Viral Epitope Scanning Reveals Correlation between Seasonal HCoVs and SARS-CoV-2 Antibody Responses among Cancer and Non-Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Subjects, and Samples
2.2. HIV-1 Serological Testing
2.3. Immunofluorescence Assay against SARS-CoV-2 Spike and Nucleocapsid Proteins
2.4. Pseudovirus Production and SARS-CoV-2 Spike Protein Neutralization Assay
2.5. Phage Immunoprecipitation and Sequencing (PhIP-Seq)
2.6. PhIP-seq Data Processing and Statistical Analyses
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Study Participants
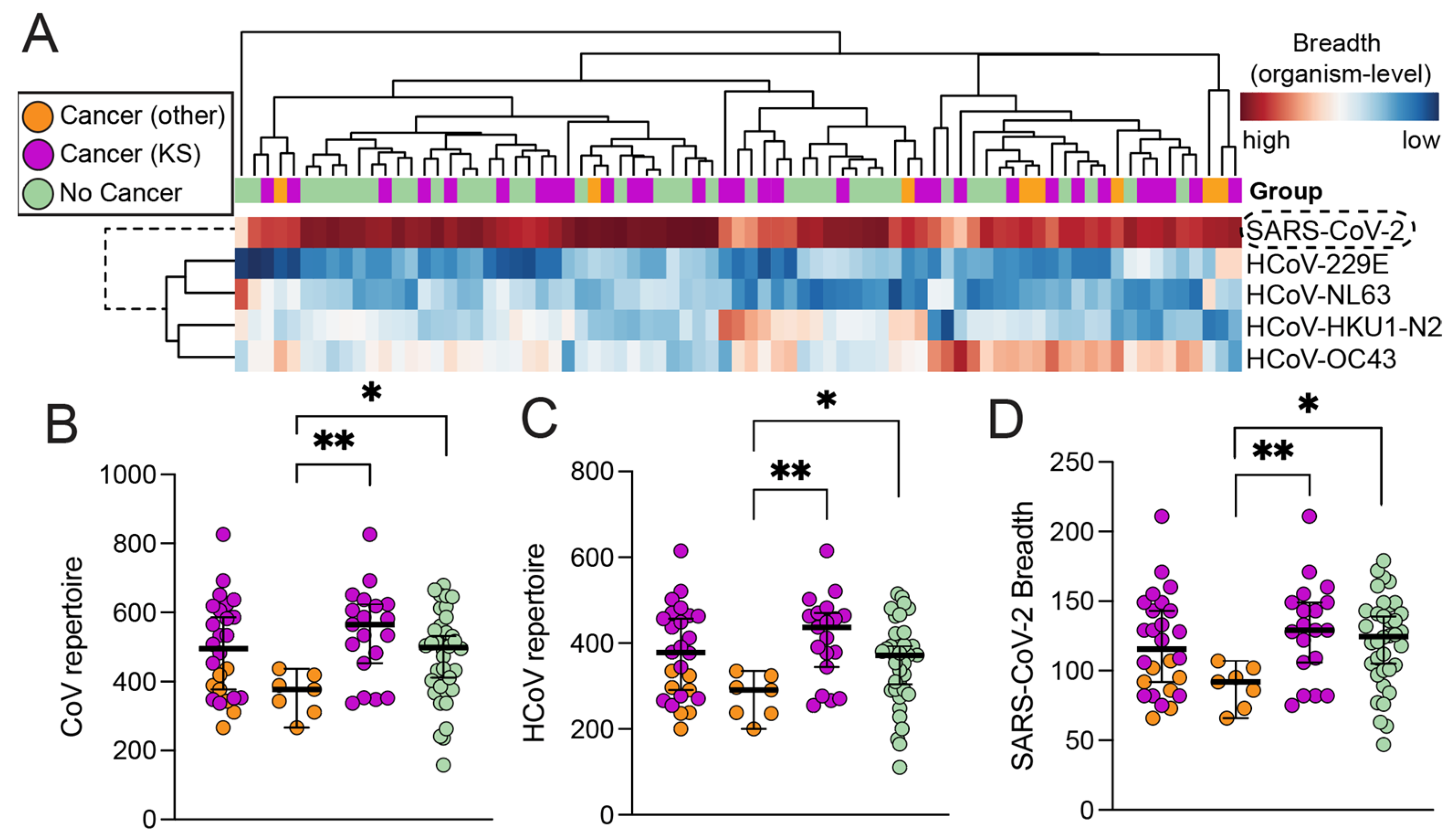
3.2. High-Resolution Humoral Antibody Responses against SARS-CoV-2 and HCoVs
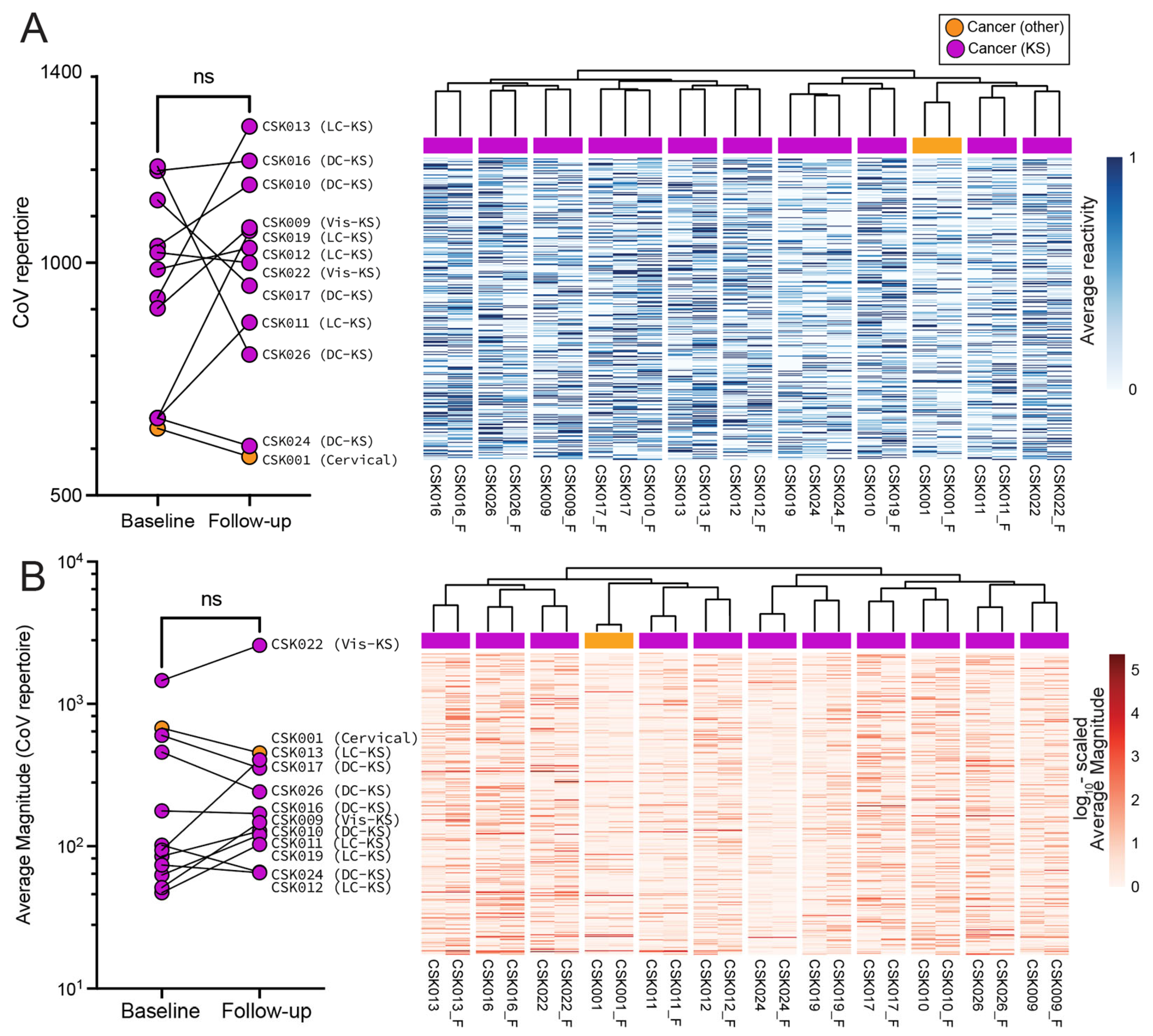
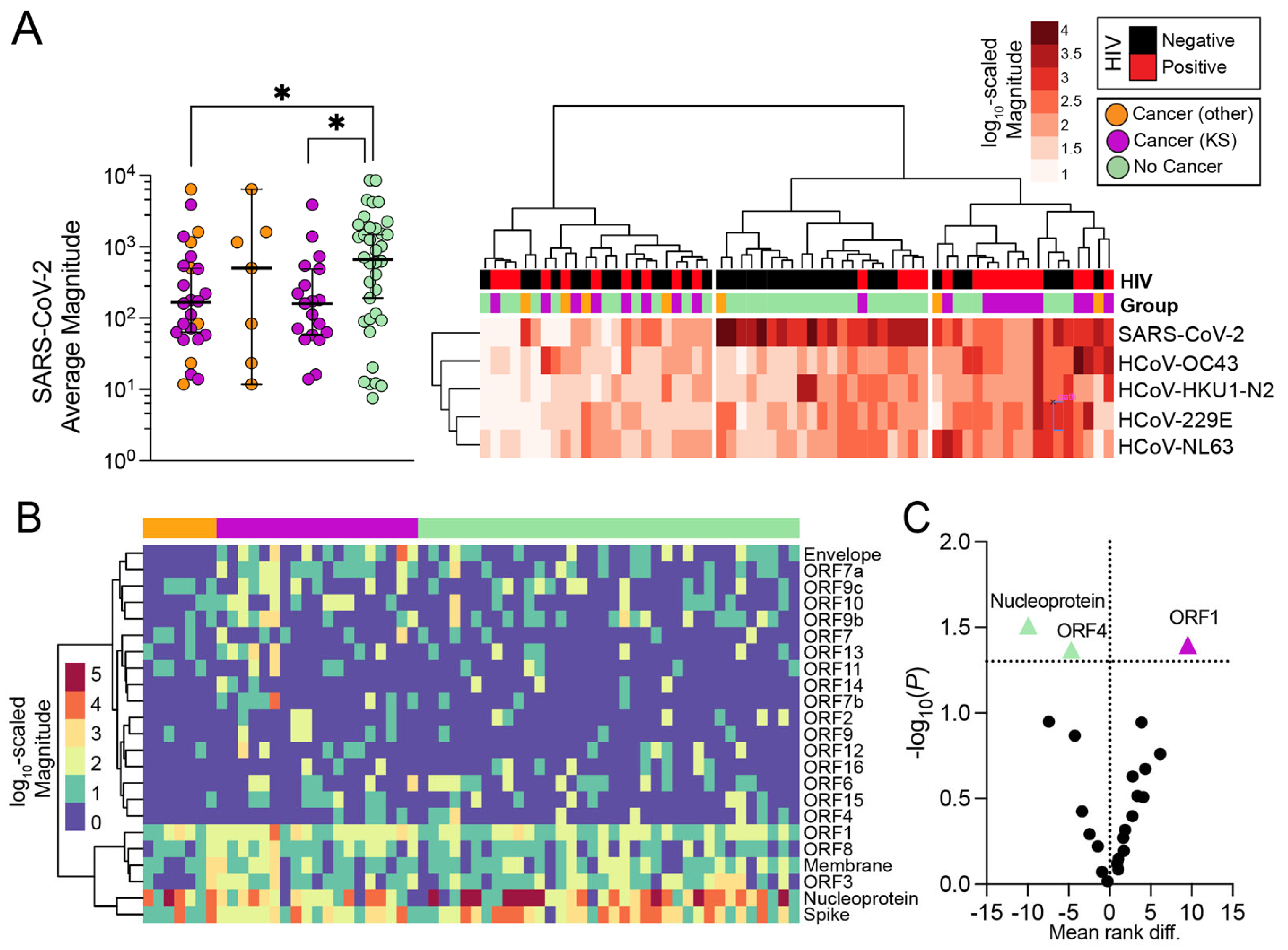
3.3. Magnitude of Antibody Responses in Individuals with and without Cancer
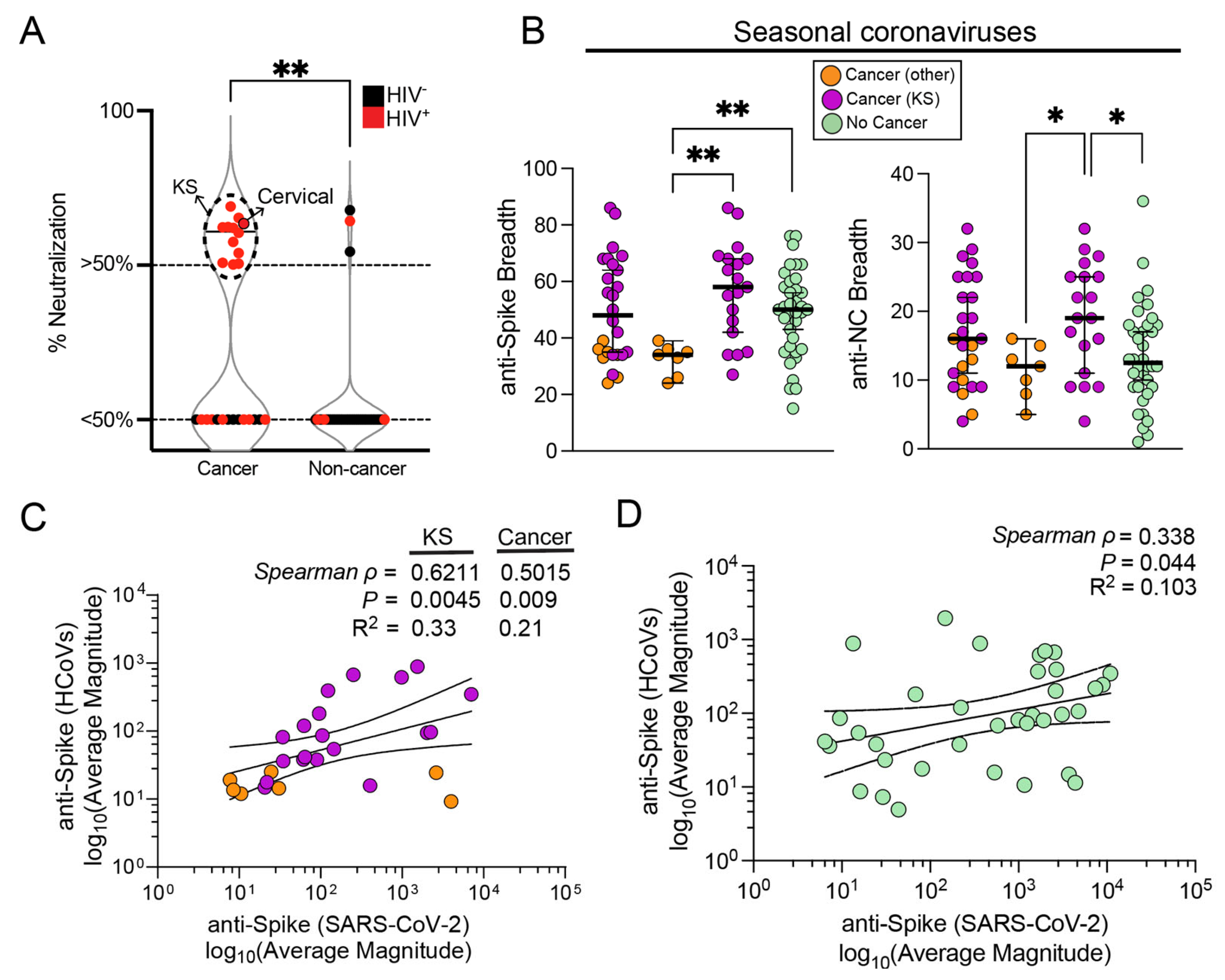
3.4. Influence of Seasonal Coronavirus Repertoire on SARS-CoV-2 Specific Antibody Responses and Neutralization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Worldometer—Real Time World Statistics. Available online: https://www.worldometers.info/ (accessed on 12 May 2021).
