Human and Viral microRNA Expression in Acute and Chronic HIV Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Virological Characterization
2.2. MiRNA Extraction and Reverse Transcription
2.3. MiRNA Library Construction and NGS
2.4. Bioinformatics Analysis
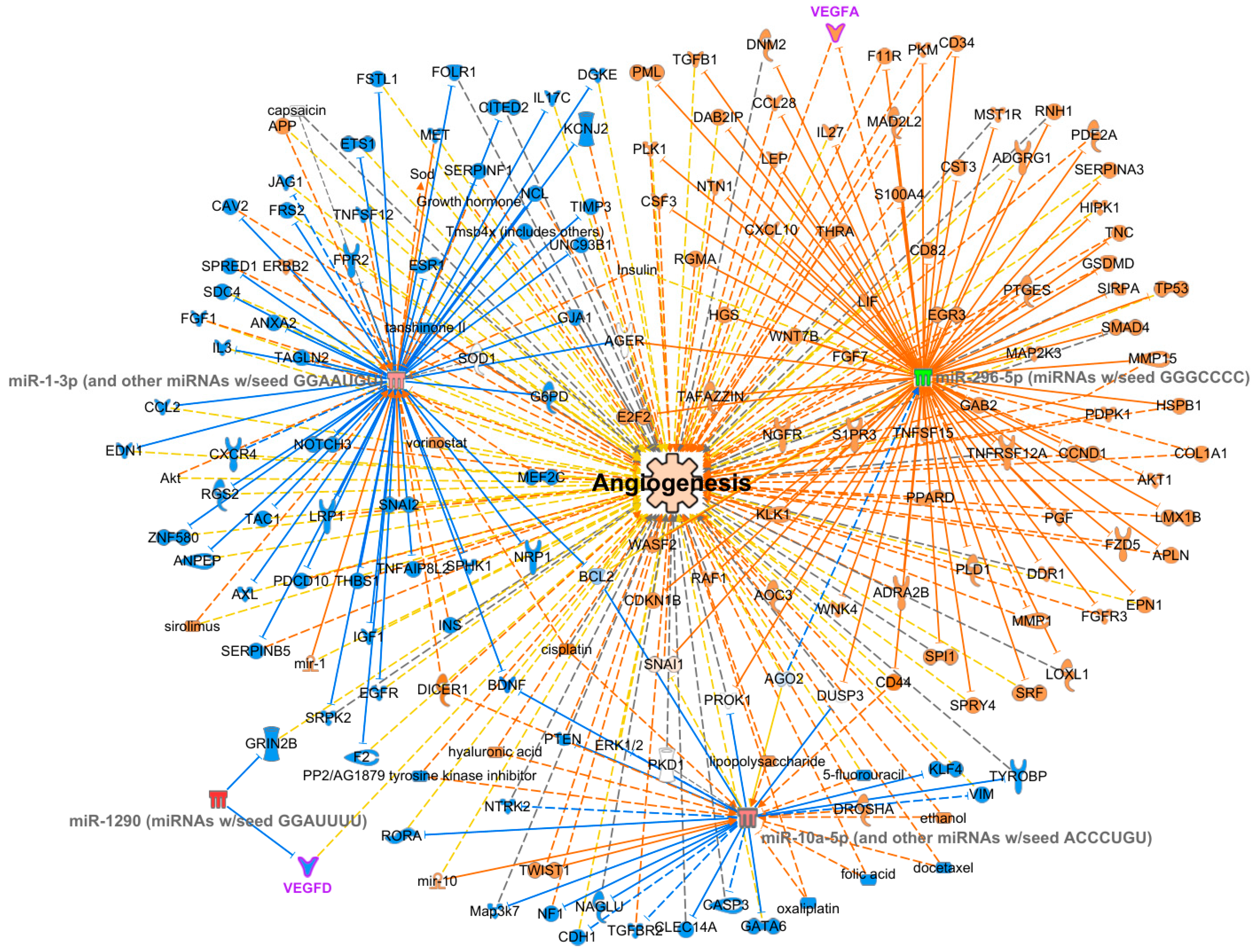
2.5. MiRNA Differential Expression Analysis and Gene Ontology (GO) Enrichment Analysis
- Build–Grow tool: miRNA Confidence Level: Experimentally Observed, High (Predicted); Diseases: Antimicrobial Response, Infectious Disease, Inflammatory Response.
- Overlay: analyses, datasets, and the List tool was used to import the DE analysis data onto the graph. Then, the Build–Connect tool was used to link angiogenesis to target mRNAs.
