Performance of Three Anti-SARS-CoV-2 Anti-S and One Anti-N Immunoassays for the Monitoring of Immune Status and Vaccine Response
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Population
2.2. Specimen Collection and Storage
2.3. Sample Analysis
2.4. Anti-SARS-CoV-2 Spike (Anti-S) or Receptor Binding Domain (Anti-RBD) Antibody Assays
2.4.1. Beckman
2.4.2. Siemens
2.4.3. Ortho
2.5. Anti-SARS-CoV-2 Nucleocapsid (Anti-N) Antibody Assay
Bio-Rad
2.6. Data Analysis
3. Results
3.1. Participants Demographics
3.2. Assay Performance for Study Population
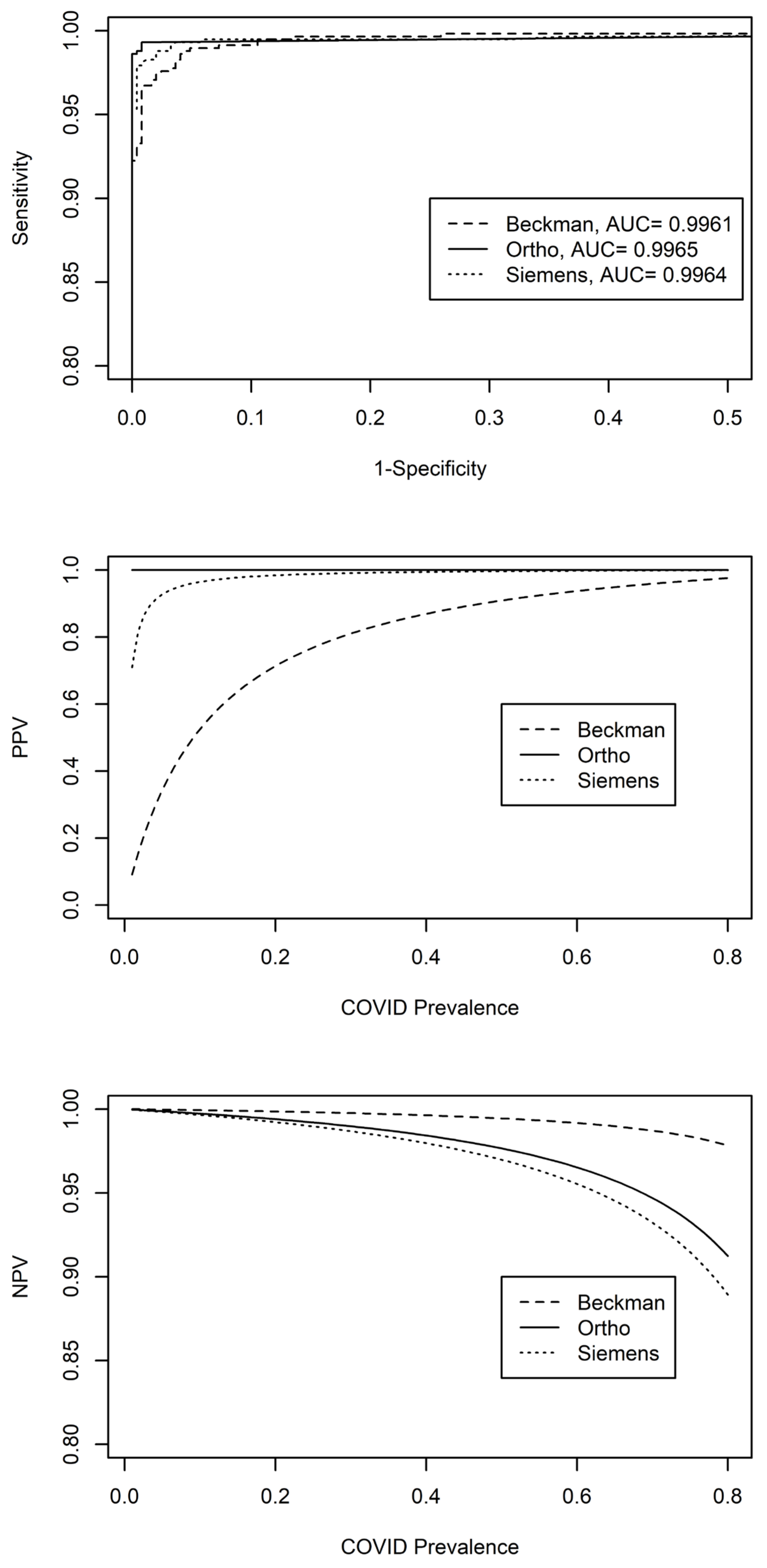
3.2.1. Anti-S Assays
3.2.2. Anti-N Assay
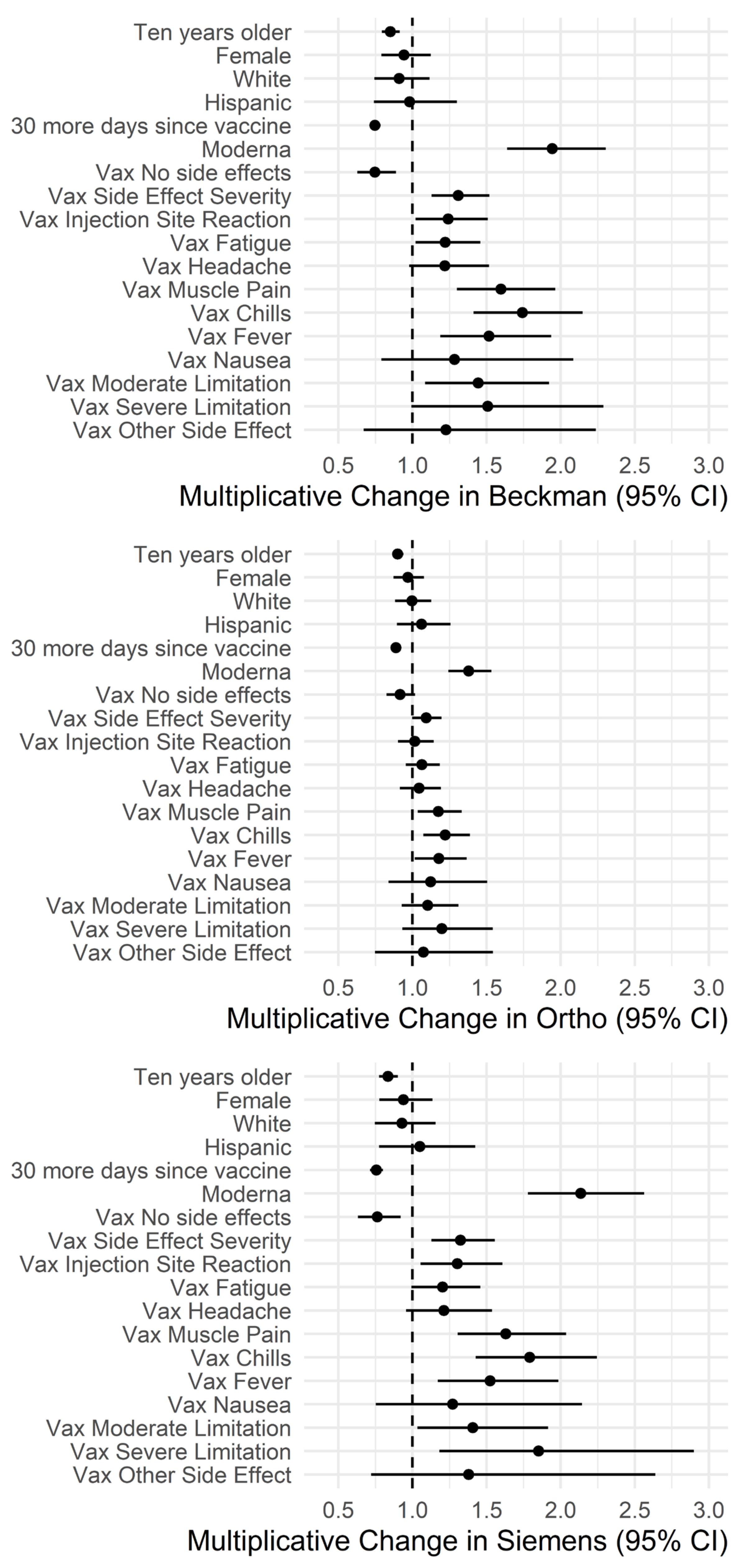
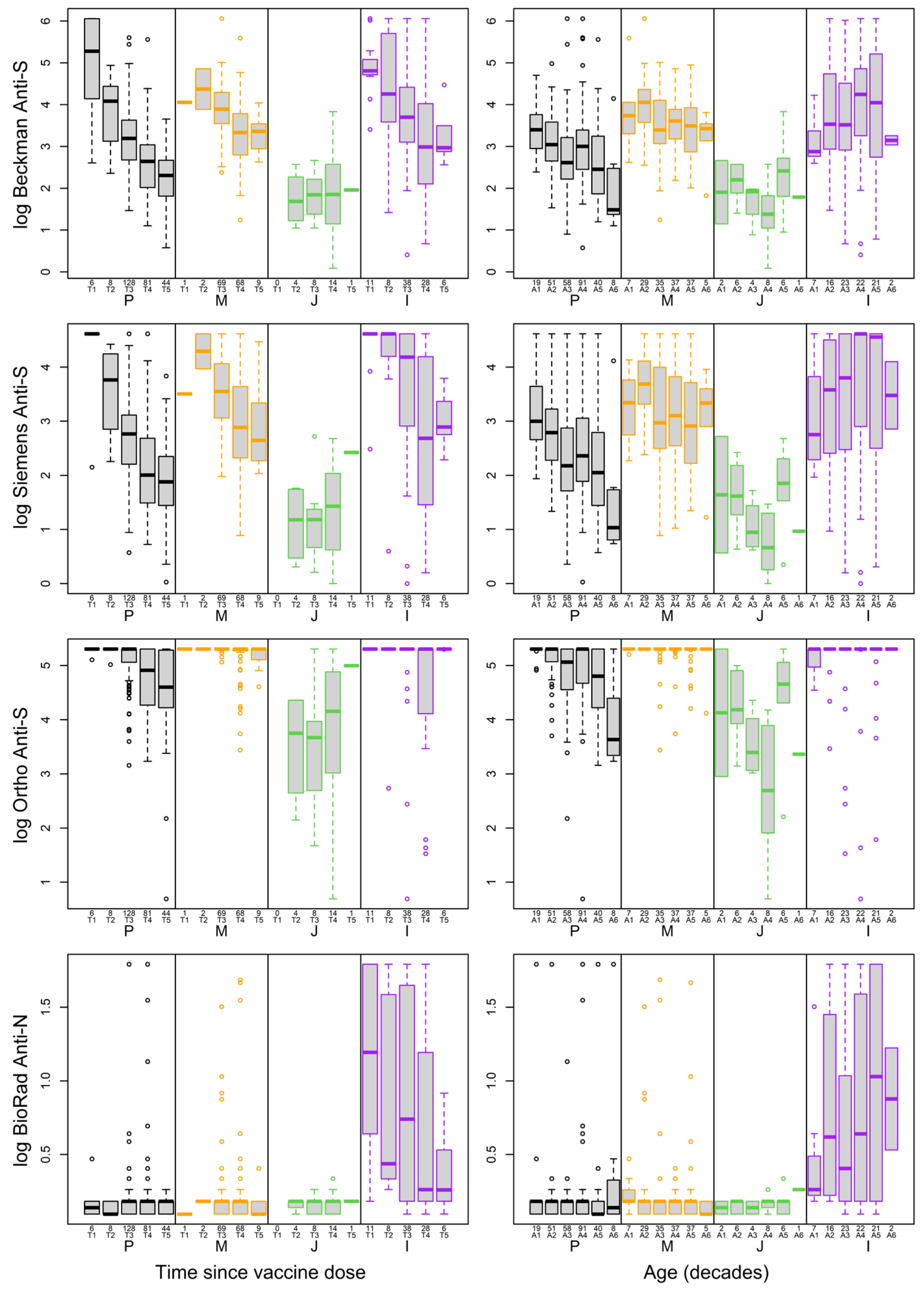
3.2.3. Impact of Vaccine Types on Antibody Levels
3.3. Impact of Demographics and Side Effects on Antibody Levels
3.4. Impact of Age and Time since Vaccine on Antibody Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X.; et al. Association of Public Health Interventions with the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA 2020, 323, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Rabi, F.A.; Al Zoubi, M.S.; Kasasbeh, G.A.; Salameh, D.M.; Al-Nasser, A.D. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.V.; Wiencek, J.; Meng, Q.H.; Theel, E.S.; Babic, N.; Sepiashvili, L.; Pecora, N.D.; Slev, P.; Cameron, A.; Konforte, D.; et al. AACC Practical Recommendations for Implementing and Interpreting SARS-CoV-2 Emergency Use Authorization and Laboratory-Developed Test Serologic Testing in Clinical Laboratories. Clin. Chem. 2021, 67, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Slev, P.; Wheeler, S.; Couturier, M.R.; Wong, S.J.; Kadkhoda, K. The Role of Antibody Testing for SARS-CoV-2: Is There One? J. Clin. Microbiol. 2020, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.-C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kruttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imohl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef]
