Abstract
The frequency of respiratory viruses in people living with HIV (PLHIV) and their impact on lung function remain unclear. We aimed to determine the frequency of respiratory viruses in bronchoalveolar lavage and induced sputum samples in PLHIV and correlate their presence with lung function. A prospective cohort of adults hospitalized in Medellín between September 2016 and December 2018 included three groups: group 1 = people diagnosed with HIV and a diagnosis of community-acquired pneumonia (CAP), group 2 = HIV, and group 3 = CAP. People were followed up with at months 1, 6, and 12. Clinical, microbiological, and spirometric data were collected. Respiratory viruses were detected by multiplex RT-PCR. Sixty-five patients were included. At least 1 respiratory virus was identified in 51.9%, 45.1%, and 57.1% of groups 1, 2 and 3, respectively. Among these, 89% of respiratory viruses were detected with another pathogen, mainly Mycobacterium tuberculosis (40.7%) and Pneumocystis jirovecii (22.2%). The most frequent respiratory virus was rhinovirus (24/65, 37%). On admission, 30.4% of group 1, 16.6% of group 2, and 50% of group 3 had airflow limitation, with alteration in forced expiratory volume at first second in both groups with pneumonia compared to HIV. Respiratory viruses are frequent in people diagnosed with HIV, generally coexisting with other pathogens. Pulmonary function on admission was affected in patients with pneumonia, improving significantly in the 1st, 6th, and 12th months after CAP onset.
1. Introduction
Respiratory diseases are the leading cause of morbidity and mortality in individuals with human immunodeficiency virus infection in both the pre- and post-HAART (highly active antiretroviral therapy) stage [1,2,3,4]. The overall rate of community-acquired pneumonia (CAP) in adults without human immunodeficiency virus (HIV) is as high as 11 cases per 1000 person-years (PY) and increases with age and the presence of comorbidities [5,6]. In individuals diagnosed with HIV, the risk of developing pneumonia is 25 times higher than in the general population, increasing with decreasing CD4 count [4,7].
Despite the development of new diagnostic methods and antimicrobial agents, CAP continues to be the leading cause of death of infectious origin in all age groups with and without HIV, with an estimated 3.1 million deaths worldwide in 2012, with mortality attributable to CAP ranging from 1 to 5% with outpatient treatment, 5 to 25% in hospital and up to 50% in ICU [7,8], and in 2011 represented costs of more than 10 billion dollars in the United States [7]. In Colombia, among the causes of hospitalization in people diagnosed with HIV, opportunistic infections are the leading category, with 23 to 30% attributed to tuberculosis, Pneumocystis jirovecii pneumonia accounting for 6 to 21%, and bacterial pneumonia accounting for 14% [8,9]. As in individuals without HIV, identifying the microorganisms involved in pulmonary infection allows for the establishment of measures such as providing adequate targeted treatment [10], de-escalation of broad spectrum antimicrobial therapy, more rational use of antibiotics [11], and implementing prophylaxis and vaccination measures. In individuals living with HIV, not identifying the etiology of pneumonia has been considered an independent mortality factor [10]. Unfortunately, despite the use of all available diagnostic tools, fewer than half of episodes receive a definitive etiological diagnosis, reaching up to 70% in the best-case scenario [4,9,12,13].
In people living with HIV who are diagnosed with bacterial pneumonia, the most frequently identified microorganisms are similar to those of individuals without HIV, first Streptococcus pneumoniae in 20 to 40%, followed by Haemophilus Influenzae (10 to 30%), Staphylococcus aureus and Pseudomonas aeruginosa (3 to 10%). Respiratory viruses (respiratory syncytial virus, parainfluenza virus, influenza virus, human metapneumovirus, adenovirus, coronavirus, and rhinovirus) have traditionally been considered infrequent in individuals living with HIV [14], probably due to the low sensitivity of serological tests in these patients, the difficulty or impossibility of culturing them, and the limited and non-standardized use of molecular biology techniques [4,9,15,16]. Studies using nucleic acid-based testing such as polymerase chain reaction (PCR) have improved the identification of respiratory viruses, making it possible to demonstrate the role of epidemic viruses such as influenza A H1N1, the presence of rhinoviruses, and the identification of others such as cytomegalovirus (CMV) and herpes simplex virus (HSV) [13,17]. However, their prevalence and role in individuals living with HIV, and the role they play in a normal virome, co-pathogens or principal causative pathogens, is still unclear.
HIV infection independently affects lung function. Several studies report that individuals living with HIV have a higher incidence of infectious and non-infectious lung diseases such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, cancer, and pulmonary hypertension, with divergent results for asthma [1,18]. It is generally accepted that HIV infection is an independent risk factor for developing COPD with an odds ratio ranging from 1.47 to 10.93 after adjusting for other variables [19] such as smoking, cannabis or intravenous drug use, sex, age, and other pulmonary infections. More recent longitudinal cohort studies have demonstrated accelerated respiratory function decline and doubling of the incidence of COPD that correlated with unsuppressed viral replication and smoking [20,21]. The development of P. jirovecii pneumonia and CAP is considered an additive factor to lung function impairment, which has been demonstrated in retrospective studies, mostly without follow-up of pulmonary function tests [22]. There are no studies on the effect of atypical bacteria or respiratory viruses on lung function in individuals diagnosed with HIV.
We sought to determine the frequency of respiratory viruses in people diagnosed with HIV and pneumonia, and their effect on lung function. To achieve these objectives, we determined the frequency of respiratory viruses by real-time PCR (RT-PCR) in respiratory samples of bronchoalveolar lavage (BAL) and induced sputum from persons diagnosed with HIV and pneumonia, HIV, and pneumonia, and correlated the presence of these respiratory viruses with airflow limitation and the effect on spirometric variables assessing lung function at 1-, 6-, and 12-month follow-ups.
2. Materials and Methods
A prospective cohort study conducted between September 2016 and December 2018 included three groups: group 1 = people diagnosed with HIV and pneumonia, group 2 = HIV, and group 3 = pneumonia. Participants were recruited in two high-complexity institutions in Medellin, Colombia.
Individuals over 18 years of age who voluntarily agreed to participate in the study and who, according to the assigned group, were diagnosed with HIV or had a diagnosis of community-acquired pneumonia (CAP) were included. For this study, CAP was defined as acute lower respiratory tract infection associated with radiographic changes that were not explained by another condition, and excluding healthcare or ventilator-associated pneumonia. HIV infection was defined as those individuals with serologic demonstration of HIV by ELISA or positive viral load. Exclusion criteria were individuals with hospitalization in the previous two weeks, antibiotic use for more than 72 h in the last week, other causes of known immunosuppression other than HIV (use of prednisone ≥0.3 mg/kg/day for 3 weeks or more or ≥1 mg/kg/day for more than seven days, or its equivalent in other steroids; cytotoxic agents (except low-dose methotrexate: ≤15 mg/week), presence of hematologic malignancies, granulocytopenia <500 cells/mm3), obstructive cancer pneumonia, significant chronic lung disease (cystic fibrosis, severe COPD (forced expiratory volume at first second measured in liters-FEV1 < 50%), bronchiectasis and asthma), orotracheal intubation at study entry, major contraindication to induced sputum collection, inability to perform spirometry due to clinical condition, high probability of loss to follow-up (history of elopement or voluntary discharge, substance-related disorder classified as serious [23], severe mental disorder with progressive and marked deterioration in functioning) [24], not having stable or permanent housing, or living outside the metropolitan area of Medellin.
