, diarrhea diseases were attributed to 9.1% of cases (95% CI 7.9–9.9) and occurred mostly in low-income countries [2,3].
The genus Rotavirus A (RV-A) is an RNA virus (Reoviridae family) containing several structural viral proteins, of which VP4 (protease-cleaved protein or P protein) and VP7 (glycoprotein or G protein) strands are determinants of genetic variability and viral serotype classification (P- and G-serotypes). These proteins have been extensively studied in the past decades as targets for neutralizing antibodies, and grounded the development of live attenuated rotavirus vaccines. Between 2008 and 2009, the World Health Organization (WHO) prequalified a pentavalent bovine-human reassortant vaccine (RotaTeq27-RV5) and a monovalent vaccine based on a human RV-A strain (Rotarix-RV1). In 2018, two additional vaccines (Rotavac and ROTASIIL) were licensed, being increasingly recommended by national immunization programs [4], especially for high-risk mortality populations [5]. As of January 2022, 114 countries (including 79% of those from Africa) have introduced RV vaccination services [4,6]. In fact, a systematic review on the impact of immunization programs in sub-Saharan Africa demonstrated that the inclusion of RV1 and RV5 vaccines led to significant reductions in the proportion of positive cases in these regions from 42% (95% CI 38–46) (pre-vaccination period) to 21% (95% CI 17–25) [7].
However, it has been suggested that massive vaccination could lead to the replacement of circulating genotypes or the emergence of new variants or neutralizing antibodies escape mutants, which may reduce the effectiveness of the vaccine [8]. Moreover, a very heterogeneous distribution of genotypes of RV-A in Africa exist—and often differ from circulating strains and G-P combinations from other regions in the globe [9,10,11,12]. Additionally, dissimilar socioeconomic conditions and cultures may lead to differences in the pattern of circulation of RV-A genotypes [7]. Previous reviews conducted between 1975 and 1992 reported three quarters of rotavirus strains in Africa belonging to one of the four globally common G types circulating at that time, namely serotypes G1, G2, G3, or G4 [13]. Later studies showed that the genotypes G1, G2, G3, G9, and G12 were the most common, together with P[8], P[6], and P[11]. It seems that the combinations G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] are responsible for around 90% of all RV-A infections in the continent [9,14,15].
Yet, the literature lacks further synthesized and more updated evidence on the genetic profile of RV-A in African countries, especially for the pediatric population. Only one systematic review, without a published protocol (2017) and assessing the genotype profile of the virus in Africa during 2006–2016, has been published [13,16]. Thus, we aimed to compile the current evidence on the genetic profile of RV-A in children up to 5 years old living in Africa and determine the prevalence of the genotypes according to the different regions by means of a broad systematic review.
2. Materials and Methods
A systematic review to synthesize the pooled prevalence of circulating RV-A in children under 5 in Africa was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses—PRISMA guidelines and Cochrane Collaboration recommendations [17,18]. The protocol of this systematic review was registered in the international prospective register of systematic reviews—PROSPERO (CRD42022346530)—and is available at Open Science Framework (DOI 10.17605/OSF.IO/RSZC6). Two authors conduct, independently, all steps of this studies’ selection and data extraction. Disagreements were resolved by discussion with a third author arbitrating in the circumstance of unresolved discrepancies.
2.1. Search Strategy
This study review was conducted by searching the following electronic databases for primary peer-reviewed studies: MEDLINE (PubMed), Scopus, Web of Science, and Scielo (updated searches in December 2022). The search was not limited by any filter tool, language, or country. Trial registration databases (www.clinicaltrials.gov; accessed on 10 December 2022), internet-based relevant databases (WHO Global Health Library, which encompasses African Index Medicus), and the reference lists of the included studies were also searched as part of the manual searching process. A comprehensive search strategy was developed using subject headings related to four sets of descriptors (rotavirus, genotyping, children, Africa), combined with Boolean Operators AND and OR. The search strategies adapted for each database are available in the Supplemental Materials (Table S1).
2.2. Study Selection
Records retrieved from the databases were exported to a reference management program (EndNote version X9.2, Clarivate, London, UK) where duplicates were removed. Thereafter, the management of references and data extraction used Excel sheets (Microsoft 2020, Redmond, WA, USA). Titles and abstracts of the studies were independently screened by two authors to remove irrelevant records. The full text of potentially eligible studies was retrieved and independently assessed for eligibility by two of the authors. The three inclusion criteria were (i) peer-reviewed primary articles reporting data on the genetic profile (genotyping) of RV-A; (ii) articles including children under 5 years old; (iii) studies carried out in at least one African country. Studies conducted in other populations (adults or children older than 5 years), in vitro or in vivo studies, as well as those without clear evidence of the type of technique used for genotyping were excluded. Discussion papers, letters, editorials, reviews, and articles in non-Roman characters were also excluded.
2.3. Data Extraction and Quality Assessment
A standardized form in Excel sheets (Microsoft 2020, Redmond, WA, USA) was used to extract information on: articles’ general data (author’s name, year of publication, country, sample size, study duration); participants and their characteristics (age, in- or outpatients); genotyping methods used, number of samples tested, absolute numbers and percentages for the relevant genotypes. Whenever necessary, indirect data from figures and charts were collected.
The methodological quality of the included studies was assessed by means of the JBI—Jonna Briggs Institute Critical Appraisal tool [16] for the domains of appropriateness of study design, selection bias, appropriate statistical analysis, and presentation of study findings. For each study, the grading of each component and the global study rating was assigned to the categories: low (<5), moderate (5–6), and high quality (7–8). To assign the final quality score, authors also verified if the stated objectives of the paper matched the reporting of outcomes within the paper (Supplementary Figure S1).
2.4. Statistical Analysis and Synthesis
A narrative synthesis of the findings from the included studies, structured around the population characteristics, geographical region, and genotype profiling, was developed. Prevalence calculations of identified genotypes were performed (i.e., by dividing the number of positive cases of a given genotype by the number of samples tested) and reported with a 95% confidence interval (95% CI—upper and lower limits) (Comprehensive Meta-analysis software version 3.0; Microsoft Excel). Location maps of G and P genotypes for the six African regions of the United Nations were built. Analyses were performed in IBM SPSS statistics version 26 software.