- Cui, J.; Li, F.; Shi, Z.L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, R.; Dar, Z.; Bijarnia, R.K.; Dhingra, N.; Kaur, T. Genetic Comparison among Various Coronavirus Strains for the Identification of Potential Vaccine Targets of SARS-CoV-2. Infect. Genet. Evol. 2021, 89, 104490. [Google Scholar] [CrossRef]
- Ljubin-Sternak, S.; Meštrović, T.; Lukšić, I.; Mijač, M.; Vraneš, J. Seasonal Coronaviruses and Other Neglected Respiratory Viruses: A Global Perspective and a Local Snapshot. Front. Public Health 2021, 9, 691163. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Nickbakhsh, S.; Ho, A.; Marques, D.F.P.; McMenamin, J.; Gunson, R.N.; Murcia, P.R. Epidemiology of Seasonal Coronaviruses: Establishing the Context for the Emergence of Coronavirus Disease 2019. J. Infect Dis. 2020, 222, 17. [Google Scholar] [CrossRef]
- Hodgens, A.; Gupta, V. Severe Acute Respiratory Syndrome; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lombardi, A.F.; Afsahi, A.M.; Gupta, A.; Gholamrezanezhad, A. Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), Influenza, and COVID-19, beyond the Lungs: A Review Article. Radiol. Med. 2021, 126, 561. [Google Scholar] [CrossRef]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- De Thoisy, A.; Woudenberg, T.; Pelleau, S.; Donnadieu, F.; Garcia, L.; Pinaud, L.; Tondeur, L.; Meola, A.; Arowas, L.; Clement, N.; et al. Seroepidemiology of the Seasonal Human Coronaviruses NL63, 229E, OC43 and HKU1 in France. Open Forum Infect. Dis. 2023, 10, ofad34. [Google Scholar] [CrossRef]
- Nichols, G.L.; Gillingham, E.L.; Macintyre, H.L.; Vardoulakis, S.; Hajat, S.; Sarran, C.E.; Amankwaah, D.; Phalkey, R. Coronavirus Seasonality, Respiratory Infections and Weather. BMC Infect. Dis. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Tso, F.Y.; Lidenge, S.J.; Peña, P.B.; Clegg, A.A.; Ngowi, J.R.; Mwaiselage, J.; Ngalamika, O.; Julius, P.; West, J.T.; Wood, C. High Prevalence of Pre-Existing Serological Cross-Reactivity against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) in Sub-Saharan Africa. Int. J. Infect. Dis. 2021, 102, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Schulien, I.; Kemming, J.; Oberhardt, V.; Wild, K.; Seidel, L.M.; Killmer, S.; Sagar; Daul, F.; Salvat Lago, M.; Decker, A.; et al. Characterization of Pre-Existing and Induced SARS-CoV-2-Specific CD8+ T Cells. Nat. Med. 2021, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Klumpp-Thomas, C.; Kalish, H.; Shunmugavel, A.; Mehalko, J.; Denson, J.P.; Snead, K.R.; Drew, M.; Corbett, K.S.; Graham, B.S.; et al. Serologic Cross-Reactivity of SARS-CoV-2 with Endemic and Seasonal Betacoronaviruses. J. Clin. Immunol. 2021, 41, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Contopoulos-Ioannidis, D.G. Prepandemic Cross-Reactive Humoral Immunity to SARS-CoV-2 in Africa: Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2023, 134, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wu, N.C.; Tsang, O.T.Y.; Yuan, M.; Perera, R.A.P.M.; Leung, W.S.; So, R.T.Y.; Chan, J.M.C.; Yip, G.K.; Chik, T.S.H.; et al. Cross-Reactive Antibody Response between SARS-CoV-2 and SARS-CoV Infections. Cell Rep. 2020, 31, 107725. [Google Scholar] [CrossRef]
- Turtle, L.; Bali, T.; Buxton, G.; Chib, S.; Chan, S.; Soni, M.; Hussain, M.; Isenman, H.; Fadnis, P.; Venkataswamy, M.M.; et al. Human T Cell Responses to Japanese Encephalitis Virus in Health and Disease. J. Exp. Med. 2016, 213, 1331. [Google Scholar] [CrossRef]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular Immune Correlates of Protection against Symptomatic Pandemic Influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]