All hospitalized patients who met the inclusion criteria and who agreed to participate and signed the informed consent form were admitted consecutively. Variables were collected at the time of admission, including demographic information (sex, age, occupation, origin, socioeconomic stratum and health insurance [contributive or subsidized]), comorbidities (COPD, diabetes, renal failure, heart failure, hepatopathies, collagenopathies, neoplasms, pregnancy, hypo- and hypersplenism, epilepsy, altered consciousness or swallowing, previous pneumonia), clinical characteristics (respiratory symptoms, vital signs, weight, height, presence of lymphadenopathy, or organomegaly), vaccination status, previous antibiotic use, smoking, psychoactive substance or alcohol use, radiological and laboratory studies (CD4 count, HIV viral load, electrolytes, renal and liver function, arterial blood gases, and tests for diagnosis of other sexually transmitted and blood-borne infections such as syphilis and hepatitis B and C). The severity of pneumonia was assessed with the pneumonia severity index (PSI) and the CURB-65 [6,25].
During hospitalization, patients underwent routine diagnostic studies according to the concept of their treating physicians. In addition, venous blood and urine samples were collected at admission. Per protocol, all people living with HIV and with a diagnosis of pneumonia (group 1) that required hospitalization underwent BAL performed by the respiratory medicine group of each institution following a standardized protocol [9]. In all groups, we collected induced sputum (IS) samples if the person did not have a major contraindication (history of massive epistaxis necessitating an emergency room visit, history of bleeding disorders, history of heart failure, chest tube drainage for pneumothorax, recent eye surgery, and history of severe asthma requiring treatment in the intensive care unit) following previous research [26]. To detect respiratory viruses, RNAlater® was added to each sample of BAL and IS, thus guaranteeing the stability of the RNA.
Conventional microbiological diagnostic studies were performed at the discretion of the treating physician and included direct studies of respiratory secretions in sputum and/or BAL (Gram, KOH, ZN, methenamine silver, toluidine blue, Wright) [9,27], cultures for aerobic bacteria, fungi and mycobacteria, and molecular tests for tuberculosis (GeneXpert® or Anyplex®). In addition, treating physicians ordered blood cultures, stains and cultures in pleural fluid, lung biopsy, pleura and lymph nodes, PCR for tuberculosis in urine, and urinary antigen for Histoplasma capsulatum in some patients based on their clinical history and findings. All people received treatment accordingly, and the antibiotics and other treatments were decided by the treating physician(s).
Likewise, respiratory viruses were identified in BAL and IS samples by molecular tests. RNA/DNA extraction was performed with the commercial kit RibospinTM vRD (GeneAll Biotechnology Co., Ltd., Seoul, Repulic of Korea) according to the manufacturer’s recommendations. Prior to extraction, the RNAlater® present in the sample was removed by adding the same amount of cold PBS and centrifuging at 5000× g. If remnants were still observed, a second wash was performed. In each extraction run, a negative control was added as a quality control. A multiplex one-step RT-PCR Allplex™ Respiratory Panel 1, 2, and 3 (Seegene®, Seoul, Repulic of Korea) was used to detect respiratory viruses in BAL and IS samples. Allplex™ Respiratory Panel 1 detected influenza A virus (Flu A), influenza A-H1 (Flu A-H1), influenza A-H1pdm09 (Flu A-H1pdm09), influenza A-H3 (Flu A-H3), influenza B virus (Flu B), respiratory syncytial virus A (RSV A), respiratory syncytial virus B (RSV B), and internal control (IC). Allplex™ Respiratory Panel 2 detected adenovirus (AdV), enterovirus (HEV), metapneumovirus (MPV), parainfluenza virus 1 (PIV 1), parainfluenza virus 2 (PIV 2), parainfluenza virus 3 (PIV 3), parainfluenza virus 4 (PIV 4), and internal control (IC). Allplex™ Respiratory Panel 3 detected bocavirus 1/2/3/4 (HBoV), coronavirus 229E (229E), coronavirus NL63 (NL63), coronavirus OC43 (OC43), human rhinovirus (HRV), and internal control (IC).
Each participant underwent simple spirometry at baseline and 1, 6, and 12 months using the Maryland, U.S. FDA (Food and Drug Administration)-approved, EasyOneTM ultrasonic spirometer (ndd Medical Technologies, Zurich, Switzerland) in accordance with the guidelines of the American Thoracic Society and the European Respiratory Society [28], ensuring measurements of forced expiratory volume at first second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio of acceptable quality, from which the best of three tests were selected for analysis [29]. The presence of airflow limitation was established according to the recommendations of the Global Lung Function Initiative (GOLD) guidelines using FEV1/FVC < lower limit of the normal range (LLN) and FEV1/FVC < 0.70, standardized for Latin population [30]. An individual was considered to have airflow limitation when the person had an FEV1/CVF ratio lower than the LLN; this LLN is equivalent to the 5th percentile of the reference population (healthy and non-smoking) standardized by age (equivalent to minus 1.64 z-score). The other definition accepted by these guidelines is the FEV1/FVC ratio < 0.70; however this option tends to underestimate the presence of COPD in young individuals and overestimate it in those older than 45 years, which should be taken into account for the analysis of people diagnosed with HIV who are generally young.
In individuals who met the criteria for airflow limitation, severity was quantified according to the percentage of FEV1% predicted. Severity assessment was defined as the percentage of FEV1 (L) of predicted, and defined as follows: mild ≥ 80%, moderate between 50 to 79%, severe between 30 to 49% and very severe <30%. In addition, independent FEV1 and FVC values and variations were analyzed to identify changes below the definition of airflow limitation (COPD), correlating these results with sex, age, body mass index (BMI), previous pulmonary infection, HIV infection, cigarette smoking, CD4 count, HIV viral load, and presence or absence of respiratory viruses.
Statistical Analysis
We designed a database in Microsoft Access® version 2019, which was used to record sociodemographic, clinical, and microbiological characteristics, including the respiratory viruses identified. We performed descriptive statistics estimating measures of total and relative frequency, and median and interquartile range (IQR, percentile 25–75) because the quantitative variables did not have a normal distribution. The Chi-square test was used to analyze the differences between groups in terms of the presence of airflow limitation (FEV1/FVC < LLN or 0.70) both at admission and at the 1-, 6-, and 12-month follow-ups. In addition, variation in spirometric measures (FEV1, FVC, in liters) and FEV1/FVC ratio were analyzed, using the Friedman test, for minimal changes that did not meet the criteria for airflow limitation. In all comparisons, a p-value < 0.05 was considered significant, and the analyses were performed in IBM SPSS® version 24.0 and Stata® version 14.1.