3. Results
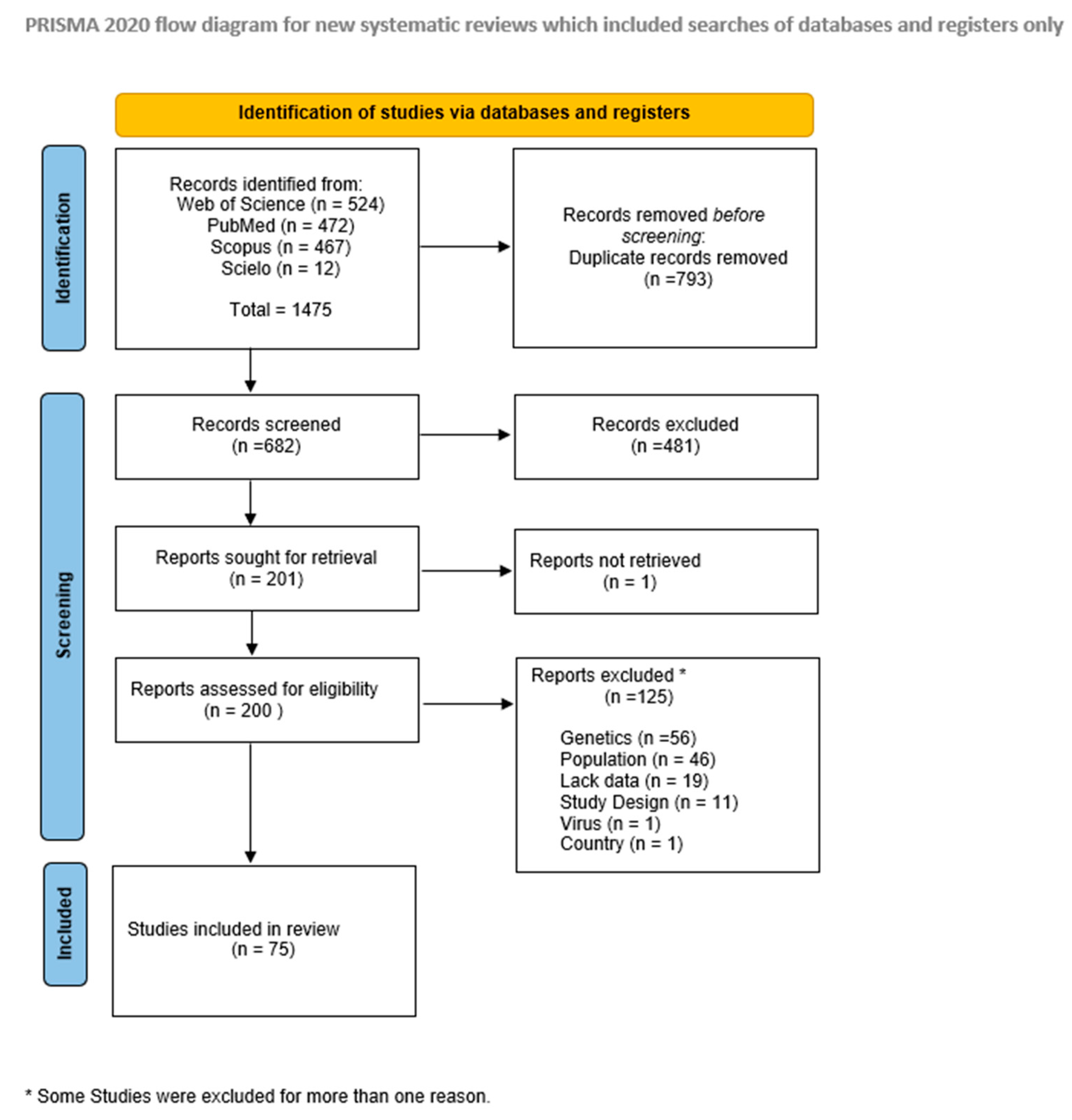
Overall, 682 records were retrieved from the databases after duplicates removal, from which 200 were fully assessed during the eligibility phase, resulting in 75 studies included for evidence synthesis (see Figure 1).
Figure 1.
Study selection flow diagram.
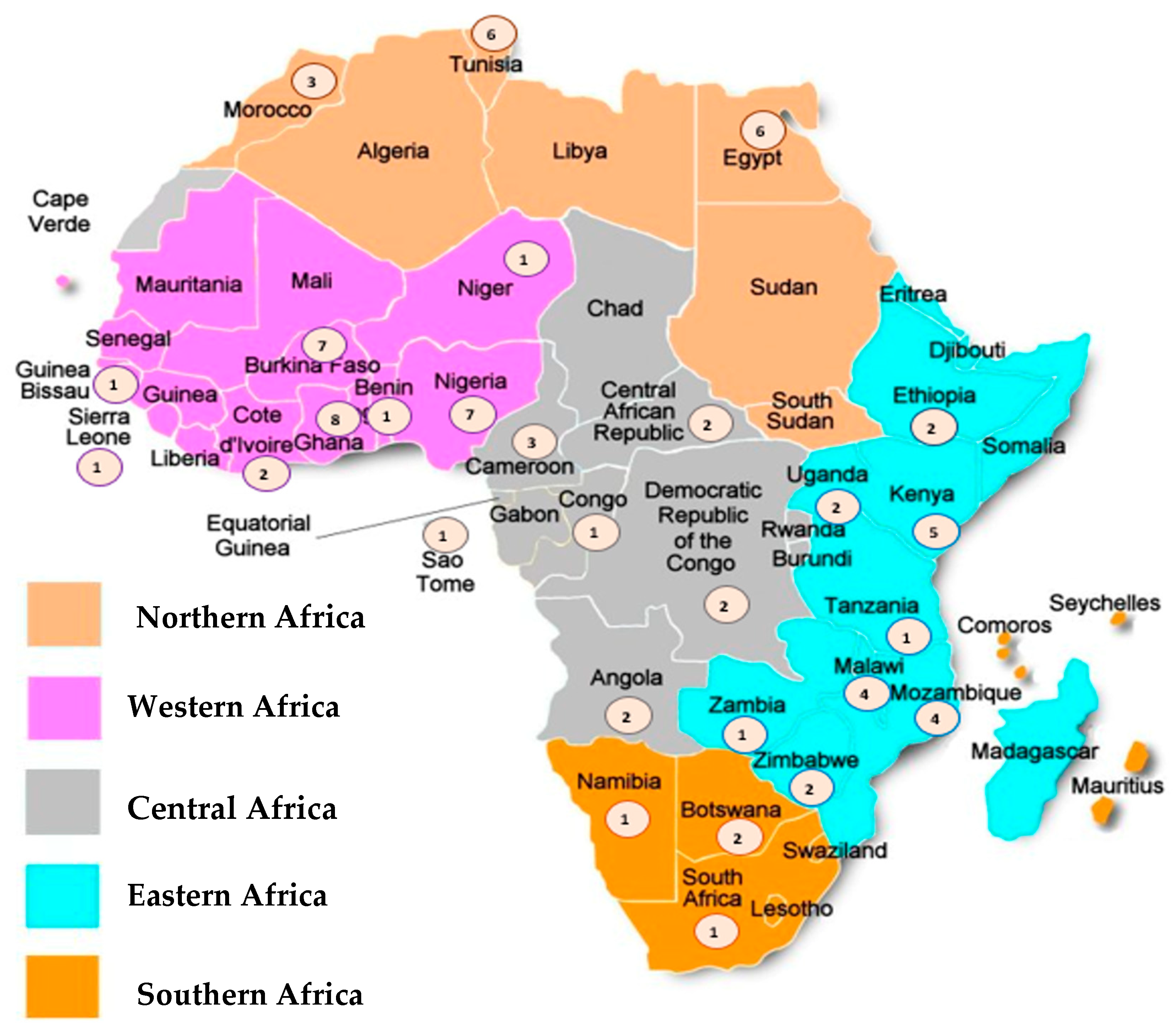
These studies were published between 1999 and 2022 and were conducted in 28 countries located in the five African regions, namely: 25 (33.3%) studies assessing the Western region, 21 (28.0%) from the Eastern region, 15 (20.0%) from the Northern region, 11 (14.7%) from the Central Region, and 3 (4.0%) studies from the Southern region of the continent (Figure 2). Around half (n = 36; 48%) of the studies were performed for 24 months or more, the total sample size generally being less than 250 patients. Most studies (n = 31; 41.3%) evaluated RV-A cases in hospital settings, while outpatient department visits were reported in 30.6% of the studies (n = 23); children attending any of these settings were assessed by n = 21; 28% of studies. Most studies (n = 55; 73.3%) evaluated RV-A cases before the introduction of the vaccines in the respective countries (vaccine introduction dates in Supplementary Table S2), while around 20% of studies (n = 13) presented data after the vaccine approval in each country. Only seven (9.3%) studies compared evidence from both periods (pre-and post-vaccine introduction). Genotyping methods to assess RV-A varied between RT-PCR, nested or multiplex RT-PCR. Most studies tested only the most common P and G-types (Table 1).

Figure 2.
Geographical distribution of the studies. Note: The circles represent the number of studies included for each country.

Table 1.