- Coste, A.T.; Jaton, K.; Papadimitriou-Olivgeris, M.; Greub, G.; Croxatto, A. Comparison of SARS-CoV-2 serological tests with different antigen targets. J. Clin. Virol. 2021, 134, 104690. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Hou, Y.L.; Li, D.T.; Li, F.Z. Diagnostic efficacy of anti-SARS-CoV-2 IgG/IgM test for COVID-19: A meta-analysis. J. Med. Virol. 2021, 93, 366–374. [Google Scholar] [CrossRef]
- Choe, J.Y.; Kim, J.W.; Kwon, H.H.; Hong, H.L.; Jung, C.Y.; Jeon, C.H.; Park, E.J.; Kim, S.K. Diagnostic performance of immunochromatography assay for rapid detection of IgM and IgG in coronavirus disease 2019. J. Med. Virol. 2020, 92, 2567–2572. [Google Scholar] [CrossRef]
- Haselmann, V.; Kittel, M.; Gerhards, C.; Thiaucourt, M.; Eichner, R.; Costina, V.; Neumaier, M. Comparison of test performance of commercial anti-SARS-CoV-2 immunoassays in serum and plasma samples. Clin. Chim. Acta 2020, 510, 73–78. [Google Scholar] [CrossRef]
- Walker, G.J.; Naing, Z.; Ospina Stella, A.; Yeang, M.; Caguicla, J.; Ramachandran, V.; Isaacs, S.R.; Agapiou, D.; Bull, R.A.; Stelzer-Braid, S.; et al. SARS Coronavirus-2 Microneutralisation and Commercial Serological Assays Correlated Closely for Some but Not All Enzyme Immunoassays. Viruses 2021, 13, 247. [Google Scholar] [CrossRef]
- Macedo, A.C.L.; Prestes, G.D.S.; Colonetti, T.; Candido, A.C.R.; Uggioni, M.L.R.; Gomes, A.C.; Silva, F.R.; Ceretta, L.B.; Grande, A.J.; da Rosa, M.I. A systematic review and meta-analysis of the accuracy of SARS-CoV-2 IGM and IGG tests in individuals with COVID-19. J. Clin. Virol. 2022, 148, 105121. [Google Scholar] [CrossRef]
- Hörber, S.; Soldo, J.; Relker, L.; Jürgens, S.; Guther, J.; Peter, S.; Lehmann, R.; Peter, A. Evaluation of three fully-automated SARS-CoV-2 antibody assays. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 2113–2120. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Peserico, D.; Pighi, L.; Nitto, S.D.; Henry, B.M.; Porru, S.; Lippi, G. Comprehensive assessment of humoral response after Pfizer BNT162b2 mRNA COVID-19 vaccination: A three-case series. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; Nitto, S.D.; Gianfilippi, G.L.; Lippi, G. Monitoring of the immunogenic response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers with Snibe SARS-CoV-2 S-RBD IgG chemiluminescent immunoassay. Clin. Chem. Lab. Med. (CCLM) 2021, 59, e377–e379. [Google Scholar] [CrossRef]
- Farnsworth, C.W.; O’Neil, C.A.; Dalton, C.; McDonald, D.; Vogt, L.; Hock, K.; Arter, O.; Wallace, M.A.; Muenks, C.; Amor, M.; et al. Association between SARS-CoV-2 Symptoms, Ct Values, and Serological Response in Vaccinated and Unvaccinated Healthcare Personnel. J. Appl. Lab. Med. 2023, 8, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, A.; Miller, J.J.; Fabros, A.; Brinc, D.; Hall, V.; Pinzon, N.; Ierullo, M.; Ku, T.; Ferreira, V.H.; Kumar, D.; et al. Evaluation of Three Anti-SARS-CoV-2 Serologic Immunoassays for Post-Vaccine Response. J. Appl. Lab. Med. 2021, 7, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Jeong, S.; Lee, S.K.; Cho, E.-J.; Hyun, J.; Park, M.-J.; Song, W.; Kim, H.S. Quantitative Analysis of Anti-N and Anti-S Antibody Titers of SARS-CoV-2 Infection after the Third Dose of COVID-19 Vaccination. Vaccines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef]
- Asamoah-Boaheng, M.; Goldfarb, D.M.; Barakauskas, V.; Kirkham, T.L.; Demers, P.A.; Karim, M.E.; Lavoie, P.M.; Marquez, A.C.; Jassem, A.N.; Jenneson, S.; et al. Evaluation of the Performance of a Multiplexed Serological Assay in the Detection of SARS-CoV-2 Infections in a Predominantly Vaccinated Population. Microbiol. Spectr. 2022, 10, e01454-21. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek, J.; Skorupa, M.; Goroncy, A.; Jarkiewicz-Tretyn, J.; Wypych, A.; Sandomierz, D.; Jarkiewicz-Tretyn, A.; Dejewska, J.; Ciechanowska, K.; Pałgan, K.; et al. Anti-SARS-CoV-2 IgG against the S Protein: A Comparison of BNT162b2, mRNA-1273, ChAdOx1 nCoV-2019 and Ad26.COV2.S Vaccines. Vaccines 2022, 10, 99. [Google Scholar] [CrossRef]