3. Results
3.1. Clinical and Virological Features of the Subjects
3.2. Human miRNA Analysis
3.3. Viral miRNA Analysis in Plasma from AHI and CHI Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, F.; Zaongo, S.D.; Song, F.; Chen, Y. The Diverse Roles of miRNAs in HIV Pathogenesis: Current Understanding and Future Perspectives. Front. Immunol. 2022, 13, 1091543. [Google Scholar] [CrossRef] [PubMed]
- Ameres, S.L.; Zamore, P.D. Diversifying microRNA Sequence and Function. Nat. Rev. Mol. Cell Biol. 2013, 14, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Duy, J.; Koehler, J.W.; Honko, A.N.; Schoepp, R.J.; Wauquier, N.; Gonzalez, J.-P.; Pitt, M.L.; Mucker, E.M.; Johnson, J.C.; O’Hearn, A.; et al. Circulating microRNA Profiles of Ebola Virus Infection. Sci. Rep. 2016, 6, 24496. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Jiang, X.; Gu, D.; Zhang, Y.; Kong, S.K.; Jiang, C.; Xie, W. Dysregulated Serum MiRNA Profile and Promising Biomarkers in Dengue-Infected Patients. Int. J. Med. Sci. 2016, 13, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bruscella, P.; Bottini, S.; Baudesson, C.; Pawlotsky, J.-M.; Feray, C.; Trabucchi, M. Viruses and miRNAs: More Friends than Foes. Front. Microbiol. 2017, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Karampoor, S.; Korotkova, N.L. The Emerging Role of miRNA-122 in Infectious Diseases: Mechanisms and Potential Biomarkers. Pathol. Res. Pract. 2023, 249, 154725. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.W.; Pantoja, C.; Beliakova-Bethell, N. An Evaluation on the Role of Non-Coding RNA in HIV Transcription and Latency: A Review. HIV AIDS 2023, 15, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Witwer, A.W.; Jn, B.; Je, C. Relationships of PBMC microRNA Expression, Plasma Viral Load, and CD4+ T-Cell Count in HIV-1-Infected Elite Suppressors and Viremic Patients. Retrovirology 2012, 9, 5. [Google Scholar] [CrossRef]
- Reynoso, R.; Laufer, N.; Hackl, M.; Skalicky, S.; Monteforte, R.; Turk, G.; Carobene, M.; Quarleri, J.; Cahn, P.; Werner, R.; et al. MicroRNAs Differentially Present in the Plasma of HIV Elite Controllers Reduce HIV Infection in Vitro. Sci. Rep. 2014, 4, 5915. [Google Scholar] [CrossRef]
- Di Carlo, D.; Falasca, F.; Mazzuti, L.; Guerrizio, G.; Migliara, G.; Santori, M.; Lazzaro, A.; Mezzaroma, I.; D’Ettorre, G.; Fimiani, C.; et al. MicroRNA Expression Levels in Peripheral Blood Mononuclear Cells from Human Immunodeficiency Virus Type 1 Positive Individuals and Relationship with Different Levels of Viral Suppression. AIDS Res. Hum. Retroviruses, 2023; ahead of print. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Pfeffer, S.; Sewer, A.; Lagos-Quintana, M.; Sheridan, R.; Sander, C.; Grässer, F.A.; van Dyk, L.F.; Ho, C.K.; Shuman, S.; Chien, M.; et al. Identification of microRNAs of the herpesvirus family. Nat. Methods 2005, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kumar, A.; Ingle, H.; Kumar, H. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection. Front. Immunol. 2020, 10, 3079. [Google Scholar] [CrossRef] [PubMed]
- Dass, D.; Dhotre, K.; Chakraborty, M.; Nath, A.; Banerjee, A.; Bagchi, P.; Mukherjee, A. miRNAs in Herpesvirus Infection: Powerful Regulators in Small Packages. Viruses 2023, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Bennasser, Y.; Le, S.-Y.; Yeung, M.L.; Jeang, K.-T. HIV-1 Encoded Candidate Micro-RNAs and Their Cellular Targets. Retrovirology 2004, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Schopman, N.C.T.; Willemsen, M.; Liu, Y.P.; Bradley, T.; van Kampen, A.; Baas, F.; Berkhout, B.; Haasnoot, J. Deep Sequencing of Virus-Infected Cells Reveals HIV-Encoded Small RNAs. Nucleic Acids Res. 2012, 40, 414–427. [Google Scholar] [CrossRef] [PubMed]
- QIAGEN CLC Genomics Workbench, version 23.0.5. Software for Bioinformatics Analyses. QIAGEN: Aarhus, Denmark, 2024.