Summary of the studies’ characteristics.
A total of 17,418 genotyped samples were analyzed for prevalence of various RV-A genotypes, 14,759 strains being characterized for the G specificity, 14,258 for P specificity, and 13,003 for both P and G antigens. Considering the very low incidence of other non-typeable RV strains and mixed infections, they were not included in the final synthesis.
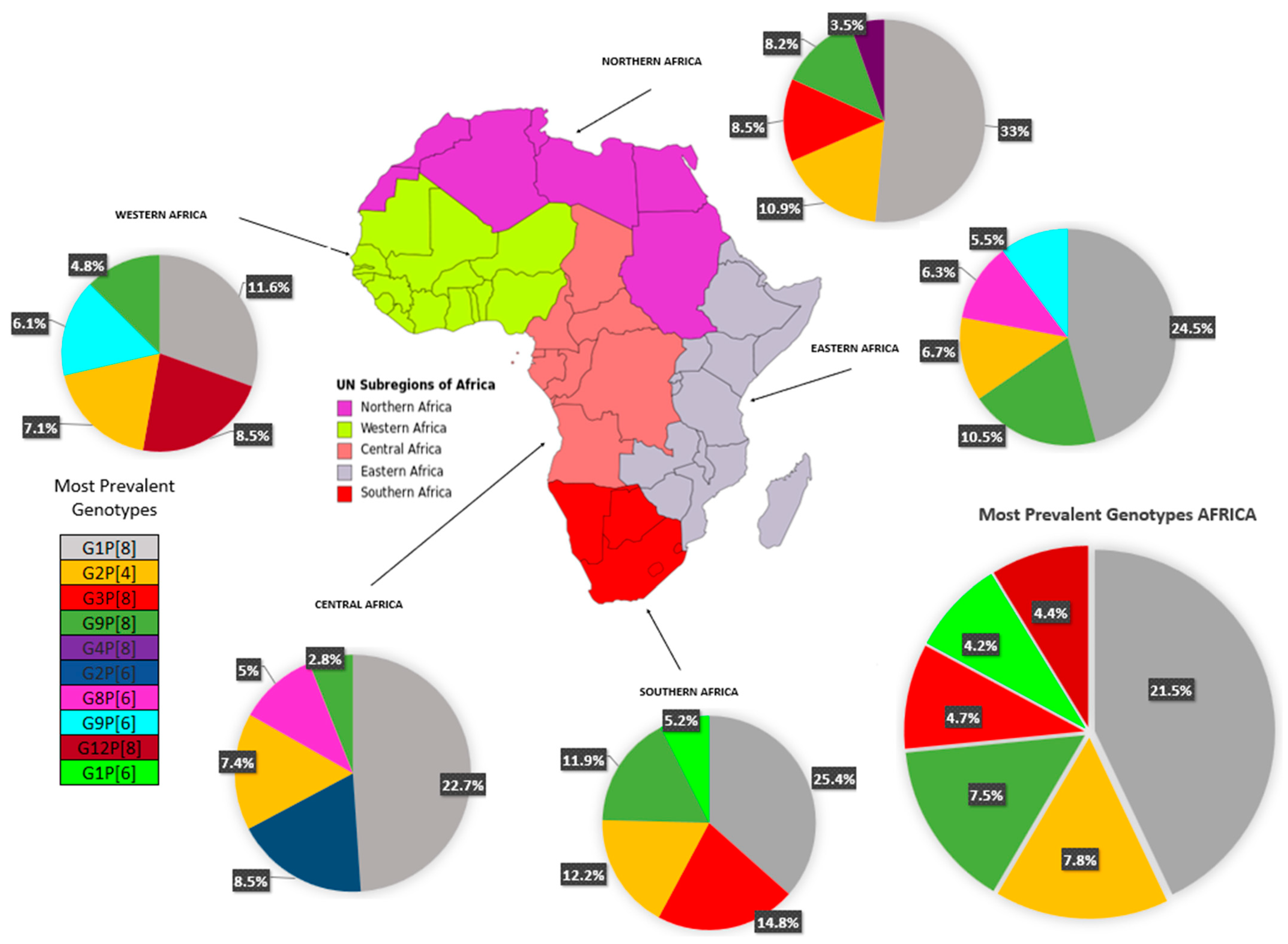
Table 2 depicts the complete data on RV-A genotypes from 28 of 54 African countries (51.8%) and Figure 3 summarizes the evidence distributed according to the regions of the African continent. Overall, G1 was the most prevalent strain (n = 5352 cases; 30.73% [95% CI 24.2–37.2]), with the highest prevalence in the North region (40.5% [95% CI 33.9–47.1]), followed by G2 (n = 2588 cases; 14.9% [95% CI 11.0–18.7]), found especially in the West (18.5%) and Central (18.1%) regions. The rotavirus G3 strain represented the third most prevalent strain (11.0%, [95% CI 5.6–19.1]), being most detected in the South (20%) (see Table 2). Regarding P genotypes, P[8] strains were highly reported (n = 7465 cases; 42.8% [95% CI 33.0–52.7]) in all regions, followed by P[6] (24.9%) and P[4] (13.7%) strains. The most prevalent combination was G1P[8] (n = 3745 cases; 21.5% [95% CI 12.9–30]), with rates ranging from 11.6% in the West region to 33.0% in the North. Other combined strains such as G2P[4] (n = 1364; 7.8% [95% CI 5—10.6]) and G9P[8] (n = 1309; 7.5% [95% CI 3.3–11.7]) were also fairly reported.

Table 2.
Rotavirus A genotypes in Africa.
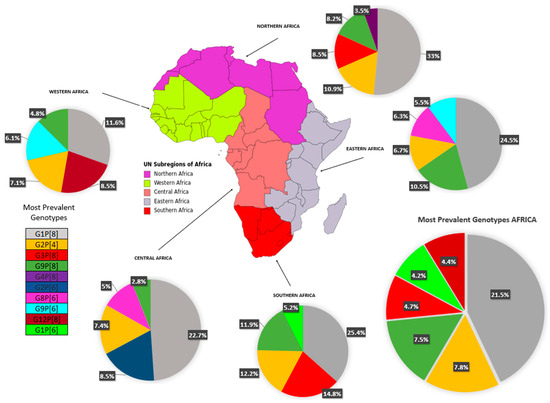
Figure 3.
Circulating rotavirus strains in the African regions.
After vaccine introduction, G1P[8] continued to be the most prevalent (20.4%) globally, due to the Northern (51.7%) and Eastern (24.3%) regions. In the Western region, G12P[8] was the most prevalent (23%), and G2P[4] was most prevalent in the Southern region (27.3%). A small number of post-vaccine studies (13), and the absence of studies from the central region may have created an important bias in the regional analysis.
The methodological quality assessment of studies demonstrated most reports as high and medium quality. Of the assessed domains, the identification of limitations, confounding factors, strategies to deal with limitations, and the lack of next steps were the most problematic. These aspects should be improved in future reports (full assessment in Supplemental Materials).
4. Discussion
This systematic review synthesized and critically assessed the evidence of 75 primary studies on RV-A genotypes in children under 5 in Africa over a 23-year period and demonstrated the prevalence of the infection is still commonplace. This highlights the need of further measures for reducing the health, social, and economic burdens related to this condition in the continent—including the development of vaccines and immunization programs targeting circulating genotypes.
We observed G1 and P[8] to be the most prevalent strains in Africa, with values around 31% and 43%, respectively. Yet if all the genotypes with the following highest prevalence were added, (G1 + G2, G3, G9) and (P[8] + P[6], P[4]), these figures would represent 80% and 99% of the total prevalence. The combination G1P[8] was the most reported in the studies (around 22%). Previous studies similarly reported these genotypes as commonly found in the continent (comparable prevalence rates varying from 25 to 45% for G1 and P[8]), yet their occurrence may significantly differ across geographical regions worldwide [13,14,90,91]. For instance, higher rates of G1P[8] infection have been found in North America, Europe, and Australia (70% of all circulating strains), while this figure is about 30% in South America and Asia [91].
