Stability of Genotube® Swabs for African Swine Fever Virus Detection Using Loop-Mediated Isothermal (LAMP) Laboratory Testing on Samples Stored without Refrigeration
Abstract
:1. Introduction
2. Materials and Methods
2.1. ASFV LAMP Detection
2.2. Preparation of ASFV Synthetic DNA Spiked Genotube® Swabs for Assessment of Stability over Four Weeks at Room Temperature (Five Replicates at Five Time Points) at Agriculture Victoria
2.3. Preparation of Naturally Acquired ASFV-Positive-Serum Genotube® Swabs for Assessment of Stability over Four Weeks at Room Temperature (Dili)
2.4. Preparation of ASFV-Negative-Serum Genotube® Swabs for Assessment of Stability over Four Weeks at Room Temperature (Five Replicates at Five Time Points) at Agriculture Victoria
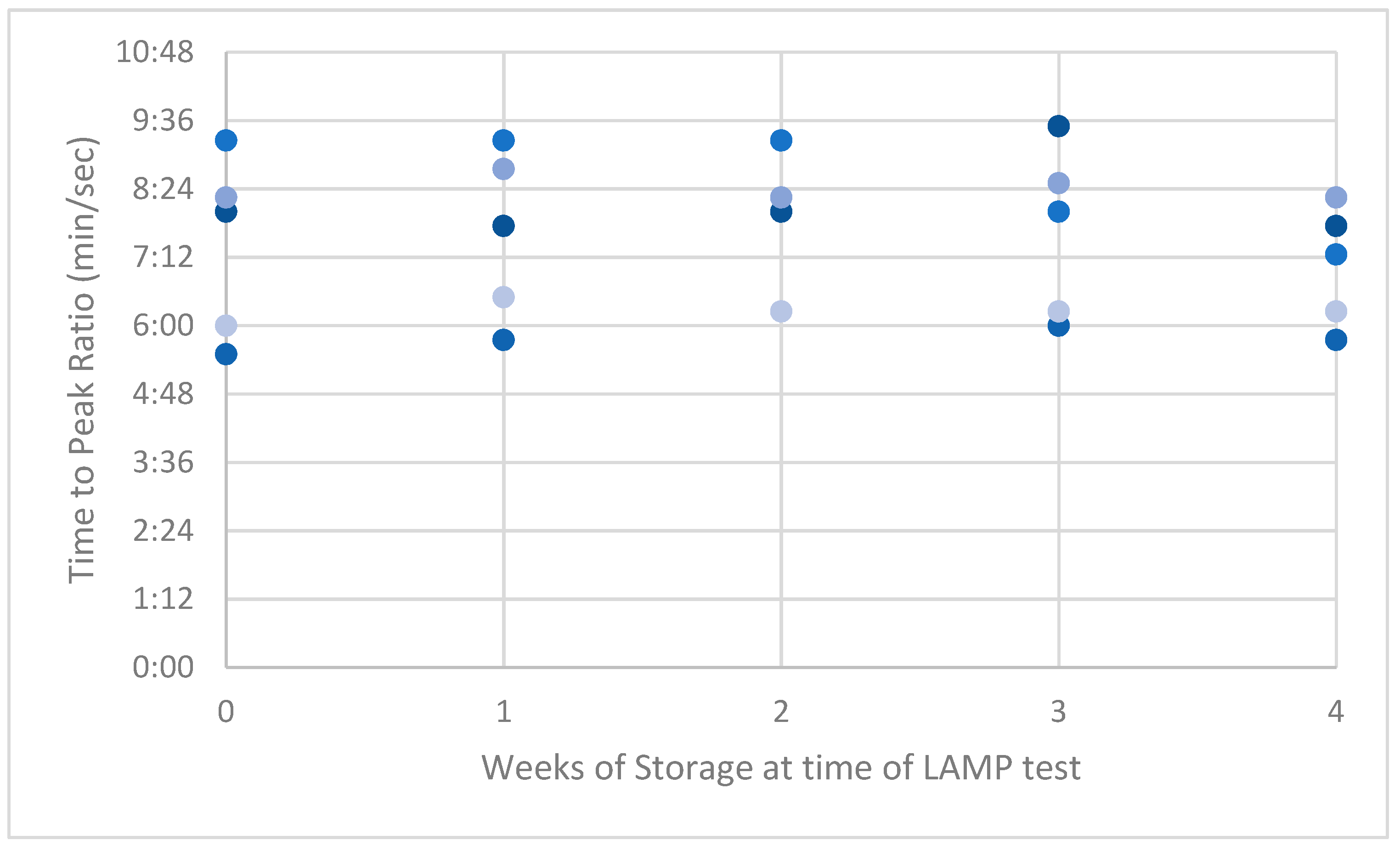
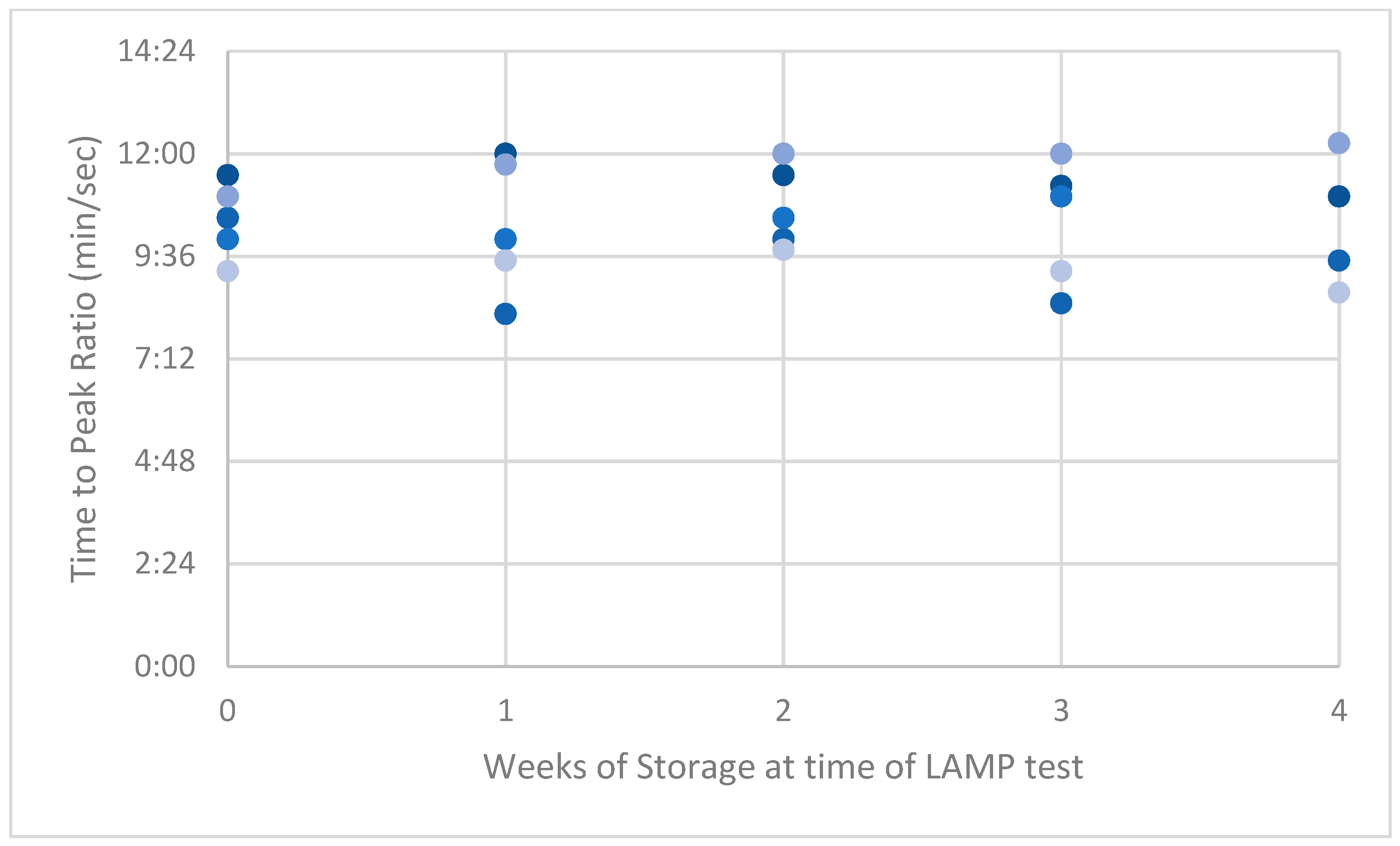
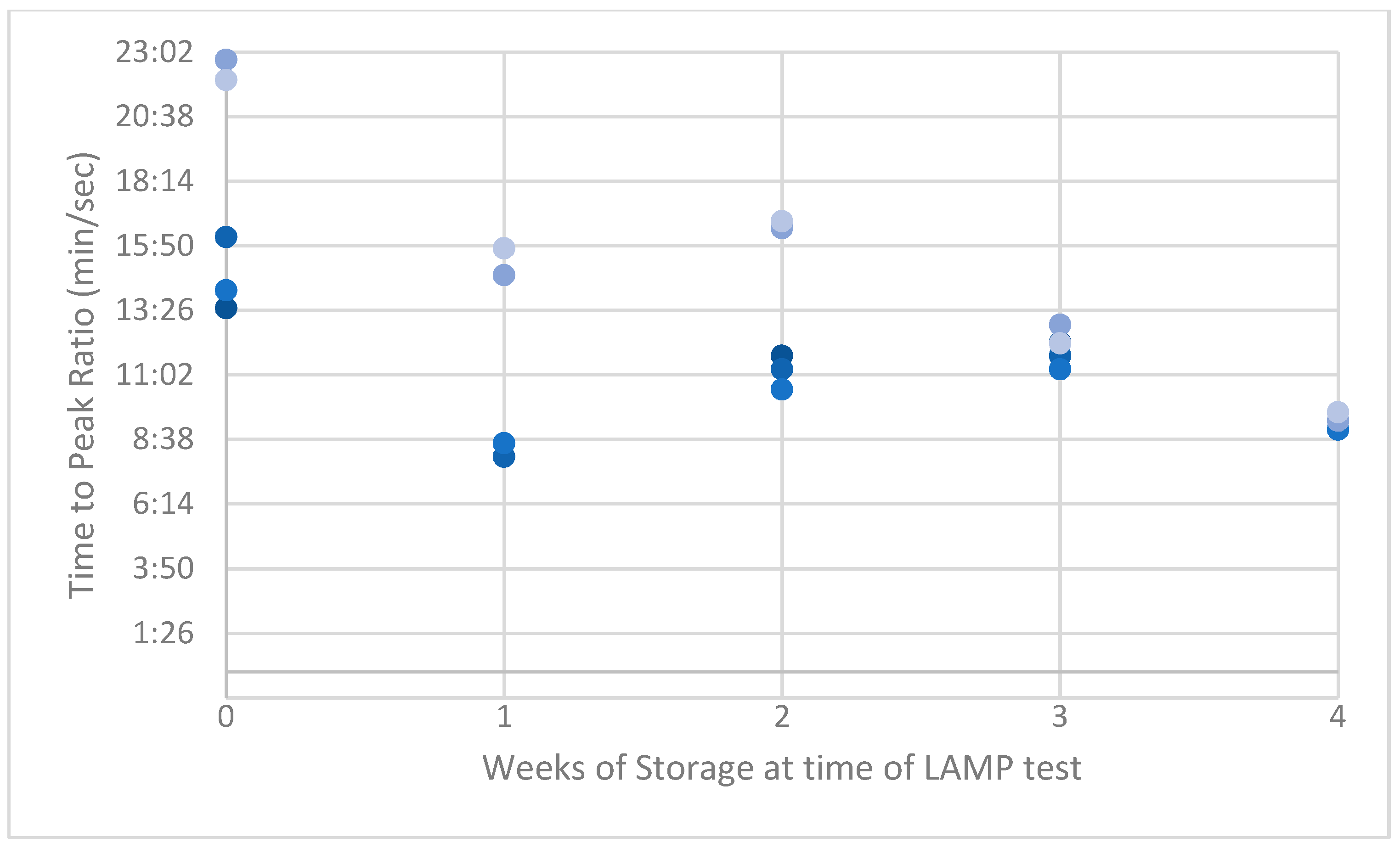
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- World Organisation for Animal Health. OIE Technical Disease Card: African Swine Fever; World Organisation for Animal Health: Paris, France, 2022. [Google Scholar]
- FAO. African Swine Fever (ASF) Situation Update in Asia & Pacific. 11 January 2024. Available online: https://www.fao.org/animal-health/situation-updates/asf-in-asia-pacific#:~:text=According%20to%20the%20Department%20of,companies%20supplying%20ASF%20vaccines%20to (accessed on 24 January 2024).
- World Organisation for Animal Health African Swine Fever. Available online: https://www.woah.org/en/disease/african-swine-fever/#ui-id-2 (accessed on 8 September 2023).
- Guinat, C.; Vergne, T.; Jurado-Diaz, C.; Sánchez-Vizcaíno, J.M.; Dixon, L.; Pfeiffer, D.U. Effectiveness and practicality of control strategies for African swine fever: What do we really know? Vet. Rec. 2017, 180, 97. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L.; van Heerden, J.; Pfeiffer, D.U.; Oļševskis, E.; Depner, K.; Chenais, E. Innovative Research Offers New Hope for Managing African Swine Fever Better in Resource-Limited Smallholder Farming Settings: A Timely Update. Pathogens 2023, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Hanh, T.X. NAVETCO Researches to Produce ASF Vaccine in Vietnam. Available online: https://vietnamagriculture.nongnghiep.vn/navetco-researches-to-produce-asf-vaccine-in-vietnam-d309586.html (accessed on 24 January 2023).
- James, H.E.; Ebert, K.; McGonigle, R.; Reid, S.M.; Boonham, N.; Tomlinson, J.A.; Hutchings, G.H.; Denyer, M.; Oura, C.A.L.; Dukes, J.P.; et al. Detection of African swine fever virus by loop-mediated isothermal amplification. J. Virol. Methods 2010, 164, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lamberga, K.; Depner, K.; Zani, L.; Oļševskis, E.; Seržants, M.; Ansonska, S.; Šteingolde, Ž.; Bērziņš, A.; Viltrop, A.; Blome, S. A practical guide for strategic and efficient sampling in African swine fever-affected pig farms. Transbound. Emerg. Dis. 2022, 69, e2408–e2417. [Google Scholar] [CrossRef] [PubMed]
- Madden, D.W.; Sunwoo, S.-Y.; Gaudreault, N.N.; Trujillo, J.D.; Morozov, I.; Gallardo, C.; Richt, J.A. Development of a chromatographic lateral flow immunoassay for detection of African swine fever virus antigen in blood. Anim. Dis. 2022, 2, 14. [Google Scholar] [CrossRef]
- Mee, P.T.; Wong, S.; O’riley, K.J.; da Conceição, F.; Jong, J.B.d.C.; Phillips, D.E.; Rodoni, B.C.; Rawlin, G.T.; Lynch, S.E. Field verification of an african swine fever virus loop-mediated isothermal amplification (Lamp) assay during an outbreak in timor-leste. Viruses 2020, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Zhang, J.; Li, N.; Chen, T.; Wang, L.; Zhang, F.; Mi, L.; Zhang, J.; Wang, S.; Wang, Y.; et al. Rapid and Sensitive Recombinase Polymerase Amplification Combined With Lateral Flow Strip for Detecting African Swine Fever Virus. Front. Microbiol. 