- miRBase, version v.22 Database of Identified miRNAs; University of Manchester: Manchester, UK. Available online: https://mirbase.org/ (accessed on 12 January 2024).
- QIAGEN IPA Ingenuity Pathway Analysis (IPA), version V01-23 01; QIAGEN Inc.: Aarhus, Denmark, 2024.
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Cuesta-Sancho, S.; Márquez-Ruiz, D.; Illanes-Álvarez, F.; Campaña-Gómez, I.; Martín-Aspas, A.; Trujillo-Soto, M.T.; Romero, A.; Galán, F.; Rodríguez-Iglesias, M.; Márquez-Coello, M.; et al. Expression Profile of microRNAs Related with Viral Infectivity, Inflammatory Response, and Immune Activation in People Living with HIV. Front. Microbiol. 2023, 14, 1136718. [Google Scholar] [CrossRef]
- Biswas, S.; Haleyurgirisetty, M.; Lee, S.; Hewlett, I.; Devadas, K. Development and Validation of Plasma miRNA Biomarker Signature Panel for the Detection of Early HIV-1 Infection. eBioMedicine 2019, 43, 307–316. [Google Scholar] [CrossRef]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Wolinsky, S.M.; Gabuzda, D. Small RNA Sequencing of Extracellular Vesicles Identifies Circulating miRNAs Related to Inflammation and Oxidative Stress in HIV Patients. BMC Immunol. 2020, 21, 57. [Google Scholar] [CrossRef]
- Marquez-Pedroza, J.; Cárdenas-Bedoya, J.; Morán-Moguel, M.C.; Escoto-Delgadillo, M.; Torres-Mendoza, B.M.; Pérez-Ríos, A.M.; González-Enriquez, G.V.; Vázquez-Valls, E. Plasma microRNA Expression Levels in HIV-1-Positive Patients Receiving Antiretroviral Therapy. Biosci. Rep. 2020, 40, BSR20194433. [Google Scholar] [CrossRef] [PubMed]
- Gilles, M.-E.; Slack, F.J. Let-7 microRNA as a Potential Therapeutic Target with Implications for Immunotherapy. Expert Opin. Ther. Targets 2018, 22, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, S.-J.; Xie, S.-J.; Zhang, Y.; Yang, J.-H.; Zhang, W.-Q.; Zheng, M.-N.; Zhou, H.; Qu, L.-H. MicroRNA-122 Supports Robust Innate Immunity in Hepatocytes by Targeting the RTKs/STAT3 Signaling Pathway. eLife 2019, 8, e41159. [Google Scholar] [CrossRef]
- Lee, T.-C.; Lin, Y.-L.; Liao, J.-T.; Su, C.-M.; Lin, C.-C.; Lin, W.-P.; Liao, C.-L. Utilizing Liver-Specific microRNA-122 to Modulate Replication of Dengue Virus Replicon. Biochem. Biophys. Res. Commun. 2010, 396, 596–601. [Google Scholar] [CrossRef]
- Murray, D.D.; Suzuki, K.; Law, M.; Trebicka, J.; Neuhaus Nordwall, J.; Johnson, M.; Vjecha, M.J.; Kelleher, A.D.; Emery, S. Circulating miR-122 and miR-200a as Biomarkers for Fatal Liver Disease in ART-Treated, HIV-1-Infected Individuals. Sci. Rep. 2017, 7, 10934. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Xiao, X.; He, Y.; Jiang, Y.; Zhang, M.; Peng, F.; Tian, Y.; Xu, Y.; Gong, G. HIV Vpr Protein Upregulates microRNA-122 Expression and Stimulates Hepatitis C Virus Replication. J. Gen. Virol. 2015, 96, 2453. [Google Scholar] [CrossRef]
- Franco, S.; Buccione, D.; Pluvinet, R.; Mothe, B.; Ruiz, L.; Nevot, M.; Jordan-Paiz, A.; Ramos, L.; Aussó, S.; Morillas, R.M.; et al. Large-Scale Screening of Circulating microRNAs in Individuals with HIV-1 Mono-Infections Reveals Specific Liver Damage Signatures. Antivir. Res. 2018, 155, 106–114. [Google Scholar] [CrossRef]