We additionally identified a potential increasing trend in the proportion of novel strains, such as G12 (7.2%) and G8 (5.9%), but also the combinations G2P[4] (7.8%) and G9P[8] (7.5%), that have been similarly detected globally [21]. Moreover, we found a fair prevalence (3–5%) of unusual strain combinations such as G1P[6], G3P[8], G2P[6], G3P[8], G12P[8], and G8P[6] that may reflect the diversity and nature of RV-A in Africa and its unique distribution compared with other regions. The identification of uncommon genotypes such as G5 (0.01%), G6 (0.6%), and G10 (0.8%) raise further awareness about the heterogeneity of RV-A in Africa and the need for active epidemiological surveillance among children under 5.
This review study demonstrated an increased strain diversity in the past two decades, when compared with data from before 1997–2006 in Africa [11]. As studies suggested, this increase in strain diversity can be explained by the introduction and prevalent use of more sensitive RT-PCR genotyping methods that enable the detection of common and uncommon strain types, lending multiple infections and rotavirus evolution in vivo, and that common strains may have evolved by genetic drift [11].
A number of rotavirus strains remained non-typeable (14.5%); less than the 16% in a review study carried out in Africa from 1997 to 2006, and more likely than the 14.8% found globally [11], allowing the possibility that other serotypes have not yet been identified.
Even though the most common G/P combinations reported in different geographical regions appear similar, the proportions vary per geographic region and over time.
The complexity of the molecular epidemiology of rotavirus strains and its variability was shown in this review study, with only 13 post-vaccine introduction studies conducted in four African regions, and the predominant genotypes varying in the regional analysis with an overall predominant prevalence being G1P[8] (20.4%). The recognition that the circulating strains will fluctuate over time and in different regions of the continent is important for the monitoring of strain diversity in the period after rotavirus vaccines introduction.
In the analysis of the relevance of strain diversity to rotavirus vaccine programs, the current oral, live attenuated rotavirus vaccines have greatly reduce the burden of severe rotavirus disease in Africa. These include a monovalent human rotavirus G1P[8] vaccine (Rotarix) and a (RotaTeq) pentavalent human-bovine reassortant vaccine that covers serotypes G1, G2, G3, G4, and P[8]. It will be important to demonstrate vaccine efficacy in settings where strains share neither G or P type with these vaccines. In this review, of the single rotavirus strains examined, 12.7% did not share either G or P type with RotaTeq and 29% did not share a G or P type with Rotarix. However, many other factors may be involved in the protective immunity and further study is needed in less developed settings to study the cross-protection of non-vaccine strains [8,92,93,94,95].
This study has some limitations, as data were available from only 51.8% (28/54) of the African countries and only 13 studies were conducted after the vaccine introduction. Also, the majority of the studies only detect common G and P-types and sequencing tests were performed in very few studies, probably because of not typing strains. Although the studies included in this review provide an indication of genotypes circulating throughout the African continent, they may not represent all countries in the region. Different definitions of genotypes, as well as the consideration of a wide variety of sequencing techniques of the rotavirus, whose numbers of eligible studies may be limited, may affect the characterization of some genotypes.
5. Conclusions
This systematic review compiled the most recent findings from primary studies of genetic identification of RV-A circulating in Africa in the past 23 years and presented the pooled prevalence of circulating rotavirus genotypes in children under 5 years old in the five African geographic regions.
In the African continent, 43 of 54 (79.6%) countries have introduced rotavirus vaccines into their immunization programs [96], representing over half of all countries in the world [8].
The high prevalence of mixed infections observed in this study as well as other studies in the continent [57] constitutes an optimal moment for the reassortment of the rotavirus genome that can lead to the generation of new rotavirus strains and may generate new genome constellations that allow rotavirus type A to expand its host range or evade immune responses [97]. The diversity of rotavirus strains in the continent, that carry a higher burden of rotavirus mortality, could represent a challenge to the efficacy of current vaccines.
African surveillance studies post-vaccine introduction are crucial to understanding the impact of the vaccine on rotavirus circulating strains, and assure vaccine efficacy. It is fundamental to maintain an efficient rotavirus surveillance network and update the information of circulating strains in each country of the African region.
We, thus, believe that our findings may directly contribute towards the updating of evidence on rotavirus circulating strains, contributing to the development of next generation rotavirus vaccines, surveillance mechanisms, and health policy measures.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v16020243/s1, Table S1: Search Strategies; Table S2: Characteristics of the Included Studies; Table S3: Vaccine Introduction Dates-African Countries (October 2023); Figure S1: Critical Appraisal of the Included Studies.
Author Contributions
All authors contributed to the design and conceptualization of this review. S.M. and F.S.T. drafted the protocol and the Systematic Review with primary support by M.B. (review guarantor) C.P.-S. and E.F.-G. The following authors: S.M., F.S.T., M.B., E.F.-G. and C.P.-S. were involved in checking various steps of the search strategy, including keywords, as well as the final version of the review study. F.S.T. and M.B. were involved in the statistical strategy for data analysis. S.M., F.S.T., E.F.-G. and M.B. were involved in establishing eligibility criteria and data extraction forms. All authors provided feedback on the manuscript, at all stages. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its Supplemental Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bernadeta, D.; Hannah, R.; Max, R. Diarrheal Diseases—Our World in Data November 2019. 2019. Available online: https://ourworldindata.org/diarrheal-diseases#burden-of-diarrheal-diseases (accessed on 14 May 2021).
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; David, P.-M.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Damtie, D.; Melku, M.; Tessema, B.; Vlasova, A.N. Prevalence and Genetic Diversity of Rotaviruses among under-Five Children in Ethiopia: A Systematic Review and Meta-Analysis. Viruses 2020, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Manual for the Surveillance of Vaccine-Preventable Diseases|CDC. Available online: https://www.cdc.gov/vaccines/pubs/surv-manual/index.html (accessed on 1 December 2022).
- World Health Organization. Rotavirus vaccines: WHO position paper—July 2021—Vaccines antirotavirus: Note de synthèse de l’OMS—Juillet 2021. Wkly Epidemiol. Rec. 2021, 96, 219–301. [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, O.; Zhang, W.; Amponsem-Boateng, C.; Oppong, T.B.; Zhao, Q.L.; Li, D. Evidence of rotavirus vaccine impact in sub-Saharan Africa: Systematic review and meta-analysis. PLoS ONE 2020, 15, e0232113. [Google Scholar] [CrossRef]
- Technical Resources|Rota Council. Available online: https://preventrotavirus.org/resources/technical-resources/ (accessed on 16 January 2021).