3. Results
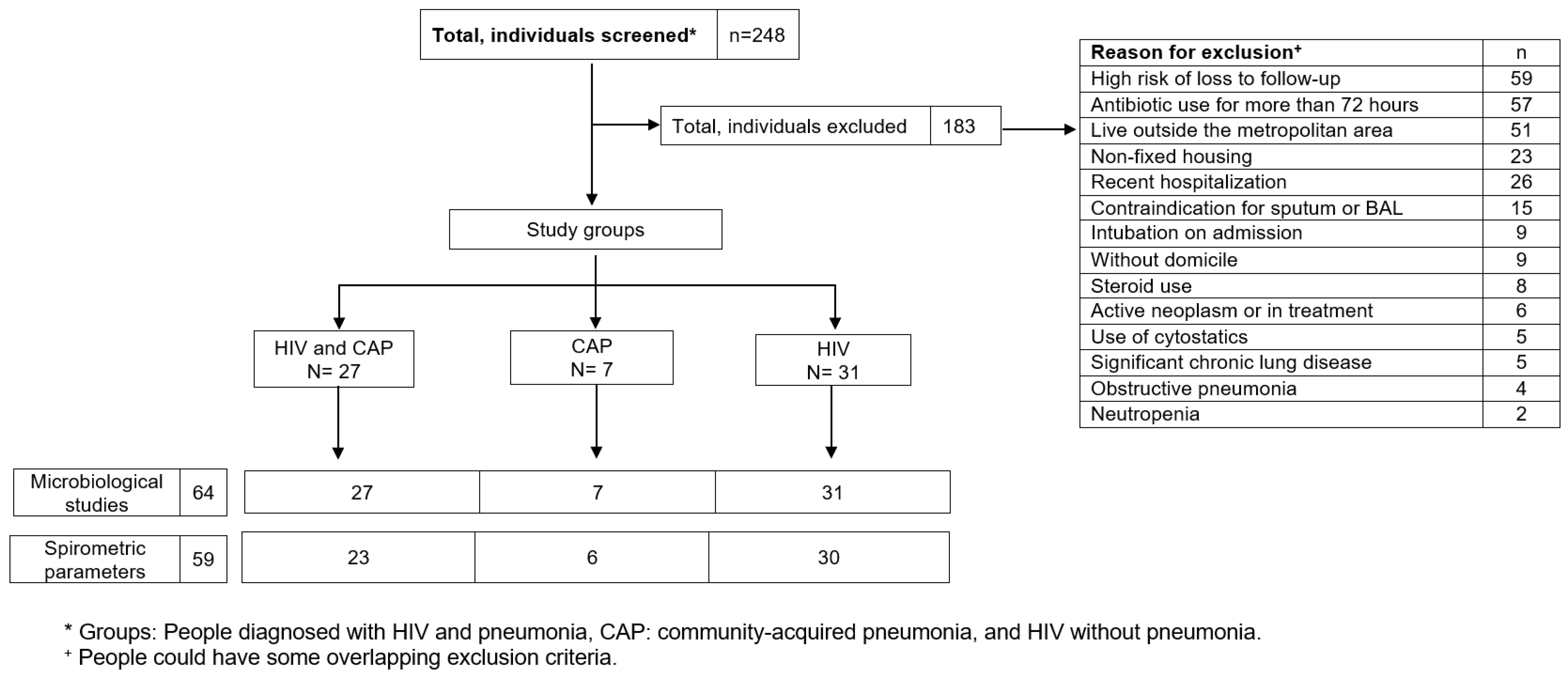
Two hundred and forty-eight (248) people were screened consecutively (Figure 1), of which 65 were included, forming the groups of HIV and pneumonia (n = 27; group 1), HIV (n = 31; group 2), and pneumonia (n = 7; group 3). The main reasons for exclusion were high risk of loss to follow-up, antibiotic use for more than 72 h, and living outside the metropolitan area (Table S1 describes the causes of exclusion).
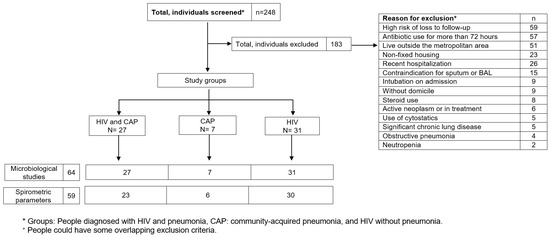
Figure 1.
Patient recruitment and follow-up flowchart.
Of all those included, 71% were men with a median age of 39 years (IQR 27–51); 14% were smokers; 30% had received vaccination against pneumococcus and influenza; 22% had some comorbidity (COPD, diabetes, renal or cardiac failure or chronic liver disease). The body mass index (BMI) of individuals in the HIV group was lower than in those in the other groups (20.3; IQR 16.7–23.3). The pneumonia group had higher antibiotic use in the last 3 months. In individuals diagnosed with HIV, the HIV/pneumonia group had the lowest CD4 count (median 56 vs. 414 cells/µL), and only 38% of this group received antiretrovirals vs. 73% in the HIV group (Table 1).

Table 1.
Baseline characteristics of people diagnosed with HIV and/or pneumonia in Colombia.
There were no differences in respiratory symptoms or clinical findings between groups with CAP or in individuals with respiratory viruses. We also found no significant differences in pneumonia severity scales (PSI and CURB65), smoking exposure, previous pneumonia, vaccination coverage, and trimethoprim sulfamethoxazole prophylaxis. An important finding was the low vaccination rate found in all three groups (without serologic confirmation or other immunization record), with vaccination against pneumococcus in 25%, 35%, and 14%; and against influenza in 22%, 41% and 14% in the HIV/pneumonia, HIV, and pneumonia groups, respectively, suggesting problems in the implementation of current immunization recommendations. Mortality at hospital discharge occurred in 6 patients, of which four were in group 1 (HIV/pneumonia group), one in group 2 (HIV), and one in group 3 (pneumonia).
3.1. Microbiological Findings
In patients with CAP, at least one microorganism (aerobic bacteria, mycobacteria, fungi, or viruses) was identified in 87.5% (24/27) of the HIV/pneumonia group, and in 57.1% (4/7) of the pneumonia group. No studies were performed for atypical bacteria or anaerobes.
In the HIV/pneumonia group, the most common microorganisms were M. tuberculosis 11/27 (40.7%), followed by P. jirovecii 6/27 (22.2%), Gram-negative bacilli 4/27 (14.8%), S. pneumoniae 3/27 (11.1%), S. aureus 2/27 (7.4%), and H. influenzae 1/27 (3.7%). In the pneumonia group, there was only one identification that was polymicrobial (more than 3 organisms determined to likely represent contamination).
We found at least 1 respiratory virus in 49.2% of all patients, with no significant differences between groups. In 5 cases, we identified 2 respiratory viruses simultaneously in the same patient. In the HIV/pneumonia group, in 2 cases, only respiratory viruses were identified as a possible etiology of pneumonia. In the pneumonia group, there were no microbiological findings other than respiratory viruses (4/7 cases). The most frequently identified respiratory viruses were rhinoviruses, with no significant difference in the 3 groups, followed by parainfluenza 1 (PIV1), parainfluenza 3 (PIV3), influenza A (FluA), metapneumovirus (MPV), parainfluenza 4 (PIV4), influenza B (FluB), coronavirus NL63, respiratory syncytial virus A (RSV A) and adenovirus; we did not detect any cases of parainfluenza 2 (Table 2).