- Maeda, K.; Amano, M.; Uemura, Y.; Tsuchiya, K.; Matsushima, T.; Noda, K.; Shimizu, Y.; Fujiwara, A.; Takamatsu, Y.; Ichikawa, Y.; et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci. Rep. 2021, 11, 22848. [Google Scholar] [CrossRef] [PubMed]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Meza-López, C.; Wang, L.-F.; Machado Sulbarán, A.C.; Torres-Hernández, P.C.; Bedolla-Barajas, M.; De la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef]
- Di Resta, C.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination—An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522. [Google Scholar] [CrossRef]
- Yang, H.S.; Costa, V.; Racine-Brzostek, S.E.; Acker, K.P.; Yee, J.; Chen, Z.; Karbaschi, M.; Zuk, R.; Rand, S.; Sukhu, A.; et al. Association of Age with SARS-CoV-2 Antibody Response. JAMA Netw. Open 2021, 4, e214302. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, Y.; Shashar, M.; Lellouche, J.; Yana, M.; Yakubovich, D.; Sharon, N. Occurrence of BNT162b2 Vaccine Adverse Reactions Is Associated with Enhanced SARS-CoV-2 IgG Antibody Response. Vaccines 2021, 9, 977. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021; 10, 100208. [Google Scholar] [CrossRef]
- Format:Caitlin, R.; Ondracek, P.D.; Stacy, E.F.; Melanson, M.D.; Doan, L.; Karen, M.; Schulz, D.C.; Kleinman, S.; Zhao, Z.; Kumanovics, A.; et al. Large-Scale Scientific Study Led by a Professional Organization during the COVID-19 Pandemic: Operations, Best Practices, and Lessons Learned. J. Appl. Lab. Med. 2023, jfad089. [Google Scholar] [CrossRef]
- Wu, A.H.B.; Apple, F.; Love, S.A.; Koch, D.; Myers, G.L.; Christenson, R.H.; on behalf of the AACC’s Biomarkers of Acute Cardiovascular Disease Division. Creation of a Universal Sample Bank for Determining the 99th Percentile for Cardiac Troponin Assays. J. Appl. Lab. Med. 2017, 1, 711–719. [Google Scholar] [CrossRef]