- Gasparinho, C.; Piedade, J.; Mirante, M.C.; Mendes, C.; Mayer, C.; Nery, S.V.; Brito, M.; Istrate, C. Characterization of rotavirus infection in children with acute gastroenteritis in Bengo province, Northwestern Angola, prior to vaccine introduction. PLoS ONE 2017, 12, e0176046. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J.; Nitiema, L.; Sharma, S.; Ouermi, D.; Traore, A.S.; Simpore, J.; Svensson, L. Emergence of Unusual G6P[6] Rotaviruses in Children, Burkina Faso, 2009–2010. Emerg. Infect. Dis. 2012, 18, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Todd, S.; Page, N.A.; Steele, A.D.; Peenze, I.; Cunliffe, N.A. Rotavirus Strain Types Circulating in Africa: Review of Studies Published during 1997–2006. J. Infect. Dis. 2010, 202, S34–S42. [Google Scholar] [CrossRef]
- João, E.D.; Strydom, A.; O’neill, H.G.; Cuamba, A.; Cassocera, M.; Acácio, S.; Mandomando, I.; Motanyane, L.; Page, N.; de Deus, N. Rotavirus A strains obtained from children with acute gastroenteritis in Mozambique, 2012-2013: G and P genotypes and phylogenetic analysis of VP7 and partial VP4 genes. Arch. Virol. 2018, 163, 153–165. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Kilgore, P.E.; Bresee, J.S.; Steele, A.D.; Luo, N.; Hart, C.A.; Glass, I.R. Epidemiology of rotavirus diarrhoea in Africa: A review to assess the need for rotavirus immunization. Bull. World Health Organ. 1998, 76, 525–537. [Google Scholar]
- Ouermi, D.; Soubeiga, D.; Nadembega, W.; Sawadogo, P.; Zohoncon, T.; Obiri-Yebo, D.; Djigma, F.; Nordgren, J.; Simpore, J. Molecular Epidemiology of Rotavirus in Children under Five in Africa (2006–2016): A Systematic Review. Pak. J. Biol. Sci. 2017, 20, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.; Nordgren, J.; Pereira, J.; Fortes, F.; Dimbu, R.; Saraiva, N.; Mendes, C.; Istrate, C. Molecular epidemiology of rotavirus in four provinces of Angola before vaccine introduction. J. Med. Virol. 2016, 88, 1511–1520. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2021. Available online: https://jbi-global-wiki.refined.site/space/MANUAL (accessed on 2 December 2022).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions. 1 January 2019, pp. 1–694. Available online: https://jhu.pure.elsevier.com/en/publications/cochrane-handbook-for-systematic-reviews-of-interventions (accessed on 2 December 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennnan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Naficy, A.B.; Abu-Elyazeed, R.; Holmes, J.L.; Rao, M.R.; Savarino, S.J.; Kim, Y.; Wierzba, T.F.; Peruski, L.; Lee, Y.J.; Gentsch, J.R.; et al. Epidemiology of rotavirus diarrhea in Egyptian children and implications for disease control. Am. J. Epidemiol. 1999, 150, 770–777. [Google Scholar] [CrossRef]
- Allayeh, A.K.; El Baz, R.M.; Saeed, N.M.; Osman, M.E.S. Detection and Genotyping of Viral Gastroenteritis in Hospitalized Children Below Five Years Old in Cairo, Egypt. Arch. Pediatr. Infect. Dis. 2018, 6, e60288. [Google Scholar] [CrossRef]
- Saudy, N.; Elshabrawy, W.O.; Megahed, A.; Foad, M.F.; Mohamed, A.F. Genotyping and Clinicoepidemiological Characterization of Rotavirus Acute Gastroenteritis in Egyptian Children. Polish J. Microbiol. 2017, 65, 433–442. [Google Scholar] [CrossRef]
- Elnady, H.; Abdelsamie, O.; Sallam, S.; Sherif, L.; Kholoussi, N.; Khoulousi, S.; Ali, M. Genotyping of rota virus causing gastroenteritis in Egyptian children. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 512–521. [Google Scholar]
- Matson, D.O.; Abdel-Messih, I.A.; Schlett, C.D.; Bok, K.; Wienkopff, T.; Wierzba, T.F.; Sanders, J.W.; Frenck, J.R.W. Rotavirus Genotypes among Hospitalized Children in Egypt, 2000–2002. J. Infect. Dis. 2010, 202, S263–S265. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mansour, A.M.; Klena, J.D.; Husain, T.S.; Hassan, K.A.; Mohamed, F.; Steele, D. Rotavirus Genotypes Associated with Acute Diarrhea in Egyptian Infants. Pediatr. Infect. Dis. J. 2014, 33, S62–S68. [Google Scholar] [CrossRef]
- Benhafid, M.; Youbi, M.; Klena, J.D.; Gentsch, J.R.; Teleb, N.; Widdowson, M.; El Aouad, R. Epidemiology of Rotavirus Gastroenteritis among Children <5 Years of Age in Morocco during 1 Year of Sentinel Hospital Surveillance, June 2006–May 2007. J. Infect. Dis. 2009, 200, S70–S75. [Google Scholar] [CrossRef]
- Benhafid, M.; Elomari, N.; Elqazoui, M.; Meryem, A.I.; Rguig, A.; Filali-Maltouf, A.; Elaouad, R. Diversity of rotavirus strains circulating in children under 5 years of age admitted to hospital for acute gastroenteritis in Morocco, June 2006 to May 2009. J. Med. Virol. 2013, 85, 354–362. [Google Scholar] [CrossRef] [PubMed]
- El Qazoui, M.; Oumzil, H.; Baassi, L.; El Omari, N.; Sadki, K.; Amzazi, S.; Benhafid, M.; El Aouad, R. Rotavirus and norovirus infections among acute gastroenteritis children in Morocco. BMC Infect. Dis. 2014, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Chouikha, A.; Fredj, M.B.H.; Fodha, I.; Mathlouthi, I.; Ardhaoui, M.; Teleb, N.; Brini, I.; Messaadi, F.; Mastouri, M.; Sfar, T.; et al. Évolution des souches de Rotavirus du groupe A en circulation en Tunisie sur une période de trois ans (2005–2007). Pathol. Biol. 2011, 59, e79–e83. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, A.; Fodha, I.; Chouikha, A.; Fredj, M.B.H.; Mastouri, M.; Ben Abdelaziz, A.; Sfar, T.; Essoussi, A.S.; Jaoua, S.; Steele, A.D. Rotavirus Strain Diversity in the Centre Coast of Tunisia from 2000 through 2003. J. Infect. Dis. 2010, 202, S252–S257. [Google Scholar] [CrossRef][Green Version]
- Soltani, M.; Bouanene, I.; Trabelsi, A.; Harbi, A.; Hachicha, M.; Amri, F.; Boussnina, S.; Gueddiche, M.N.; Sfar, M.T.; Teleb, N.; et al. Épidémiologie des gastroentérites à rotavirus chez les enfants âgés de moins de cinq ans en Tunisie—Résultats de la surveillance sentinelle hospitalière 2009 à 2011. Rev. Epidemiol. Sante Publique 2012, 60, 473–480. [Google Scholar] [CrossRef]
- Chouikha, A.; Fodha, I.; Noomen, S.; Bouzid, L.; Mastouri, M.; Peenze, I.; De Beer, M.; Dewar, J.; Geyer, A.; Sfar, T.; et al. Group A rotavirus strains circulating in the eastern center of Tunisia during a ten-year period (1995–2004). J. Med. Virol. 2007, 79, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Fredj, M.B.H.; Fodha, I.; BenHamida-Rebaï, M.; Kacem, S.; Argoubi, A.; Bennour, H.; Boujaafar, N.; Trabelsi, A. Distribution of rotavirus VP7 and VP4 genotypes circulating in Tunisia from 2009 to 2014: Emergence of the genotype G12. J. Med. Microbiol. 2016, 65, 1028–1037. [Google Scholar] [CrossRef]