- Zimmerman, M.G.; Quicke, K.M.; O’Neal, J.T.; Arora, N.; Machiah, D.; Priyamvada, L.; Kauffman, R.C.; Register, E.; Adekunle, O.; Swieboda, D.; et al. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24, 731. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Athavale, P.; Kumar, V.; Clark, J.; Mondal, S.; Sur, S. Differential Impact of COVID-19 Risk Factors on Ethnicities in the United States. Front. Public Health 2021, 9, 743003. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Starke, K.R.; Petereit-Haack, G.; Schubert, M.; Kämpf, D.; Schliebner, A.; Hegewald, J.; Seidler, A. The Age-Related Risk of Severe Outcomes Due to Covid-19 Infection: A Rapid Review, Meta-Analysis, and Meta-Regression. Int. J. Environ. Res. Public Health 2020, 17, 5974. [Google Scholar] [CrossRef] [PubMed]
- Starke, K.R.; Reissig, D.; Petereit-Haack, G.; Schmauder, S.; Nienhaus, A.; Seidler, A. The Isolated Effect of Age on the Risk of COVID-19 Severe Outcomes: A Systematic Review with Meta-Analysis. BMJ Glob. Health 2021, 6, e006434. [Google Scholar] [CrossRef]
- Sha, Z.; Chang, K.; Mi, J.; Liang, Z.; Hu, L.; Long, F.; Shi, H.; Lin, Z.; Wang, X.; Pei, X. The Impact of the COVID-19 Pandemic on Lung Cancer Patients. Ann. Palliat. Med. 2020, 9, 3373378. [Google Scholar] [CrossRef]
- Al-Quteimat, O.M.; Amer, A.M. The Impact of the COVID-19 Pandemic on Cancer Patients. Am. J. Clin. Oncol. Cancer Clin. Trials 2020, 43, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Sim, B.Z.; Lim, C.; Hocking, C.; Teo, T.; Runnegar, N.; Boan, P.; Heath, C.H.; Rainey, N.; Lyle, M.; et al. COVID-19 Infection among Patients with Cancer in Australia from 2020 to 2022: A National Multicentre Cohort Study. Lancet Reg. Health—West. Pac. 2023, 38, 100824. [Google Scholar] [CrossRef]
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; McAllister, D.A.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Risks of and Risk Factors for COVID-19 Disease in People with Diabetes: A Cohort Study of the Total Population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of Protection against COVID-19 Infection and Intensity of Symptomatic Disease in Vaccinated Individuals Exposed to SARS-CoV-2 in Households in Israel (ICoFS): A Prospective Cohort Study. Lancet Microbe 2023, 4, e309–e318. [Google Scholar] [CrossRef] [PubMed]
- Poonia, B.; Kottilil, S. Immune Correlates of COVID-19 Control. Front. Immunol. 2020, 11, 569611. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of Protection against SARS-CoV-2 Infection and COVID-19 Disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ouyang, W.; Chua, M.L.K.; Xie, C. SARS-CoV-2 Transmission in Patients with Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020, 6, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer Patients in SARS-CoV-2 Infection: A Nationwide Analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Feng, R.M.; Zong, Y.N.; Cao, S.M.; Xu, R.H. Current Cancer Situation in China: Good or Bad News from the 2018 Global Cancer Statistics? Cancer Commun 2019, 39, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Strohl, W.R.; Ku, Z.; An, Z.; Carroll, S.F.; Keyt, B.A.; Strohl, L.M. Passive Immunotherapy Against SARS-CoV-2: From Plasma-Based Therapy to Single Potent Antibodies in the Race to Stay Ahead of the Variants. BioDrugs 2022, 36, 231–323. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A. Protective Roles and Protective Mechanisms of Neutralizing Antibodies against SARS-CoV-2 Infection and Their Potential Clinical Implications. Front. Immunol. 2023, 14, 1055457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, R.; Chen, Z. Human Neutralizing Antibodies for SARS-CoV-2 Prevention and Immunotherapy. Immunother. Adv. 2022, 2, ltab027. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J.; Moore, J.P. Antibodies to SARS-CoV-2 and Their Potential for Therapeutic Passive Immunization. eLife 2020, 9, e57877. [Google Scholar] [CrossRef] [PubMed]
- Tso, F.Y.; Lidenge, S.J.; Poppe, L.K.; Peña, P.B.; Privatt, S.R.; Bennett, S.J.; Ngowi, J.R.; Mwaiselage, J.; Belshan, M.; Siedlik, J.A.; et al. Presence of Antibody-Dependent Cellular Cytotoxicity (ADCC) against SARS-CoV-2 in COVID-19 Plasma. PLoS ONE 2021, 16, e0247640. [Google Scholar] [CrossRef]
- United Republic of Tanzania, Ministry of Health and Social Welfare, NACP. Guidelines on HIV Testing and Counseling in Clinical Setting; NACP: Dar es Salaam, Tanzania, 2007.