- Melanson, S.E.F.; Zhao, Z.; Kumanovics, A.; Love, T.; Meng, Q.H.; Wu, A.H.B.; Apple, F.; Ondracek, C.R.; Schulz, K.M.; Wiencek, J.R.; et al. Tolerance for three commonly administered COVID-19 vaccines by healthcare professionals. Front. Public Health 2022, 10, 975781. [Google Scholar] [CrossRef]
- Zhao, Z.; Kumanovics, A.; Love, T.; Melanson, S.E.F.; Meng, Q.H.; Wu, A.H.B.; Wiencek, J.; Apple, F.S.; Ondracek, C.R.; Koch, D.D.; et al. T Cell Responses Correlate with Self-Reported Disease Severity and Neutralizing Antibody Responses Predict Protection against SARS-CoV-2 Breakthrough Infection. Viruses 2023, 15, 709. [Google Scholar] [CrossRef] [PubMed]
- Platelia SARS-CoV-2 Total Ab—Instructions for Use. Available online: https://www.fda.gov/media/137493/download (accessed on 18 December 2023).
- SARS-CoV-2 IgG (sCOVG)—Instructions for Use. Available online: https://www.fda.gov/media/150238/download (accessed on 18 December 2023).
- ACCESS SARS-CoV-2 IgG—Instructions for Use. Available online: https://www.fda.gov/media/139627/download (accessed on 18 December 2023).
- VITROS Anti-SARS-CoV-2 IgG Quantitative—Instructions for Use. Available online: https://www.fda.gov/media/150675/download (accessed on 18 December 2023).
- Mekonnen, D.; Mengist, H.M.; Derbie, A.; Nibret, E.; Munshea, A.; He, H.; Li, B.; Jin, T. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, e2181. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination with BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Adjobimey, T.; Meyer, J.; Sollberg, L.; Bawolt, M.; Berens, C.; Kovacevic, P.; Trudic, A.; Parcina, M.; Hoerauf, A. Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization with Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 Vaccines. Front. Immunol. 2022, 13, 917905. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. COVID-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Bonfante, F.; Pagliari, M.; Bortolami, A.; Negrini, D.; Zuin, S.; Bozzato, D.; Cosma, C.; Sciacovelli, L.; Plebani, M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine 2020, 62, 103101. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.; Grebe, E.; Sulaeman, H.; Di Germanio, C.; Dave, H.; Kelly, K.; Biggerstaff, B.J.; Crews, B.O.; Tran, N.; Jerome, K.R.; et al. Evaluation of Commercially Available High-Throughput SARS-CoV-2 Serologic Assays for Serosurveillance and Related Applications. Emerg. Infect. Dis. 2022, 28, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Saadat, S.; Rikhtegaran Tehrani, Z.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and Neutralization Antibody Titers after a Single Vaccine Dose in Health Care Workers Previously Infected with SARS-CoV-2. JAMA 2021, 325, 1467–1469. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef] [PubMed]
- Alfego, D.; Sullivan, A.; Poirier, B.; Williams, J.; Adcock, D.; Letovsky, S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine 2021, 36, 100902. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat. Commun. 2021, 12, 6250. [Google Scholar] [CrossRef]


| COVID-19 Samples | Pre-COVID-19 Samples | ||
|---|---|---|---|
| N | 580 | 247 | |
| Age a | 50 (39–59) | 41 (32–53) | |
| Gender (Male %) | 270 (46.6%) | 111 (44.9%) | |
| Race (White %) | 437 (75.3%) | 187 (75.7%) | |
| Ethnicity (Hispanic/Latino %) | 81 (14.0%) | 31 (12.6%) | |
| Vaccine type | Pfizer | 336 (57.9%) | - |
| Moderna | 175 (30.2%) | - | |
| J&J | 43 (7.4%) | - | |
| Others b | 26 (4.5%) | - | |
| Days since last dose of vaccine a | 174 (148–214) | - | |
| Any side effects (%) | 299 (51.6%) | - | |
| Moderate limitations (%) | 275 (47.4%) | - | |
| Severe limitations (%) | 24 (4.1%) | - | |
| COVID-19 a | Pre-COVID-19 | Sensitivity | Specificity | ||
|---|---|---|---|---|---|
| A. Overall Population | |||||
| Beckman b | Pos | 575 | 24 | 99.5% | 90.0% |
| Neg | 3 | 215 | |||
| Ortho | Pos | 566 | 0 | 97.6% | 100% |
| Neg | 14 | 247 | |||
| Siemens | Pos | 562 | 1 | 96.9% | 99.6% |
| Neg | 18 | 246 | |||
| B. Breakdown by Vaccine Types c | |||||
| Beckman | |||||
| Pfizer (n = 267) | Pos | 266 | 24 | 99.6% | 90.0% |
| Moderna (n = 150) | Pos | 150 | 100% | ||
| J&J (n = 27) | Pos | 26 | 96.3% | ||