- Bennour, H.; Fodha, I.; Bouazizi, A.; Ben Hamida-Rebaï, M.; Jerbi, A.; Fredj, M.B.H.; Lakhal, S.; Dhiflaoui, A.; Abdelberi, S.; Abbassi, F.; et al. Molecular characterization of group A rotavirus among children aged under 5 years in Tunisia, 2015–2017. J. Med. Microbiol. 2019, 68, 1240–1243. [Google Scholar] [CrossRef]
- Agbla, J.M.; Esona, M.D.; Agbankpe, A.J.; Capo-Chichi, A.; Gautam, R.; Dougnon, T.V.; Razack, O.; Bowen, M.D.; Bankole, H.S. Molecular characteristics of rotavirus genotypes circulating in the south of Benin, 2016–2018. BMC Res. Notes 2020, 13, 485. [Google Scholar] [CrossRef]
- Steele, A.D.; Page, N.; de Beer, M.; Sawadogo, S. Antigenic and Molecular Characterization of Unusual Rotavirus Strains in Burkina Faso in 1999. J. Infect. Dis. 2010, 202, S225–S230. [Google Scholar] [CrossRef]
- Bonkoungou, I.J.; Damanka, S.; Sanou, I.; Tiendrébéogo, F.; Coulibaly, S.O.; Bon, F.; Haukka, K.; Traoré, A.S.; Barro, N.; Armah, G.E. Genotype diversity of group A rotavirus strains in children with acute diarrhea in urban Burkina Faso, 2008–2010. J. Med. Virol. 2011, 83, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Rönnelid, Y.; Bonkoungou, I.J.O.; Ouedraogo, N.; Barro, N.; Svensson, L.; Nordgren, J. Norovirus and rotavirus in children hospitalised with diarrhoea after rotavirus vaccine introduction in Burkina Faso. Epidemiol. Infect. 2020, 148, e245. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J.; Bonkoungou, I.J.O.; Nitiema, L.W.; Sharma, S.; Ouermi, D.; Simpore, J.; Barro, N.; Svensson, L. Rotavirus in diarrheal children in rural Burkina Faso: High prevalence of genotype G6P[6]. Infect. Genet. Evol. 2012, 12, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Bonkoungou, I.J.O.; Ouédraogo, N.; Tamini, L.; Teguera, R.K.; Yaméogo, P.; Drabo, M.K.; Medah, I.; Barro, N.; Sharma, S.; Svensson, L.; et al. Rotavirus and norovirus in children with severe diarrhea in Burkina Faso before rotavirus vaccine introduction. J. Med. Virol. 2018, 90, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Asmah, R.H.; Green, J.; Armah, G.E.; Gallimore, C.I.; Gray, J.J.; Iturriza-Gόmara, M.; Anto, F.; Oduro, A.; Binka, F.N.; Brown, D.W.G.; et al. Rotavirus G and P Genotypes in Rural Ghana. J. Clin. Microbiol. 2001, 39, 1981–1984. [Google Scholar] [CrossRef]
- Armah, G.E.; Pager, C.T.; Asmah, R.H.; Anto, F.R.; Oduro, A.R.; Binka, F.; Steele, D. Prevalence of unusual human rotavirus strains in Ghanaian children. J. Med. Virol. 2001, 63, 67–71. [Google Scholar] [CrossRef]
- Binka, F.N.; Anto, F.K.; Oduro, A.R.; Awini, E.A.; Nazzar, A.K.; Armah, G.E.; Asmah, R.H.; Hall, A.J.; Cutts, F.; Alexander, N.; et al. Incidence and risk factors of paediatric rotavirus diarrhoea in northern Ghana. Trop. Med. Int. Health 2003, 8, 840–846. [Google Scholar] [CrossRef]
- Enweronu-Laryea, C.C.; Sagoe, K.W.; Damanka, S.; Lartey, B.; Armah, G. E Rotavirus genotypes associated with childhood severe acute diarrhoea in southern Ghana: A cross-sectional study. Virol. J. 2013, 10, 287. [Google Scholar] [CrossRef]
- Lartey, B.L.; Damanka, S.; Dennis, F.E.; Enweronu-Laryea, C.C.; Addo-Yobo, E.; Ansong, D.; Kwarteng-Owusu, S.; Sagoe, K.W.; Mwenda, J.M.; Diamenu, S.K.; et al. Rotavirus strain distribution in Ghana pre- and post- rotavirus vaccine introduction. Vaccine 2018, 36, 7238–7242. [Google Scholar] [CrossRef]
- Letsa, V.; Damanka, S.; Dennis, F.; Lartey, B.; Armah, G.E.; Betrapally, N.; Gautam, R.; Esona, M.D.; Bowen, M.D.; Quaye, O. Distribution of rotavirus genotypes in the postvaccine introduction era in Ashaiman, Greater Accra Region, Ghana, 2014–2016. J. Med. Virol. 2019, 91, 2025–2028. [Google Scholar] [CrossRef]
- Damanka, S.; Adiku, T.K.; Armah, G.E.; Rodrigues, O.; Donkor, E.S.; Nortey, D.; Asmah, R. Rotavirus Infection in Children with Diarrhea at Korle-Bu Teaching Hospital, Ghana. Jpn. J. Infect. Dis. 2016, 69, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Eugen-Olsen, J.; Aaby, P.; Mølbak, K.; Rodrigues, A.; Fischer, T.K. Characterisation of rotavirus strains among hospi-talised and non-hospitalised children in Guinea-Bissau, 2002: A high frequency of mixed infections with serotype G8. J. Clin. Virol. 2005, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Boni-Cisse, C.; Meite, S.; Mlan, A.B.; Zaba, F.; N’guessan, R.; Lepri, N.A.; Lartey, B. Genotypic characterization of rotavirus in children under 5 years circulating in Côte D’Ivoire from 2010 to 2013. Virol. J. 2018, 15, 78. [Google Scholar] [CrossRef]
- Page, A.L.; Jusot, V.; Mamaty, A.A.; Adamou, L.; Kaplon, J.; Pothier, P.; Djibo, A.; Manzo, M.L.; Toure, B.; Langendorf, C.; et al. Rotavirus Surveillance in Urban and Rural Areas of Niger, April 2010–March 2012. Emerg. Infect. Dis. 2014, 20, 573–580. [Google Scholar] [CrossRef]
- Audu, R.; Omilabu, S.A.; de Beer, M.; Peenze, I.; Steele, A.D. Diversity of human rotavirus VP6, VP7, and VP4 in Lagos State, Nigeria. J. Health Popul. Nutr. 2002, 20, 59–64. [Google Scholar] [PubMed]
- Ianiro, G.; Delogu, R.; Baba, M.; Oderinde, B.S.; Dawurung, J.; Ruggeri, F.M.; Fiore, L. Molecular characterization of group A rotavirus strains detected in children with diarrhea admitted to Nigerian hospitals in 2013. Arch. Virol. 2015, 160, 1511–1517. [Google Scholar] [CrossRef]
- Ayolabi, C.I. Genetic diversity of rotavirus strains in children with diarrhea in Lagos, Nigeria. Asian Pac. J. Trop. Dis. 2016, 6, 517–520. [Google Scholar] [CrossRef]
- Uzoma, E.B.; Chukwubuikem, C.; Omoyibo, E.; Tagbo, O. Rota virus genotypes and the clinical severity of Diarrhoea among children under 5 years of age. Niger. Postgrad. Med. J. 2016, 23, 1–5. [Google Scholar] [CrossRef]
- Japhet, M.O.; Famurewa, O.; Iturriza-Gomara, M.; Adesina, O.A.; Opaleye, O.O.; Niendorf, S.; Bock, C.T.; Marques, A.M. Group A rotaviruses circulating prior to a national immunization programme in Nigeria: Clinical manifestations, high G12P[8] frequency, intra-genotypic divergence of VP4 and VP7. J. Med. Virol. 2017, 90, 239–249. [Google Scholar] [CrossRef]