2019, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Onyilagha, C.; Nguyen, K.; Luka, P.D.; Hussaini, U.; Adedeji, A.; Odoom, T.; Ambagala, A. Evaluation of a lateral flow assay for rapid detection of African swine fever virus in multiple sample types. Pathogens 2022, 11, 138. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Edwards, L.; Batten, C.A. Virological diagnosis of African swine fever—Comparative study of available tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef]
- Petrov, A.; Schotte, U.; Pietschmann, J.; Dräger, C.; Beer, M.; Anheyer-Behmenburg, H.; Goller, K.V.; Blome, S. Alternative sampling strategies for passive classical and African swine fever surveillance in wild boar. Vet. Microbiol. 2014, 173, 360–365. [Google Scholar] [CrossRef]
- Pikalo, J.; Deutschmann, P.; Fischer, M.; Roszyk, H.; Beer, M.; Blome, S. African Swine Fever Laboratory Diagnosis—Lessons Learned from Recent Animal Trials. Pathogens 2021, 10, 177. [Google Scholar] [CrossRef]
- Sastre, P.; Gallardo, C.; Monedero, A.; Ruiz, T.; Arias, M.; Sanz, A.; Rueda, P. Development of a novel lateral flow assay for detection of African swine fever in blood. BMC Vet. Res. 2016, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, P.; Fan, H.; Dang, L.; Wan, W.; Liu, S.; Li, Y.; Yu, W.; Li, X.; Ma, X.; et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Commun. Biol. 2020, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, J.; Wang, Y.; Zhang, M.; Li, P.; Liu, M.; Liu, Y. Development of a real-time loop-mediated isothermal amplification (LAMP) assay and visual LAMP assay for detection of African swine fever virus (ASFV). J. Virol. Methods 2020, 276, 113775. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, L.; Wang, Y.; Yang, Z.; Yao, X.; Peng, B. Development of a Rapid and Sensitive Method for Detection of African Swine Fever Virus Using Loop-Mediated Isothermal Amplification. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Guo, J.; Li, D.; Wang, L.; Wang, X.; Xing, G.; Deng, R.; Zhang, G. An Isothermal Molecular Point of Care Testing for African Swine Fever Virus Using Recombinase-Aided Amplification and Lateral Flow Assay without the Need to Extract Nucleic Acids in Blood. Front. Cell. Infect. Microbiol. 2021, 11, 633763. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Zani, L.; Schwaiger, T.; Nurmoja, I.; Viltrop, A.; Vilem, A.; Beer, M.; Blome, S. Simplifying sampling for African swine fever surveillance: Assessment of antibody and pathogen detection from blood swabs. Transbound. Emerg. Dis. 2018, 65, e165–e172. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, A.; Kobialka, R.M.; Ssekitoleko, J.; Okuni, J.B.; Blome, S.; Abd El Wahed, A.; Truyen, U. Rapid Extraction and Detection of African Swine Fever Virus DNA Based on Isothermal Recombinase Polymerase Amplification Assay. Viruses 2021, 13, 1731. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.S.; Morais, O.; Cargill, C.; Parke, C.R.; Urlings, A. First steps in managing the challenge of African Swine Fever in Timor-Leste. One Health 2020, 10, 100151. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- van Bockel, D.; Munier, C.M.L.; Turville, S.; Badman, S.G.; Walker, G.; Stella, A.O.; Aggarwal, A.; Yeang, M.; Condylios, A.; Kelleher, A.D.; et al. Evaluation of Commercially Available Viral Transport Medium (VTM) for SARS-CoV-2 Inactivation and Use in Point-of-Care (POC) Testing. Viruses 2020, 12, 1208. [Google Scholar] [CrossRef]
- Braae, U.C.; Johansen, M.V.; Ngowi, H.A.; Rasmussen, T.B.; Nielsen, J.; Uttenthal, Å. Detection of African Swine Fever Virus DNA in Blood Samples Stored on FTA Cards from Asymptomatic Pigs in Mbeya Region, Tanzania. Transbound. Emerg. Dis. 2015, 62, 87–90. [Google Scholar] [CrossRef]