| Booster (n = 26) | Pos | 26 | 100% | ||
| Prev. Infection (n = 91) | Pos | 88 | 96.7% | ||
| Ortho | |||||
| Pfizer (n = 267) | Pos | 265 | 0 | 99.3% | 100% |
| Moderna (n = 150) | Pos | 150 | 100% | ||
| J&J (n = 27) | Pos | 22 | 81.5% | ||
| Booster (n = 26) | Pos | 26 | 100% | ||
| Prev. Infection (n = 91) | Pos | 85 | 93.4% | ||
| Siemens | |||||
| Pfizer (n = 267) | Pos | 264 | 1 | 98.9% | 99.6% |
| Moderna (n = 150) | Pos | 150 | 100% | ||
| J&J (n = 27) | Pos | 19 | 70.4% | ||
| Booster (n = 26) | Pos | 26 | 100% | ||
| Prev. Infection (n = 91) | Pos | 85 | 93.4% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.V.; Kumanovics, A.; Wiencek, J.; Melanson, S.E.F.; Love, T.; Wu, A.H.B.; Zhao, Z.; Meng, Q.H.; Koch, D.D.; Apple, F.S.; et al. Performance of Three Anti-SARS-CoV-2 Anti-S and One Anti-N Immunoassays for the Monitoring of Immune Status and Vaccine Response. Viruses 2024, 16, 292. https://doi.org/10.3390/v16020292
Zhang YV, Kumanovics A, Wiencek J, Melanson SEF, Love T, Wu AHB, Zhao Z, Meng QH, Koch DD, Apple FS, et al. Performance of Three Anti-SARS-CoV-2 Anti-S and One Anti-N Immunoassays for the Monitoring of Immune Status and Vaccine Response. Viruses. 2024; 16(2):292. https://doi.org/10.3390/v16020292
Chicago/Turabian StyleZhang, Y. Victoria, Attila Kumanovics, Joesph Wiencek, Stacy E. F. Melanson, Tanzy Love, Alan H. B. Wu, Zhen Zhao, Qing H. Meng, David D. Koch, Fred S. Apple, and et al. 2024. "Performance of Three Anti-SARS-CoV-2 Anti-S and One Anti-N Immunoassays for the Monitoring of Immune Status and Vaccine Response" Viruses 16, no. 2: 292. https://doi.org/10.3390/v16020292
APA StyleZhang, Y. V., Kumanovics, A., Wiencek, J., Melanson, S. E. F., Love, T., Wu, A. H. B., Zhao, Z., Meng, Q. H., Koch, D. D., Apple, F. S., Ondracek, C. R., & Christenson, R. H. (2024). Performance of Three Anti-SARS-CoV-2 Anti-S and One Anti-N Immunoassays for the Monitoring of Immune Status and Vaccine Response. Viruses, 16(2), 292. https://doi.org/10.3390/v16020292