- Amadu, D.O.; Abdullahi, I.N.; Emeribe, A.U.; Musa, P.O.; Olayem, L.; Yunusa, T.; Okechukwu, C.E.; Salami, M.O. Molecular characterization of rotavirus genotype-A in children with acute diarrhea attending a tertiary hospital in Ilorin, Nigeria. Int. J. Health Allied Sci. 2019, 8, 187–192. [Google Scholar]
- Jere, K.C.; Sawyerr, T.; Seheri, L.M.; Peenze, I.; Page, N.A.; Geyer, A.; Steele, A.D. A first report on the characterization of rotavirus strains in Sierra Leone. J. Med. Virol. 2011, 83, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Armah, G.E.; Steele, A.D.; Esona, M.D.; Akran, V.A.; Nimzing, L.; Pennap, G. Diversity of Rotavirus Strains Circulating in West Africa from 1996 to 2000. J. Infect. Dis. 2010, 202, S64–S71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esona, M.D.; Armah, G.E.; Steele, A.D. Rotavirus VP4 and VP7 Genotypes Circulating in Cameroon: Identification of Unusual Types. J. Infect. Dis. 2010, 202, S205–S211. [Google Scholar] [CrossRef] [PubMed]
- Boula, A.; Waku-Kouomou, D.; Njiki Kinkela, M.; Esona, M.D.; Kemajou, G.; Mekontso, D.; Seheri, M.; Ndze, V.N.; Emah, I.; Ela, S.; et al. Molecular surveillance of rotavirus strains circulating in Yaoundé, Cameroon, September 2007–December 2012. Infect. Genet. Evol. 2014, 28, 470–475. [Google Scholar] [CrossRef]
- Ndze, V.N.; Papp, H.; Achidi, E.A.; Gonsu, K.H.; László, B.; Farkas, S.; Kisfali, P.; Melegh, B.; Esona, M.D.; Bowen, M.D.; et al. One year survey of human rotavirus strains suggests the emergence of genotype G12 in Cameroon. J. Med. Virol. 2013, 85, 1485–1490. [Google Scholar] [CrossRef]
- Banga-Mingo, V.; Waku-Kouomou, D.; Gody, J.C.; Esona, M.D.; Yetimbi, J.F.; Mbary-Daba, R.; Dahl, B.A.; Dimanche, L.; Koyazegbe, T.D.; Tricou, V.; et al. Molecular surveillance of rotavirus infection in Bangui, Central African Republic, October 2011–September 2013. Infect. Genet. Evol. 2014, 28, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Moure, U.A.E.; Banga-Mingo, V.; Gody, J.C.; Mwenda, J.M.; Fandema, J.; Waku-Kouomou, D.; Manengu, C.; Koyazegbe, T.D.; Esona, M.D.; Bowen, M.D.; et al. Emergence of G12 and G9 rotavirus genotypes in the Central African Republic, January 2014 to February 2016. BMC Res. Notes 2018, 11, 5. [Google Scholar] [CrossRef]
- Mayindou, G.; Ngokana, B.; Sidibé, A.; Moundélé, V.; Koukouikila-Koussounda, F.; Christevy Vouvoungui, J.; Kwedi Nolna, S.; Velavan, T.P.; Ntoumi, F. Molecular epidemiology and surveillance of circulating rotavirus and adenovirus in Congolese children with gastroenteritis. J. Med. Virol. 2016, 88, 596–605. [Google Scholar] [CrossRef]
- Kabue, J.P.; Peenze, I.; de Beer, M.; Esona, M.D.; Lunfungula, C.; Biamungu, M.; Simba, T.R.; Tamfum, J.J.M.; Steele, A.D. Characterization of Human Rotavirus Recovered from Children with Acute Diarrhea in Kinshasa, Democratic Republic of Congo. J. Infect. Dis. 2010, 202, S193–S197. [Google Scholar] [CrossRef]
- Pukuta, E.S.; Esona, M.D.; Nkongolo, A.; Seheri, M.; Makasi, M.; Nyembwe, M.; Mondonge, V.; Dahl, B.A.; Mphahlele, M.J.; Cavallaro, K.; et al. Molecular Surveillance of Rotavirus Infection in the Democratic Republic of the Congo August 2009 to June 2012. Pediatr. Infect. Dis. J. 2014, 33, 355–359. [Google Scholar] [CrossRef]
- Istrate, C.; Sharma, S.; Nordgren, J.; Videira e Castro, S.; Lopes, Â.; Piedade, J.; Zaky, A.; Lima, A.; Neves, E.; Veiga, J.; et al. High rate of detection of G8P[6] rotavirus in children with acute gastroenteritis in São Tomé and Príncipe. Arch. Virol. 2015, 160, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.; Teka, T.; Kassa, T.B.; Seheri, M.; Beyene, B.M.; Teshome, B.; Kebede, F.; Habtamu, A.; Maake, L.M.; Kassahun, A.M.; et al. Hospital-based Surveillance for Rotavirus Gastroenteritis in Children Younger Than 5 Years of Age in Ethiopia. Pediatr. Infect. Dis. J. 2014, 33, S28–S33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gelaw, A.; Pietsch, C.; Liebert, U.G. Molecular epidemiology of rotaviruses in Northwest Ethiopia after national vaccine introduction. Infect. Genet. Evol. 2018, 65, 300–307. [Google Scholar] [CrossRef]
- Nyangao, J.; Page, N.; Esona, M.; Peenze, I.; Gatheru, Z.; Tukei, P.; Steele, A.D. Characterization of Human Rotavirus Strains from Children with Diarrhea in Nairobi and Kisumu, Kenya, between 2000 and 2002. J. Infect. Dis. 2010, 202, S187–S192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wandera, E.A.; Mohammad, S.; Bundi, M.; Nyangao, J.; Galata, A.; Kathiiko, C.; Odoyo, E.; Guyo, S.; Miring’u, G.; Komoto, S.; et al. Impact of rotavirus vaccination on rotavirus hospitalisation rates among a resource-limited rural population in Mbita, Western Kenya. Trop. Med. Int. Health 2018, 23, 425–432. [Google Scholar] [CrossRef]
- Raini, S.; Nyangao, J.; Kombich, J.; Sang, C.; Gikonyo, J.; Ongus, J.; Odari, E. Human Rotavirus Group a Serotypes Causing Gastroenteritis in Children Less Than 5 Years and HIV-Infected Adults in Viwandani Slum, Nairobi. Ethiop. J. Health Sci. 2015, 25, 39–46. [Google Scholar] [CrossRef]
- Kiulia, N.M.; Nyaga, M.M.; Seheri, M.L.; Wolfaardt, M.; van Zyl, W.B.; Esona, M.D.; Irimu, G.; Inoti, M.; Gatinu, B.W.; Njenga, P.K.; et al. Rotavirus G and P types circulating in the eastern region of Kenya: Predominance of G9 and emergence of G12 genotypes. Pediatr. Infect Dis. J. 2014, 33, S85–S88. [Google Scholar] [CrossRef]
- Wandera, E.A.; Mohammad, S.; Komoto, S.; Maeno, Y.; Nyangao, J.; Ide, T.; Kathiiko, C.; Odoyo, E.; Tsuji, T.; Taniguchi, K.; et al. Molecular epidemiology of rotavirus gastroenteritis in Central Kenya before vaccine introduction, 2009-2014. J. Med. Virol. 2016, 89, 809–817. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Gondwe, J.S.; Broadhead, R.L.; Molyneux, M.E.; Woods, P.A.; Bresee, J.S.; Glass, R.I.; Gentsch, J.R.; Hart, C.A. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: Predominance of novel P[6]G8 strains. J. Med. Virol. 1999, 57, 308–312. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Gondwe, J.S.; Graham, S.M.; Thindwa, B.D.M.; Dove, W.; Broadhead, R.L.; Molyneux, M.E.; Hart, C.A. Rotavirus Strain Diversity in Blantyre, Malawi, from 1997 to 1999. J. Clin. Microbiol. 2001, 39, 836–843. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Ngwira, B.M.; Dove, W.; Thindwa, B.D.M.; Turner, A.M.; Broadhead, R.L.; Molyneux, M.E.; Hart, C.A. Epidemiology of Rotavirus Infection in Children in Blantyre, Malawi, 1997–2007. J. Infect. Dis. 2010, 202, S168–S174. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Ngwira, B.; Witte, D.; Mwapasa, M.; Dove, W.; Cunliffe, N. Surveillance of rotavirus gastro-enteritis in children in Blantyre, Malawi. Ann. Trop. Paediatr. 2013, 33, 42–45. [Google Scholar] [CrossRef] [PubMed]