- Yalcin, D.; Bennett, S.J.; Sheehan, J.; Trauth, A.J.; Tso, F.Y.; West, J.T.; Hagensee, M.E.; Ramsay, A.J.; Wood, C. Longitudinal Variations in Antibody Responses against SARS-CoV-2 Spike Epitopes upon Serial Vaccinations. Int. J. Mol. Sci. 2023, 24, 7292. [Google Scholar] [CrossRef] [PubMed]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral Epitope Profiling of COVID-19 Patients Reveals Cross-Reactivity and Correlates of Severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef]
- Bennett, S.J.; Yalcin, D.; Privatt, S.R.; Ngalamika, O.; Lidenge, S.J.; West, J.T.; Wood, C. Antibody Epitope Profiling of the KSHV LANA Protein Using VirScan. PLoS Pathog. 2022, 18, e1011033. [Google Scholar] [CrossRef]
- Mohan, D.; Wansley, D.L.; Sie, B.M.; Noon, M.S.; Baer, A.N.; Laserson, U.; Larman, H.B. PhIP-Seq Characterization of Serum Antibodies Using Oligonucleotide-Encoded Peptidomes. Nat. Protoc. 2018, 13, 1958–1978. [Google Scholar] [CrossRef]
- Xu, G.J.; Kula, T.; Xu, Q.; Li, M.Z.; Vernon, S.D.; Ndung’u, T.; Ruxrungtham, K.; Sanchez, J.; Brander, C.; Chung, R.T.; et al. Viral Immunology. Comprehensive Serological Profiling of Human Populations Using a Synthetic Human Virome. Science 2015, 348, aaa0698. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The Impact of Pre-Existing Cross-Reactive Immunity on SARS-CoV-2 Infection and Vaccine Responses. Nat. Rev. Immunol. 2022, 23, 304–316. [Google Scholar] [CrossRef]
- Ma, Z.; Li, P.; Ji, Y.; Ikram, A.; Pan, Q. Cross-Reactivity towards SARS-CoV-2: The Potential Role of Low-Pathogenic Human Coronaviruses. Lancet Microbe 2020, 1, e151. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Kode, V.; Bhojak, K.; Karunakaran, C.; Lee, K.; Manoharan, M.; Ramesh, A.; Hv, S.; Srivastava, A.; Sathian, R.; et al. Immunodominant T-Cell Epitopes from the SARS-CoV-2 Spike Antigen Reveal Robust Pre-Existing T-Cell Immunity in Unexposed Individuals. Sci. Rep. 2021, 11, 13164. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Kumar, S.; Singh, S.; Bansal, T.; Jain, N.; Saluja, S.; Kumar, R.; Bhattacharyya, S.; Palanichamy, J.K.; Mir, R.A.; et al. Cross-Neutralization of SARS-CoV-2 by HIV-1 Specific Broadly Neutralizing Antibodies and Polyclonal Plasma. PLoS Pathog. 2021, 17, e1009958. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Díez, C.; Martín-Vicente, M.; Micán, R.; Pérez-Elías, M.J.; García-Fraile, L.J.; Vidal, F.; Suárez-García, I.; Podzamczer, D.; Del Romero, J.; et al. Prevalence and Factors Associated with SARS-CoV-2 Seropositivity in the Spanish HIV Research Network Cohort. Clin. Microbiol. Infect. 2021, 27, 1678. [Google Scholar] [CrossRef]
- Nasrullah, A.; Patel, S.; Ud Din, M.T.; Javed, A.; Arshad, H.; Raja, A.; Dumont, T. A Case of Acquired Immunodeficiency Syndrome-Related Kaposi Sarcoma in a Patient with COVID-19—A Brief Review of HIV-COVID Co-Infection and Its Therapeutic Challenges! Respir. Med. Case Rep. 2021, 34, 101524. [Google Scholar] [CrossRef]
- Chanda, D.; Minchella, P.A.; Kampamba, D.; Itoh, M.; Hines, J.Z.; Fwoloshi, S.; Boyd, M.A.; Hamusonde, K.; Chirwa, L.; Nikoi, K.; et al. COVID-19 Severity and COVID-19–Associated Deaths Among Hospitalized Patients with HIV Infection—Zambia, March–December 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 807. [Google Scholar] [CrossRef]