- Michaud, V.; Gil, P.; Kwiatek, O.; Prome, S.; Dixon, L.; Romero, L.; Le Potier, M.F.; Arias, M.; Couacy-Hymann, E.; Roger, F.; et al. Long-term storage at tropical temperature of dried-blood filter papers for detection and genotyping of RNA and DNA viruses by direct PCR. J. Virol. Methods 2007, 146, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.Y.; Kim, U.; Oh, S.W. Diverse methods of reducing and confirming false-positive results of loop-mediated isothermal amplification assays: A review. Anal. Chim. Acta 2023, 1280, 341693. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher Scientific Inc. Evaluation of GenoTubes for Transport, Storage and Extraction of Nucleic Acids from Porcine Oral Fluids. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fposters%2Fevaluation-genotubes-porcine-oral-fluids-poster.pdf (accessed on 7 December 2023).
- Skalina, K.A.; Goldstein, D.Y.; Jaffar, S.; Hahm, E.; Narlieva, M.; Szymczak, W.; Fox, A.S. Extended storage of SARS-CoV-2 nasopharyngeal swabs does not negatively impact results of molecular-based testing across three clinical platforms. J. Clin. Pathol. 2022, 75, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Parikh, B.A.; Wallace, M.A.; McCune, B.T.; Burnham, C.-A.D.; Anderson, N.W. The Effects of “Dry Swab” Incubation on SARS-CoV-2 Molecular Testing. J. Appl. Lab. Med. 2021, 6, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.A.; Baumann, R.E.; Borillo, G.A.; Kagan, R.M.; Batterman, H.J.; Galdzicka, M.M.; Marlowe, E.M. Evaluation of Transport Media and Specimen Transport Conditions for the Detection of SARS-CoV-2 by Use of Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2020, 58, e00708-20. [Google Scholar] [CrossRef]
- Millar, J.; Morais, O.; Da Silva, H.; Hick, P.; Foster, A.; Jong, J.B.d.C.; Pereira, A.; Ting, S.; da Conceição, F.; Toribio, J.-A.L.M.L. Community engagement strengthens pig disease knowledge and passive surveillance in Timor-Leste. Front. Vet. Sci. 2023, 9, 1024094. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Colling, A.; Gurung, R.B.; Allen, J. The potential of diagnostic point-of-care tests (POCTs) for infectious and zoonotic animal diseases in developing countries: Technical, regulatory and sociocultural considerations. Transbound. Emerg. Dis. 2021, 68, 1835–1849. [Google Scholar] [CrossRef]