Table 2.
Detection of respiratory viruses in people diagnosed with HIV and/or pneumonia.
Eighty-nine percent of respiratory viruses were associated with another respiratory pathogen (M. tuberculosis, P. jirovecii, aerobic bacteria); respiratory viruses were found without another microorganism in only 4 cases (3 of rhinovirus and 1 of parainfluenza 3) (Table S3).
In the HIV group, a wide variety of conditions led to hospitalization. The most frequent were syphilis, acute diarrhea, headache of unclear cause, heart failure, nodal tuberculosis, intestinal tuberculosis, cerebral toxoplasmosis, and Paracoccidioides brasiliensis (Table S5).
3.2. Lung Function Studies-Spirometric Findings
There were four deaths in group 1, one in group 2, and one in group 3 during hospitalization; therefore, spirometry data were obtained at admission in 23 individuals in group 1 (HIV/pneumonia group), 30 in group 2 (HIV), and 6 in group 3 (pneumonia). At follow-up, there were two withdrawals in group 2 and one in group 3, and there were additional deaths and loss to follow-up.
Using the definition of airflow limitation based on FEV1/FVC < LLN, 7 patients (30.4%) in the HIV/pneumonia group, 5 (16.6%) in the HIV group, and 3 (50%) in the pneumonia group were identified in the admission spirometry. Follow-up spirometry at 1, 6, and 12 months showed a decrease in the number of cases of airflow limitation in the three groups, demonstrating the improvement in spirometric variables over time. Using the FEV1/FVC < 0.70 definition, fewer patients with airflow limitation were identified, suggesting differences in applying the two definitions to diagnose COPD (Table 3).

Table 3.
Airflow limitation according to spirometric parameters at admission and at 1, 6, and 12 months in people living with HIV and/or pneumonia.
In the subgroup of patients who at admission had FEV1/FVC < LLN meeting the definitions of COPD, an analysis was performed to evaluate severity, finding most patients in the moderate category; see Table S2.
In the pneumonia groups (with and without HIV), the effect of the acute condition (pneumonia) on FEV1 was evident. On admission, spirometry in the HIV/pneumonia group had a median FEV1 of 1.80 L (IQR 1.03–2.78), and in the pneumonia group, a median of 1.38 L (IQR 0.70–1.68) was found, which contrasts with the median FEV1 of the HIV group, which was 3.21 L (IQR 2.46–3.82) (Table 4).

Table 4.
Comparison of FEV1 in people diagnosed with HIV and/or pneumonia, measured at admission and at months 1, 6, and 12.
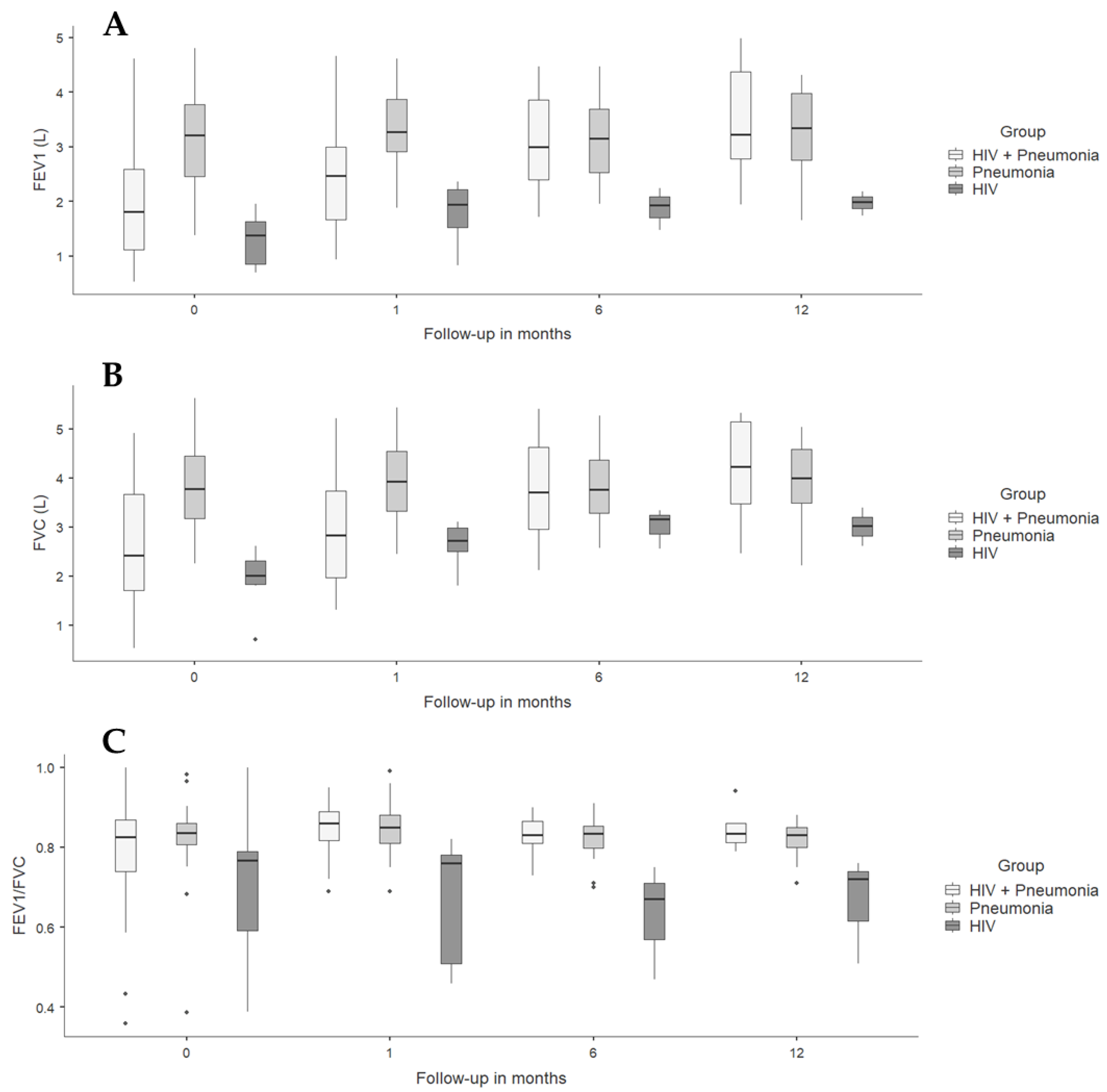
Spirometric follow-up in the pneumonia groups (with and without HIV) showed a progressive increase in FEV1, probably due to the expected recovery of lung function after resolution of acute pulmonary infection, which was strikingly faster in individuals with HIV and CAP. The HIV group had a higher FEV1 than the other groups from admission and remained without significant changes over time; similar findings were found when analyzing FVC and FEV1/FVC ratio (Table 4 and Figure 2).