- Aran, D.; Beachler, D.C.; Lanes, S.; Overhage, J.M. Prior Presumed Coronavirus Infection Reduces COVID-19 Risk: A Cohort Study. J. Infect. 2020, 81, 923–930. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent Endemic Coronavirus Infection Is Associated with Less-Severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J.E.; et al. Seasonal Human Coronavirus Antibodies Are Boosted upon SARS-CoV-2 Infection but Not Associated with Protection. Cell 2021, 184, 1858–1864.e10. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; van Deuren, R.C.; van Werkhoven, C.H.; Jaeger, M.; Debisarun, P.; Taks, E.; Mourits, V.P.; Koeken, V.A.C.M.; de Bree, L.C.J.; ten Doesschate, T.; et al. Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: A Retrospective Cohort Study. Cell Rep. Med. 2020, 1, 100073. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; van Eyk, J.E.; Cheng, S.; Arditi, M. BCG Vaccination History Associates with Decreased SARS-CoV-2 Seroprevalence across a Diverse Cohort of Health Care Workers. J. Clin. Investig. 2021, 131, e145157. [Google Scholar] [CrossRef] [PubMed]
| Variable | All (N = 62) | Cancer (n = 26) | Non-Cancer (n = 36) | p-Value |
|---|---|---|---|---|
| Age|Median (IQR), years | 44 (30.25) | 38 (16.5) | 55.5 (33.75) | 0.0027 |
| Sex|n (%) | 0.4425 | |||
| Female | 28 (45.2) | 10 (38.5) | 18 (50) | |
| Male | 34 (54.8) | 16 (61.5) | 18 (50) | |
| HIV Status|n (%) | <0.0001 | |||
| Negative | 37 (59.7) | 7 (29.9) | 30 (83.3) | |
| Positive | 25 (40.3) | 19 (73.1) | 6 (16.7) | |
| Vaccinated TB BCG|n (%) | 0.5501 | |||
| Yes | 46 (75.4) | 21 (80.8) | 25 (71.4) | |
| No | 15 (24.6) | 5 (19.2) | 10 (28.6) | |
| Unknown | 1 (1.6) | 0 | 1 (2.8) | |
| Vaccinated SARS-CoV-2|n (%) | 0.1516 | |||
| Yes | 5 (8.1) | 4 (15.4) | 1 (2.8) | |
| No | 57 (91.9) | 22 (84.6) | 35 (97.2) | |
| COVID-19 Like Symptoms|n (%) | 0.0874 | |||
| Yes | 18 (29.0) | 11 (42.3) | 7 (19.4) | |
| Cough | 6 (9.7) | 5 (19.2) | 1 (2.8) | |
| DIB | 1 (1.6) | 1 (3.9) | 0 | |
| Cough and DIB | 2 (2/62) | 1 (3.9) | 1 (2.8) | |
| Fever | 8 (12.9) | 4 (15.4) | 4 (11.1) | |
| Fever and cough | 1 (1.6) | 0 | 1 (2.8) | |
| No | 44 (71) | 15 (57.7) | 29 (80.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidenge, S.J.; Yalcin, D.; Bennett, S.J.; Ngalamika, O.; Kweyamba, B.B.; Mwita, C.J.; Tso, F.Y.; Mwaiselage, J.; West, J.T.; Wood, C. Viral Epitope Scanning Reveals Correlation between Seasonal HCoVs and SARS-CoV-2 Antibody Responses among Cancer and Non-Cancer Patients. Viruses 2024, 16, 448. https://doi.org/10.3390/v16030448
Lidenge SJ, Yalcin D, Bennett SJ, Ngalamika O, Kweyamba BB, Mwita CJ, Tso FY, Mwaiselage J, West JT, Wood C. Viral Epitope Scanning Reveals Correlation between Seasonal HCoVs and SARS-CoV-2 Antibody Responses among Cancer and Non-Cancer Patients. Viruses. 2024; 16(3):448. https://doi.org/10.3390/v16030448
Chicago/Turabian StyleLidenge, Salum J., Dicle Yalcin, Sydney J. Bennett, Owen Ngalamika, Brenda B. Kweyamba, Chacha J. Mwita, For Yue Tso, Julius Mwaiselage, John T. West, and Charles Wood. 2024. "Viral Epitope Scanning Reveals Correlation between Seasonal HCoVs and SARS-CoV-2 Antibody Responses among Cancer and Non-Cancer Patients" Viruses 16, no. 3: 448. https://doi.org/10.3390/v16030448
APA StyleLidenge, S. J., Yalcin, D., Bennett, S. J., Ngalamika, O., Kweyamba, B. B., Mwita, C. J., Tso, F. Y., Mwaiselage, J., West, J. T., & Wood, C. (2024). Viral Epitope Scanning Reveals Correlation between Seasonal HCoVs and SARS-CoV-2 Antibody Responses among Cancer and Non-Cancer Patients. Viruses, 16(3), 448. https://doi.org/10.3390/v16030448