| Constituent Name | Sample (Wells 1–3) | Sample and IAC (Wells 4–6) | Positive Control (Well 7) | Negative Control (Well 8) |
|---|---|---|---|---|
| LAMP ASFV master mix | 15 µL | 15 µL | 15 µL | 15 µL |
| 10× primer mix | 2.5 µL | 2.5 µL | 2.5 µL | 2.5 µL |
| Template | 7.5 µL | 7.5 µL | 0 | 0 |
| IAC | 0 | 2.0 µL | 0 | 0 |
| ASFV synthetic positive | 0 | 0 | 2.0 µL | 0 |
| Water | 2.0 µL | 0 | 7.5 µL | 9.5 µL |
| Total | 27 µL | 27 µL | 27 µL | 27 µL |
| Swab ID | Time 0 (25 August 2022) | Week 1 (1 September 2022) | Week 2 (8 September 2022) | Week 3 (15 September 2022) | Week 4 (22 September 2022) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | Ta | PR | Ta | PR | Ta | PR | Ta | PR | Ta | |
| 1 | 8:00 | 86.9 | 5:30 | 87.5 | 9:15 | 86.9 | 8:15 | 86.8 | 6:00 | 86.9 |
| 2 | 7:45 | 86.9 | 5:45 | 87.5 | 9:15 | 86.8 | 8:45 | 86.8 | 6:30 | 86.9 |
| 3 | 8:00 | 86.8 | 6:15 | 87.4 | 9:15 | 86.7 | 8:15 | 86.7 | 6:15 | 86.8 |
| 4 | 9:30 | 86.9 | 6:00 | 87.4 | 8:00 | 86.7 | 8:30 | 86.7 | 6:15 | 86.8 |
| 5 | 7:45 | 86.9 | 5:45 | 87.4 | 7:15 | 86.8 | 8:15 | 86.8 | 6:15 | 86.9 |
| Positive control | 11:00 | 87.2 | 3:45 | 87.5 | 3:45 | 87 | 4:30 | 87 | 3:45 | 87.1 |
| Negative control | - | - | - | - | - | - | - | - | - | - |
| Swab ID | Time 0 (25 August 2022) | Week 1 (1 September 2022) | Week 2 (8 September 2022) | Week 3 (15 September 2022) | Week 4 (22 September 2022) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | Ta | PR | Ta | PR | Ta | PR | Ta | PR | Ta | |
| 1 | 11:30 | 86.9 | 10:30 | 87.5 | 10:00 | 86.8 | 11:00 | 86.8 | 9:15 | 86.9 |
| 2 | 12:00 | 87.0 | 8:15 | 87.5 | 10:00 | 86.8 | 11:45 | 86.8 | 9:30 | 86.8 |