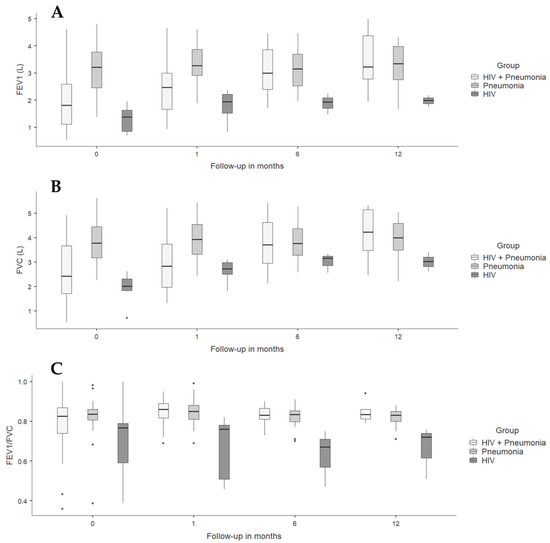
Figure 2.
Spirometric variables in people diagnosed with HIV and/or pneumonia at admission, and at 1- and 6-month follow-ups. (A) FEV1 L: forced expiratory volume at first second measured in liters. (B) FVC: forced vital capacity. (C) FEV1/FVC.
Analysis of FEV1 and FVC as a percentage of predicted both at admission and at 1-, 6-, and 12-month follow-ups showed similar results (Figure S1).
When correlating the presence of respiratory viruses in the three study groups with the variables FEV1, predicted FEV1, FVC, and predicted FVC, at admission and in the follow-up spirometries, there were no differences between the groups. However, the FEV1/FVC ratios in individuals with respiratory viruses revealed lower values in the three groups without being statistically significant, maintaining the previously mentioned trends of improvement over time (Table 5).

Table 5.
Pulmonary function tests according to the presence of respiratory viruses at admission and at months 1, 6, and 12 in people diagnosed with HIV and/or pneumonia.
The type of microorganism identified (mycobacteria, fungi, bacteria, or virus) in any of the groups was not associated with particular patterns of spirometry results. Patients with a history of previous pneumonia in all groups had lower FEV1 values on admission spirometry. No significant differences were found between spirometry results with age, sex, smoking exposure, CD4 count, or HIV viral load.
4. Discussion
This study demonstrated the presence of respiratory viruses in similar proportions in individuals with HIV with or without pneumonia and those without HIV with pneumonia. In addition, it showed the reduction of pulmonary function evaluated by spirometry after acute pneumonia and recovery therefrom during follow-up.
The study is notable for the high percentage of microorganisms identified in the group with HIV and pneumonia. We found at least one respiratory virus in 51.9%, 45.1%, and 57.1% of the patients in the HIV/pneumonia, HIV, and pneumonia groups, respectively. One of the few and most recent studies of etiologic investigation of pneumonia in people with HIV demonstrated the presence of respiratory viruses in 6% of patients using traditional techniques (serology, cultures), which increased to 21% when molecular biology testing (PCR) was used [13]. A similar finding was reported by Garbino et al. in BAL samples, finding at least one respiratory virus in 18.6% of people diagnosed with HIV with respiratory symptoms [31]. In Spain, another study of individuals with HIV and pneumonia detected the presence of respiratory viruses in 20.8% of patients using serological tests, cultures and PCR for adenovirus, rhinovirus, influenza, enterovirus, respiratory syncytial virus, and coronavirus in nasopharyngeal swab samples [16]. Respiratory viruses have also been reported in children with HIV and lower respiratory symptoms in Africa at higher frequencies, with at least one respiratory virus in 53% of children according to PCR on nasal swabs, a finding that may be explained in part by colonization of the upper airway [32]. Recently, Maartens et al. in Cape Town, South Africa prospectively studied 284 patients with HIV and lower respiratory infections, in whom they applied multiplex PCR to induced sputum samples, finding a prevalence of pneumonia of 52%, of PjP of 34%, and of tuberculosis of 41%. Respiratory viruses were detected in 71% of all patients without establishing their pathogenic role. Coinfections were common and diverse [17]. On the other hand, in patients with pneumonia without HIV, the identification of respiratory viruses is very variable, reported between 15% to 37% in different studies [33]; moreover, their pathogenic role is not conclusive, either. One explanation for the higher proportion of respiratory viruses found in our study may be the use of multiplex real-time PCR with the capacity to identify 16 different viruses.
The main virus we Identified was rhinovirus in 37% of HIV/pneumonia patients, 35% of HIV patients, and 42% of pneumonia patients, similar to what has been found in other studies [13,16,32,34]. Another important aspect was the co-infection of respiratory viruses with other viruses, M. tuberculosis, P. jirovecii, and bacteria (nearly 90%), a similar finding reported in other publications, such as the study by Camps et al., where three etiological categories were defined, one of which included the combination of virus with other agent [34] and the aforementioned study by Maartens [17]. In our study, the most common combination was a respiratory virus with M. tuberculosis, followed by respiratory viruses and P. jirovecii.
Multiple hypotheses can explain the effect of HIV and the presence of other microorganisms on the progression of airflow limitation, which can be clinically translated into COPD. The main factor appears to be systemic immune activation in which the CD8+ T cell and macrophage response increases generating chronic lymphocytic alveolitis with release of proteases and inflammatory cytokines (IFNγ) that cause lung damage. There is an imbalance with decreased antioxidant levels and increased oxidants that directly damage the lung parenchyma, and there is increased susceptibility to apoptosis stimulated in endothelial cells by HIV Tat and Naf proteins. Additionally, pulmonary infections such as tuberculosis, P. jirovecii pneumonia, and bacterial pneumonia promote the inflammatory response and lung damage [35], which can generate a further reduction in lung function, between 109 mL to 264 mL of FEV1 and 117 mL to 254 mL of FVC [36,37]. Other factors with a possible effect on lung function impairment are colonization of the airway by other microorganisms, such as atypical bacteria and changes in the pulmonary microbiome [19]. A cohort study following 265 individuals for a median of 8.1 years used a data-driven model to describe longitudinal pulmonary function phenotypes among PLHIV. They found that current smoking and past years of smoking were predictors of adverse FEV1 and FEV1/FVC, and that detectable viremia was the only HIV infection marker associated with the adverse DlCO phenotype. The authors identified two trajectories each for FEV1 and FVC, characterized by a faster rate of decline in participants with higher baseline lung function. These findings are consistent with observations in population-based and COPD cohorts. They also found a correlation between CRP and endothelin-1 levels, representing inflammation and endothelial dysfunction, respectively, with adverse FEV and FVC phenotypes [38].