- João, E.D.; Munlela, B.; Chissaque, A.; Chilaúle, J.; Langa, J.; Augusto, O.; Boene, S.S.; Anapakala, E.; Sambo, J.; Guimarães, E.; et al. Molecular Epidemiology of Rotavirus A Strains Pre- and Post-Vaccine (Rotarix®) Introduction in Mozambique, 2012–2019: Emergence of Genotypes G3P[4] and G3P[8]. Pathogens 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Chissaque, A.; Bauhofer, A.F.L.; Cossa-Moiane, I.; Sitoe, E.; Munlela, B.; João, E.D.; Langa, J.S.; Chilaúle, J.J.; Boene, S.S.; Cassocera, M.; et al. Rotavirus A infection in pre- and post-vaccine period: Risk factors, genotypes distribution by vaccination status and age of children in Nampula Province, Northern Mozambique (2015–2019). PLoS ONE 2021, 16, e0255720. [Google Scholar] [CrossRef]
- Manjate, F.; João, E.D.; Chirinda, P.; Garrine, M.; Vubil, D.; Nobela, N.; Kotloff, K.; Nataro, J.P.; Nhampossa, T.; Acácio, S.; et al. Molecular Epidemiology of Rotavirus Strains in Symptomatic and Asymptomatic Children in Manhiça District, Southern Mozambique 2008–2019. Viruses 2022, 14, 134. [Google Scholar] [CrossRef]
- Hokororo, A.; Kidenya, B.R.; Seni, J.; Mapaseka, S.; Mphahlele, J.; Mshana, S.E. Predominance of Rotavirus G1[P8] Genotype among Under-Five Children with Gastroenteritis in Mwanza, Tanzania. J. Trop. Pediatr. 2014, 60, 393–396. [Google Scholar] [CrossRef][Green Version]
- Odiit, A.M.M.; Mulindwa, A.B.; Nalumansi, E.B.; Mphahlele, M.J.; Seheri, L.M.; Mwenda, J.M.; Kisakye, A. Rotavirus Prevalence and Genotypes Among Children Younger Than 5 Years With Acute Diarrhea at Mulago National Referral Hospital, Kampala, Uganda. Pediatr. Infect. Dis. J. 2014, 33, S41–S44. [Google Scholar] [CrossRef]
- Bwogi, J.; Malamba, S.; Kigozi, B.; Namuwulya, P.; Tushabe, P.; Kiguli, S.; Byarugaba, D.K.; Desselberger, U.; Iturriza-Gomara, M.; Karamagi, C. The epidemiology of rotavirus disease in under-five-year-old children hospitalized with acute diarrhea in central Uganda, 2012–2013. Arch. Virol. 2016, 161, 999–1003. [Google Scholar] [CrossRef]
- Simwaka, J.; Seheri, M.; Mulundu, G.; Kaonga, P.; Mwenda, J.M.; Chilengi, R.; Mpabalwani, E.; Munsaka, S. Rotavirus breakthrough infections responsible for gastroenteritis in vaccinated infants who presented with acute diarrhoea at University Teaching Hospitals, Children’s Hospital in 2016, in Lusaka Zambia. PLoS ONE 2021, 16, e0246025. [Google Scholar] [CrossRef]
- Mukaratirwa, A.; Berejena, C.; Nziramasanga, P.; Ticklay, I.; Gonah, A.; Nathoo, K.; Manangazira, P.; Mangwanya, D.; Marembo, J.; Mwenda, J.M.; et al. Distribution of rotavirus genotypes associated with acute diarrhoea in Zimbabwean children less than five years old before and after rotavirus vaccine introduction. Vaccine 2018, 36, 7248–7255. [Google Scholar] [CrossRef]
- Mukaratirwa, A.; Berejena, C.; Nziramasanga, P.; Shonhai, A.; Mamvura, T.S.; Chibukira, P.; Mucheuki, I.; Mangwanya, D.; Kamupota, M.; Manangazira, P.; et al. Epidemiologic and genotypic characteristics of rotavirus strains detected in children less than 5 years of age with gastroenteritis treated at 3 pediatric hospitals in Zimbabwe during 2008–2011. Pediatr. Infect. Dis. J. 2014, 33, S45–S48. [Google Scholar] [CrossRef] [PubMed]
- Mokomane, M.; Esona, M.D.; Bowen, M.D.; Tate, J.E.; Steenhoff, A.P.; Lechiile, K.; Gaseitsiwe, S.; Seheri, L.M.; Magagula, N.B.; Weldegebriel, G.; et al. Diversity of Rotavirus Strains Circulating in Botswana before and after introduction of the Monovalent Rotavirus Vaccine. Vaccine 2019, 37, 6324–6328. [Google Scholar] [CrossRef] [PubMed]
- Page, N.; Pager, C.; Steele, A.D. Characterization of Rotavirus Strains Detected in Windhoek, Namibia during 1998–1999. J. Infect. Dis. 2010, 202, S162–S167. [Google Scholar] [CrossRef]
- Seheri, L.M.; Page, N.; Dewar, J.B.; Geyer, A.; Nemarude, A.L.; Bos, P.; Esona, M.; Steele, A.D. Characterization and Molecular Epidemiology of Rotavirus Strains Recovered in Northern Pretoria, South Africa during 2003–2006. J. Infect. Dis. 2010, 202, S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Padilla, E.; Grais, R.F.; Guerin, P.J.; Steele, A.D.; Burny, M.E.; Luquero, F.J. Burden of disease and circulating serotypes of rotavirus infection in sub-Saharan Africa: Systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 567–576. [Google Scholar] [CrossRef]
- Seheri, M.; Nemarude, L.M.; Peenze, I.M.; Netshifhefhe, L.M.; Nyaga, M.M.M.; Ngobeni, H.G.M.; Maphalala, G.M.; Maake, L.L.M.; Steele, A.D.; Mwenda, J.M.; et al. Update of Rotavirus Strains Circulating in Africa From 2007 Through 2011. Pediatr. Infect. Dis. J. 2014, 33, S76–S84. [Google Scholar] [CrossRef]
- Santos, N.; Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2004, 15, 29–56. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/rmv.448 (accessed on 6 July 2022). [CrossRef]
- Iturriza-Gómara, M.; Kang, G.; Gray, J. Rotavirus genotyping: Keeping up with an evolving population of human rotaviruses. J. Clin. Virol. 2004, 31, 259–265. [Google Scholar] [CrossRef]
- Dennehy, P.H. Rotavirus Vaccines: An Overview. Clin. Microbiol. Rev. 2008, 21, 198–208. [Google Scholar] [CrossRef]
- Burnett, E.; Jonesteller, C.L.; Tate, J.E.; Yen, C.; Parashar, U.D. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J. Infect. Dis. 2017, 215, 1666–1672. [Google Scholar] [CrossRef]
- Steele, A.D.; Armah, G.E.; Mwenda, J.M.; Kirkwood, C.D. The Full Impact of Rotavirus Vaccines in Africa Has Yet to Be Realized. Clin. Infect. Dis. 2023, 76, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Hoxie, I.; Dennehy, J.J. Rotavirus A Genome Segments Show Distinct Segregation and Codon Usage Patterns. Viruses 2021, 13, 1460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).