| 3 | 11:30 | 87.0 | 10:00 | 87.5 | 10:30 | 86.8 | 12:00 | 86.8 | 9:45 | 86.8 |
| 4 | 11:15 | 87.0 | 8:30 | 87.5 | 11:00 | 86.8 | 12:00 | 86.8 | 9:15 | 86.8 |
| 5 | 11:00 | 87.0 | 9:30 | 87.5 | 12:15 | 86.8 | 12:15 | 86.8 | 8:45 | 86.8 |
| Positive control | 4:15 | 87.0 | 4:00 | 87.6 | 4:15 | 87 | 4:30 | 87 | 3:45 | 87 |
| Negative control | - | - | - | - | - | - | - | - | - | - |
| Swab ID | Time 0 (11 March 2023) | Week 1 (17 March 2023) | Week 2 (24 March 2023) | Week 3 (31 March 2023) | Week 4 (7 April 2023) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | Ta | PR | Ta | PR | Ta | PR | Ta | PR | Ta | |
| 1 | 13:31 | 86.76 | 8:00 | 86.97 | 11:45 | 86.90 | 12:15 | 86.75 | 9:00 | 86.83 |
| 2 | 16:10 | 86.65 | 8:00 | 86.84 | 11:15 | 86:60 | 11:45 | 86.83 | 9:00 | 86.69 |
| 3 | 14:11 | 86.59 | 8:30 | 86.90 | 10:30 | 86.84 | 11:15 | 86.75 | 9:00 | 86.76 |
| 1 + IAC | 6:32 | 89.30 | 4:30 | 89.44 | 6:00 | 89:29 | 5:15 | 89.36 | 5:15 | 89.29 |
| 2 + IAC | 7:34 | 89.10 | 5:00 | 89.29 | 6:15 | 89:34 | 5:45 | 89.20 | 5:15 | 89.29 |
| 3 + IAC | 6:52 | 89.01 | 5:30 | 89.29 | 6:15 | 89.38 | 5:15 | 89.30 | 5:30 | 89.34 |
| Positive | 5:12 | 86.65 | 5:30 | 86.83 | 5:45 | 86.87 | 4:30 | 86.93 | 8:45 | 86.18 |
| Negative | 2:00 | 24:15 | ||||||||
| 4 | 22:45 | 86.55 | 14:45 | 86.73 | 16:30 | 86.86 | 12:54 | 86.66 | 9:20 | 86.45 |
| 5 | 22:00 | 86.68 | 15:45 | 86.70 | 16:45 | 86.79 | 12:13 | 86.71 | 9:39 | 86.56 |
| 4 + IAC | 9:00 | 89.31 | 8:30 | 89.30 | 9:30 | 89.28 | 6:51 | 89.15 | 5:43 | 89.21 |
| 5 + IAC | 10:00 | 89.30 | 10:00 | 89.28 | 10:00 | 89.23 | 6:37 | 89.26 | 5:22 | 89.40 |
| Positive control | 6:15 | 86.88 | 7:45 | 86.84 | 9:00 | 86.88 | 5:56 | 86.88 | 5:06 | 86.61 |
| Negative control | - | - | - | - | - | - | - | - | - | - |
| Swab ID | Time 0 (6 October 2023) | Week 1 (13 October 2023) | Week 2 (20 October 2023) | Week 3 (27 October 2023) | Week 4 (3 October 2023) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | Ta | PR | Ta | PR | Ta | PR | Ta | PR | Ta | |