In the pneumonia groups (with and without HIV), the initial FEV1 was lower than in the group without pneumonia, suggesting that the acute infectious event is directly related to the deterioration of pulmonary tests, beyond the presence of HIV or respiratory viruses. The low lung volumes at the 6-month follow-ups suggest underlying lung pathology that may have predisposed to the infection to playing a role in the persistent dysfunction. In these groups with pneumonia, progressive improvement of pulmonary function was documented at follow-up. Our limited duration of 1-year follow-up did not allow for assessment of the trajectories of functional decline. There are few studies of pulmonary function in HIV; one of them evaluated the prevalence of airflow limitation at a single moment in patients with HIV without pneumonia and healthy controls without HIV, finding 10.6% in both populations, with a slight difference in FEV1, which was lower in patients with HIV [39]. Other studies have shown decreased pulmonary function in individuals with HIV after presenting pulmonary infection by P. jirovecii and bacterial pneumonia, using spirometry and diffusing capacity for carbon monoxide (DLCO) [40,41,42]. The ALIVE study, in which spirometry (FEV1, FVC, FEV1/FVC) was performed after adjustment for clinical variables (sex, age), behavioral variables (smoking), and some infections, showed that uncontrolled HIV infection was independently associated with lung function impairment. Although not statistically significant, the annual rate of reduction in forced expiratory volume at first second (FEV1) in individuals with HIV was −35.8 mL/year (95% CI −51.2 to −20.3 mL/year) compared to −23.6 mL/year (95% CI −32.6 to −14.7 mL/year) among those not infected with HIV (p = 0.135); forced vital capacity (FVC) in people living with HIV decreased −9.29 mL/year (95% CI −25.1 to 6.5 mL/year), and in those not infected with HIV, it was +8.0 mL/year (95% CI −26.4 to 18.7 mL/year) (p = 0.05) [36]. When researchers stratified patients, the patients evidenced greater pulmonary deterioration in individuals with HIV viral load (CV-HIV) greater than 75,000 copies/mL and CD4 <100 cells/mm3, with significant reduction in FEV1 and FVC in individuals with HIV vs. those without HIV of −99.1 mL/year vs. −23.5 mL/year (p = 0.004) for FEV1 and −74.0 mL/year vs. +8.24 mL/year in FVC (p = 0.008). Varkila et al. studied individuals with HIV by post-bronchodilator spirometry vs. individuals without HIV, demonstrating greater deterioration of lung function in individuals diagnosed with HIV with independent effect on outcome in those with tuberculosis [43]. In our study we found no significant differences between pulmonary function and CD4 count or HIV viral load. Spirometric follow-up needs to be prolonged to find possible differences, perhaps at least 5 years. The subgroup of patients in the START study, in which spirometric follow-up was performed at 3.9 years, showed no significant differences [44].
The main limitations of this study are as follows: 1. Due to lack of resources, it was not possible to perform all the microbiological diagnostic tests to include identification by utilizing bacterial and H. capsulatum urinary antigens, which could have increased the proportion of identified microorganisms. 2. As in other studies, defining whether the presence of respiratory viruses corresponds to asymptomatic infection (“colonization”) is complex and will continue to be a challenge that should be evaluated in future studies, perhaps by analyzing the quantitative values of RT-PCR and using repeated measures to evaluate respiratory virus shedding after an acute infection, in particular among people with immunosuppressive conditions. Previous research has shown that certain conditions, such as older age, multiple comorbidities, solid organ transplant may delay PCR clearance, but not by degree of immunosuppression [45]; however, other studies have shown long-term shedding of influenza, parainfluenza, and respiratory syncytial viruses in people with hematological disorders [46]. 3. As for pulmonary function tests, DLCO measurement was not performed, which allows for a dynamic evaluation of pulmonary function, although it represents greater expenses and operational difficulties. 4. Finally, our short follow-up time does not allow to evaluate long-term lung dysfunction. An extended follow-up, of 2 to 5 years, would allow for changes in pulmonary function described in individuals post recovery from pneumonia to be observed. 5. The small sample size, in particular in group 3 (pneumonia), is exploratory. Therefore, our results are limited to people who have similar conditions to the participants in each group.
In conclusion, our findings demonstrate that respiratory viruses are frequent in people diagnosed with HIV, generally coexisting with other pathogens. We could not identify any difference in lung function or clinical outcomes among those coinfected with respiratory viruses. The role respiratory viruses play in lung normal microbiome or as contributors to the pathogenesis of lung infection and dysfunction in people living with HIV remain to be elucidated. In patients with CAP, pulmonary function was affected on admission and improved significantly in the first months after acute infection (CAP). Further studies with a larger number of patients and prolonged follow-up are required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v16030344/s1, Figure S1: Spirometric variables (FEV1 and FVC) according to percentage of predicted at admission and at one-month and six-month follow-ups; Table S1: Frequency of causes of exclusion to the study in the three groups; Table S2: Severity of airflow limitation (FEV1/FVC < LLN) at admission; Table S3: Microbiological identification in individuals with community-acquired pneumonia; Table S4: Microbiological isolates by the presence of respiratory viruses; Table S5: Clinical diagnosis of individuals in the HIV+/pneumonia- group.
Author Contributions
Conceptualization, I.A.R.-S., L.V., Y.K. and Z.V.R.; methodology, I.A.R.-S., R.C., Y.A., G.G., K.P.-V. and W.R.; software, L.L. and D.M.; validation, Y.A.; formal analysis, D.M.; data curation, L.L.; writing—original draft preparation, I.A.R.-S., Y.K. and Z.V.R.; writing—review and editing, R.C., D.M., L.L., Y.A., G.G., K.P.-V., W.R. and L.V.; supervision, L.V., Y.K. and Z.V.R.; project administration, Z.V.R.; funding acquisition, L.V. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Minciencias (Ministerio de Ciencia, Tecnologia e Innovacion) (Grant number: 111580763362), Universidad de Antioquia, Universidad Pontificia Bolivariana (CIDI code: 818B-06/17-55), and University of Manitoba which provided materials and reagents for sample processing. This research was also supported, in part, by the Canada Research Chairs Program for ZVR (Award # 950-232963). The funding entities had no role in the design, execution, analysis or decision to publish the article.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee of the Universidad de Antioquia (approval number 007/2016), Universidad Pontifica Bolivariana (# approval 3-2017), Hospital Universitario San Vicente Fundación (# approval 16-2016) and Clínica SOMA (# approval 57-2017).
Informed Consent Statement
The project was explained to all participants and only those who accepted and signed the informed consent for the study were included. Any individual could stop participating in the study at any time if they so wished.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons because at the time people were included in the study we did not request their permission to share their data publicly.
Acknowledgments
To the patients for their willingness and support with the project. To Deny Sánchez and Lisandra Arango for their invaluable support in patient recruitment and follow-up. To Maryluz Posada for her data management. To the pulmonologist group of the Hospital Universitario San Vicente Fundación for their support in collecting patient samples. To Mariana Herrera for her logistical support in patient follow-up. To the participating hospitals: Hospital Universitario San Vicente Fundación and Clínica SOMA.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crothers, K.; Huang, L.; Goulet, J.L.; Goetz, M.B.; Brown, S.T.; Rodriguez-Barradas, M.C.; Oursler, K.K.; Rimland, D.; Gibert, C.L.; Butt, A.A.; et al. HIV Infection and Risk for Incident Pulmonary Diseases in the Combination Antiretroviral Therapy Era. Am. J. Respir. Crit. Care Med. 2011, 183, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.; Shubber, Z.; Meintjes, G.; Grinsztejn, B.; Eholie, S.; Mills, E.J.; Davies, M.-A.; Vitoria, M.; Penazzato, M.; Nsanzimana, S.; et al. Causes of Hospital Admission among People Living with HIV Worldwide: A Systematic Review and Meta-Analysis. Lancet HIV 2015, 2, e438–e444. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Hansen, N.I.; Lavange, L.; Glassroth, J.; Browdy, B.L.; Rosen, M.J.; Kvale, P.A.; Mangura, B.T.; Reichman, L.B.; Hopewell, P.C. Respiratory Disease Trends in the Pulmonary Complications of HIV Infection Study Cohort. Pulmonary Complications of HIV Infection Study Group. Am. J. Respir. Crit. Care Med. 1997, 155, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Moreno, A.; Miro, J.M.; Torres, A. Pulmonary Infections in HIV-Infected Patients: An Update in the 21st Century. Eur. Respir. J. 2012, 39, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.M.; Shay, D.K.; Holmans, R.C.; Curns, A.T.; Anderson, L.J. Trends in Hospitalization for Pneumonia among Persons Aged 65 Years or Older in the United States, 1988–2002. JAMA 2005, 294, 2712–2719. [Google Scholar] [CrossRef]
- Asociación Colombiana de Neumología y Cirugía de Tórax (ACNCT); Asociación Colombiana de Medicina Crítica y Cuidado Intensivo (AMCI); Asociación Colombiana de Medicina Interna (ACMI); Asociación Colombiana de Infectología (ACIN). Recomendaciones para el Diagnóstico, Tratamiento y Prevención de la Neumonía Adquirida en la Comunidad en Adultos. Infectio 2013, 17, 1–38. [Google Scholar] [CrossRef]
- Hirschtick, R.; Glassroth, J. Bacterial Pneumonia in Persons Infected with the Human Immunodeficiency Virus. N. Engl. J. Med. 1995, 333, 845–851. [Google Scholar] [CrossRef]
- Fernanda, M.; Barreneche, Á.; Andrés, C.; Castro, R.; Botero, A.H.; Franco, J.P.V.; Romero, I.M.T.; Carvajal, L.R.; García, A.E.; Mesa, A.O.; et al. Hospitalization Causes and Outcomes in HIV Patients in the Late Antiretroviral Era in Colombia. AIDS Res. Ther. 2017, 14, 60. [Google Scholar] [CrossRef]
- Vélez, L.; Correa, L.T.; Maya, M.A.; Mejía, P.; Ortega, J.; Bedoya, V.; Ortega, H. Diagnostic Accuracy of Bronchoalveolar Lavage Samples in Immunosuppressed Patients with Suspected Pneumonia: Analysis of a Protocol. Respir. Med. 2007, 101, 2160–2167. [Google Scholar] [CrossRef]
- Benito, N.; Gatell, J.M. Pulmonary Infiltrates in HIV Infected Patients in the HAART Era in Spain, JAIDS 2001.Pdf. J. Acquir. Inmune Defic. Syndr. 2001, 27, 35–43. [Google Scholar] [CrossRef]
- Pretorius, M.A.; Tempia, S.; Walaza, S.; Cohen, A.L.; Moyes, J.; Variava, E.; Dawood, H.; Seleka, M.; Hellferscee, O.; Treurnicht, F.; et al. The Role of Influenza, RSV and Other Common Respiratory Viruses in Severe Acute Respiratory Infections and Influenza-like Illness in a Population with a High HIV Sero-Prevalence, South Africa 2012–2015. J. Clin. Virol. 2016, 75, 21–26. [Google Scholar] [CrossRef]
- Segal, L.N.; Methé, B.A.; Nolan, A.; Hoshino, Y.; Rom, W.N.; Dawson, R.; Bateman, E.; Weiden, M.D. HIV-1 and Bacterial Pneumonia in the Era of Antiretroviral Therapy. Proc. Am. Thorac. Soc. 2011, 8, 282–287. [Google Scholar] [CrossRef]
- Figueiredo-Mello, C.; Naucler, P.; Negra, M.D.; Levin, A.S. Prospective Etiological Investigation of Community-Acquired Pulmonary Infections in Hospitalized People Living with HIV. Medicine 2017, 96, e5778. [Google Scholar] [CrossRef]
- Masur, H.; Brooks, J.T.; Benson, C.A.; Holmes, K.K.; Pau, A.K.; Kaplan, J.E.; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 58, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Vigil, K.J.; Adachi, J.A.; Chemaly, R.F. Analytic Review: Viral Pneumonias in Immunocompromised Adult Hosts. J. Intensive Care Med. 2010, 25, 307–326. [Google Scholar] [CrossRef]
- Perelló, R.; Moreno, A.; Camps, M.; Cervera, C.; Linares, L.; Pumarola, T.; Marcos, M.Á. Implicación de Los Virus Respiratorios En La Neumonía Adquirida En La Comunidad En Pacientes Infectados Por El Virus de La Inmunodeficiencia Humana. Enfermedades Infecc. Microbiol. Clin. 2008, 26, 85–87. [Google Scholar] [CrossRef]
- Maartens, G.; Griesel, R.; Dube, F.; Nicol, M.; Mendelson, M. Etiology of Pulmonary Infections in Human Immunodeficiency Virus–Infected Inpatients Using Sputum Multiplex Real-Time Polymerase Chain Reaction. Clin. Infect. Dis. 2019, 70, 1147–1152. [Google Scholar] [CrossRef]
- Gingo, M.R.; George, M.P.; Kessinger, C.J.; Lucht, L.; Rissler, B.; Weinman, R.; Slivka, W.A.; McMahon, D.K.; Wenzel, S.E.; Sciurba, F.C.; et al. Pulmonary Function Abnormalities in HIV-Infected Patients during the Current Antiretroviral Therapy Era. Am. J. Respir. Crit. Care Med. 2010, 182, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.B.; Kunisaki, K.M.; Huang, L.; Francisco, S.; Hospital, G.; Francisco, S.; Francisco, S.; Hospital, G.; Francisco, S. Obstructive Lung Diseases in HIV: A Clinical Review and Identification of Key Future Research Needs. Semin. Respir. Crit. Care Med. 2017, 37, 277–288. [Google Scholar] [CrossRef][Green Version]
- Verboeket, S.O.; Boyd, A.; Wit, F.W.; Verheij, E.; van der Loeff, M.F.S.; Kootstra, N.; van der Valk, M.; van Steenwijk, R.P.; Drummond, M.B.; Kirk, G.D.; et al. Changes in Lung Function among Treated HIV-Positive and HIV-Negative Individuals: Analysis of the Prospective AGEhIV Cohort Study. Lancet Healthy Longev. 2021, 2, e202–e211. [Google Scholar] [CrossRef] [PubMed]