| 1 | - | - | - | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - | - | - | - |
| 3 | - | - | - | - | - | - | - | - | - | - |
| 1 + IAC | 4:00 | 89.89 | 4:45 | 89.90 | 4:00 | 89.89 | 4:00 | 89.90 | 6:00 | 89.90 |
| 2 + IAC | 4:00 | 89.94 | 4:45 | 89.94 | 4:00 | 89.94 | 4:00 | 89.84 | 6:00 | 89.85 |
| 3 + IAC | 4:00 | 89.99 | 4:45 | 89.89 | 4:00 | 89.99 | 4:00 | 89.90 | 6:00 | 89.90 |
| Positive control | 2:45 | 87.57 | 3:15 | 87.57 | 2:45 | 87.57 | 2:45 | 87.58 | 4:00 | 87.57 |
| Negative control | - | - | - | - | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - | - | - | 20:00 | - |
| 4 + IAC | 4:00 | 89.91 | 4:45 | 89.81 | 4:00 | 89.91 | 3:45 | 89.80 | 4:00 | 89.90 |
| 5 + IAC | 4:15 | 89.80 | 5:00 | 89.71 | 4:15 | 89.80 | 3:45 | 89.70 | 3:45 | 89.89 |
| Positive control | 2:45 | 87.56 | 3:15 | 87.47 | 2:45 | 87.56 | 2:30 | 87.37 | 2:45 | 87.47 |
| Negative control | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, D.; Conceicao, F.d.; Jong, J.B.d.C.; Rawlin, G.; Mee, P. Stability of Genotube® Swabs for African Swine Fever Virus Detection Using Loop-Mediated Isothermal (LAMP) Laboratory Testing on Samples Stored without Refrigeration. Viruses 2024, 16, 263. https://doi.org/10.3390/v16020263
Phillips D, Conceicao Fd, Jong JBdC, Rawlin G, Mee P. Stability of Genotube® Swabs for African Swine Fever Virus Detection Using Loop-Mediated Isothermal (LAMP) Laboratory Testing on Samples Stored without Refrigeration. Viruses. 2024; 16(2):263. https://doi.org/10.3390/v16020263
Chicago/Turabian StylePhillips, Dianne, Felisiano da Conceicao, Joanita Bendita da Costa Jong, Grant Rawlin, and Peter Mee. 2024. "Stability of Genotube® Swabs for African Swine Fever Virus Detection Using Loop-Mediated Isothermal (LAMP) Laboratory Testing on Samples Stored without Refrigeration" Viruses 16, no. 2: 263. https://doi.org/10.3390/v16020263