- Ronit, A.; Omland, L.H.; Kronborg, G.; Pedersen, G.; Nielsen, L.; Mohey, R.; Wiese, L.; Obel, N.; Ahlström, M.G. Incidence of Chronic Obstructive Pulmonary Disease in People with Human Immunodeficiency Virus and Their Parents and Siblings in Denmark. J. Infect. Dis. 2022, 225, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, G.L.; Gray, D.M. Lung Function Abnormalities in HIV-Infected Adults and Children. Respirology 2015, 20, 24–32. [Google Scholar] [CrossRef]
- American Psyquiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psyquiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Ministerio de Sanidad y Social de España. Guía de Práctica Clínica de Intervenciones Psicosociales en el Trastorno Mental Grave; Instituto Aragonés de Ciencias de la Salud-I+CS: Zaragoza, Spain, 2009. [Google Scholar]
- Almeida, A.; Almeida, A.R.; Branco, S.C.; Vesza, Z.F.; Pereira, R. CURB-65 and Other Markers of Illness Severity in Community-Acquired Pneumonia among HIV-Positive Patients. Int. J. STD AIDS 2016, 27, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Rueda, Z.V.; López, L.; Marín, D.; Vélez, L.A.; Arbeláez, M.P. Sputum Induction Is a Safe Procedure to Use in Prisoners and MGIT Is the Best Culture Method to Diagnose Tuberculosis in Prisons. A Cohort Study. Int. J. Infect. Dis. 2015, 33, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rodiño, J.; Rincón, N.; Aguilar, Y.A.; Rueda, Z.V.; Herrera, M.; Vélez, L.A. Microscopic diagnosis of Pneumocystis jirovecii pneumonia in bronchoalveolar lavage and oropharyngeal wash samples of immunocompromised patients with pneumonia. Biomed. Rev. Inst. Nac. Salud 2011, 31, 222–231. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. ATS/ERS Task Force: Standardisation of Lung Function Testing. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- ndd Medical Technologies. ndd EasyGuide Manual del Operador Espirómetro EasyOneTM: 2010; ndd Medizintechnik AG: Zurich, Switzerland, 2010. [Google Scholar]
- GOLD. Global Strategy for the Diagnosis Management, and Prevention of Chronic Obstructive Pulmonary Disease; Global Initiative for Chronic Obstructive Lung Disease, Inc.: MD, USA, 2018. [Google Scholar]
- Garbino, J.; Inoubli, S.; Mossdorf, E.; Weber, R.; Tamm, M.; Soccal, P.; Aubert, J.-D.; Bridevaux, P.-O.; Tapparel, C.; Kaiser, L. Respiratory Viruses in HIV-Infected Patients with Suspected Respiratory Opportunistic Infection. Aids 2008, 22, 701–705. [Google Scholar] [CrossRef]
- Nunes, M.C.; Kuschner, Z.; Rabede, Z.; Madimabe, R.; Van Niekerk, N.; Moloi, J.; Kuwanda, L.; Rossen, J.W.; Klugman, K.P.; Adrian, P.V.; et al. Clinical Epidemiology of Bocavirus, Rhinovirus, Two Polyomaviruses and Four Coronaviruses in HIV-Infected and HIV-Uninfected South African Children. PLoS ONE 2014, 9, e86448. [Google Scholar] [CrossRef]
- Lieberman, D.; Shimoni, A.; Shemer-Avni, Y.; Keren-Naos, A.; Shtainberg, R.; Lieberman, D. Respiratory Viruses in Adults with Community-Acquired Pneumonia. CHEST 2010, 138, 811–816. [Google Scholar] [CrossRef]
- Serra, M.C.; Cervera, C.; Pumarola, T.; Moreno, A.; Perello, R.; Torres, A.; de Anta, M.T.J.; Marcos, M.A. Virological Diagnosis in Community Acquired Pneumonia in Immunocompromised Patients. Eur. Respir. J. 2008, 31, 618–624. [Google Scholar] [CrossRef]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef]
- Drummond, M.B.; Merlo, C.A.; Astemborski, J.; Marshall, M.; Kisalu, A.; Mcdyer, J.F.; Mehta, S.H.; Brown, H.; Wise, R.A.; Kirk, G.D. The Effect of HIV Infection on Longitudinal Lung Function Decline among Injection Drug Users: A Prospective Cohort. AIDS 2013, 27, 1303–1311. [Google Scholar] [CrossRef]
- Morris, A.; George, M.P.; Crothers, K.; Huang, L.; Lucht, L.; Kessinger, C.; Kleerup, E.C. HIV and Chronic Obstructive Pulmonary Disease: Is It Worse and Why? Proc. Am. Thorac. Soc. 2011, 8, 320–325. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Qin, S.; Fitzpatrick, M.; Kessinger, C.; Gentry, H.; McMahon, D.; Weinman, R.D.; Tien, P.; Huang, L.; McCormack, M.; et al. Pulmonary Function Trajectories in People with HIV: Analysis of the Pittsburgh HIV Lung Cohort. Ann. Am. Thorac. Soc. 2022, 19, 2013–2020. [Google Scholar] [CrossRef]
- Ronit, A.; Lundgren, J.; Afzal, S.; Benfield, T.; Roen, A.; Mocroft, A.; Gerstoft, J.; Nordestgaard, B.G.; Vestbo, J.; Nielsen, S.D.; et al. Airflow Limitation in People Living with HIV and Matched Uninfected Controls. Thorax 2018, 73, 431–438. [Google Scholar] [CrossRef]
- Morris, A.M.; Huang, L.; Bacchetti, P.; Turner, J.; Hopewell, P.C.; Wallace, J.M.; Kvale, P.A.; Rosen, M.J.; Glassroth, J.; Reichman, L.B.; et al. Permanent Declines in Pulmonary Function Following Pneumonia in Human Immunodeficiency Virus-Infected Persons. Am. J. Respir. Crit. Care Med. 2000, 162, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, U.S.; Lebech, A.-M.; Mortensen, J.; Gerstoft, J.; Gutte, H.; Kjaer, A. Changes in Lung Function of HIV-Infected Patients: A 4·5-Year Follow-up Study. Clin. Physiol. Funct. Imaging 2012, 32, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.; Shaw, R.J.; Mitchell, D.M. Pulmonary Function in Human Immunodeficiency Virus Infection. Am. Rev. Respir. Dis. 1992, 146, 745–751. [Google Scholar]
- Varkila, M.R.J.; Vos, A.G.; Barth, R.E.; Tempelman, H.A.; Devillé, W.L.J.; Coutinho, R.A.; Grobbee, D.E.; Klipstein-Grobusch, K. The Association between HIV Infection and Pulmonary Function in a Rural African Population. PLoS ONE 2019, 14, e0210573. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Baker, J.V.; Collins, G.; MacDonald, D.M.; Bakowska, E.; El Filali, K.M.; Nnakelu, E.; La Rosa, A.; Connett, J.E. Lung Function Decline in Early HIV Infection: Impact of Antiretroviral Drug Timing and Drug Regimen. Am. J. Respir. Crit. Care Med. 2019, 201, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.L.; Sperring, H.; Hofman, M.; Lodi, S.; White, L.F.; Barocas, J.A.; Bouton, T.C.; Xiao, Y.; Hsu, H.E.; Miller, N.S.; et al. Time to SARS-CoV-2 PCR Clearance in Immunocompromising Conditions: Is Test-Based Removal From Isolation Necessary in Severely Immunocompromised Individuals? Open Forum Infect. Dis. 2021, 8, ofab164. [Google Scholar] [CrossRef] [PubMed]
- Lehners, N.; Tabatabai, J.; Prifert, C.; Wedde, M.; Puthenparambil, J.; Weissbrich, B.; Biere, B.; Schweiger, B.; Egerer, G.; Schnitzler, P. Long-Term Shedding of Influenza Virus, Parainfluenza Virus, Respiratory Syncytial Virus and Nosocomial Epidemiology in Patients with Hematological Disorders. PLoS ONE 2016, 11, e0148258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).