- Cairoli, V.; Valle-Millares, D.; Terrón-Orellano, M.C.; Luque, D.; Ryan, P.; Dominguez, L.; Martín-Carbonero, L.; De Los Santos, I.; De Matteo, E.; Ameigeiras, B.; et al. MicroRNA Signature from Extracellular Vesicles of HCV/HIV Co-Infected Individuals Differs from HCV Mono-Infected. J. Mol. Med. 2023, 101, 1409–1420. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Feng, B.; Wang, W.; Liu, D.; Zhao, X.; Yu, C.; Wang, X.; Gao, Y. MiR-550a-3p Restores Damaged Vascular Smooth Muscle Cells by Inhibiting Thrombomodulin in an in Vitro Atherosclerosis Model. Eur. J. Histochem. 2022, 66, 3429. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lin, F.-C.; Hsu, T.-I.; Lin, J.-T.; Guo, J.-H.; Tsai, C.-H.; Lee, Y.-C.; Lee, Y.-C.; Chen, C.-L.; Hsiao, M.; et al. MicroRNA-296-5p (miR-296-5p) Functions as a Tumor Suppressor in Prostate Cancer by Directly Targeting Pin1. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2055–2066. [Google Scholar] [CrossRef]
- Bignami, F.; Pilotti, E.; Bertoncelli, L.; Ronzi, P.; Gulli, M.; Marmiroli, N.; Magnani, G.; Pinti, M.; Lopalco, L.; Mussini, C.; et al. Stable Changes in CD4+ T Lymphocyte miRNA Expression after Exposure to HIV-1. Blood 2012, 119, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Bedoya, J.; Marquez-Pedroza, J.; Morán-Moguel, M.C.; Escoto-Delgadillo, M.; Vázquez-Valls, E.; González-Enríquez, G.V.; Pérez-Ríos, A.M.; Torres-Mendoza, B.M. MicroRNA-296-5p Is Differentially Expressed in Individuals with and without HIV-1 Infection. Genet. Mol. Biol. 2020, 43, e20200017. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Garcia-Martin, R.; Torriani, M.; Fitch, K.V.; Carlson, A.R.; Kahn, C.R.; Grinspoon, S.K. Altered Pattern of Circulating miRNAs in HIV Lipodystrophy Perturbs Key Adipose Differentiation and Inflammation Pathways. JCI Insight 2021, 6, e150399. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Walias, F.J.; Vázquez, E.; Pacheco, Y.; Rodríguez-Fernández, J.M.; Pérez-Elías, M.J.; Dronda, F.; Casado, J.L.; Moreno, A.; Hermida, J.M.; Quereda, C.; et al. Risk, Diagnostic and Predictor Factors for Classical Hodgkin Lymphoma in HIV-1-Infected Individuals: Role of Plasma Exosome-Derived miR-20a and miR-21. J. Clin. Med. 2020, 9, 760. [Google Scholar] [CrossRef]
- Ascherl, G.; Hohenadl, C.; Schatz, O.; Shumay, E.; Bogner, J.; Eckhart, L.; Tschachler, E.; Monini, P.; Ensoli, B.; Stürzl, M. Infection With Human Immunodeficiency Virus-1 Increases Expression of Vascular Endothelial Cell Growth Factor in T Cells: Implications for Acquired Immunodeficiency Syndrome-Associated Vasculopathy. Blood 1999, 93, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Henderson, S.; Lagos, D.; Nikitenko, L.; Coulter, E.; Roberts, S.; Gratrix, F.; Plaisance, K.; Renne, R.; Bower, M.; et al. KSHV-Encoded miRNAs Target MAF to Induce Endothelial Cell Reprogramming. Genes Dev. 2010, 24, 195–205. [Google Scholar] [CrossRef]
- Blümke, J.; Bauer, M.; Vaxevanis, C.; Wilfer, A.; Mandelboim, O.; Wickenhauser, C.; Seliger, B.; Jasinski-Bergner, S. Identification and Characterization of the Anti-Viral Interferon Lambda 3 as Direct Target of the Epstein-Barr Virus microRNA-BART7-3p. Oncoimmunology 2023, 12, 2284483. [Google Scholar] [CrossRef]
- Qi, M.; Qi, Y.; Ma, Y.; He, R.; Ji, Y.; Sun, Z.; Ruan, Q. Over-Expression of Human Cytomegalovirus miR-US25-2-3p Downregulates eIF4A1 and Inhibits HCMV Replication. FEBS Lett. 2013, 587, 2266–2271. [Google Scholar] [CrossRef]
- Carmi, O.; Gotlieb, Y.; Shemer-Avni, Y.; Bentwich, Z. The Role of HIV-1-Encoded microRNAs in Viral Replication. Microorganisms 2024, 12, 425. [Google Scholar] [CrossRef]
- Iizasa, H.; Kim, H.; Kartika, A.V.; Kanehiro, Y.; Yoshiyama, H. Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases. Front. Immunol. 2020, 11, 367. [Google Scholar] [CrossRef]
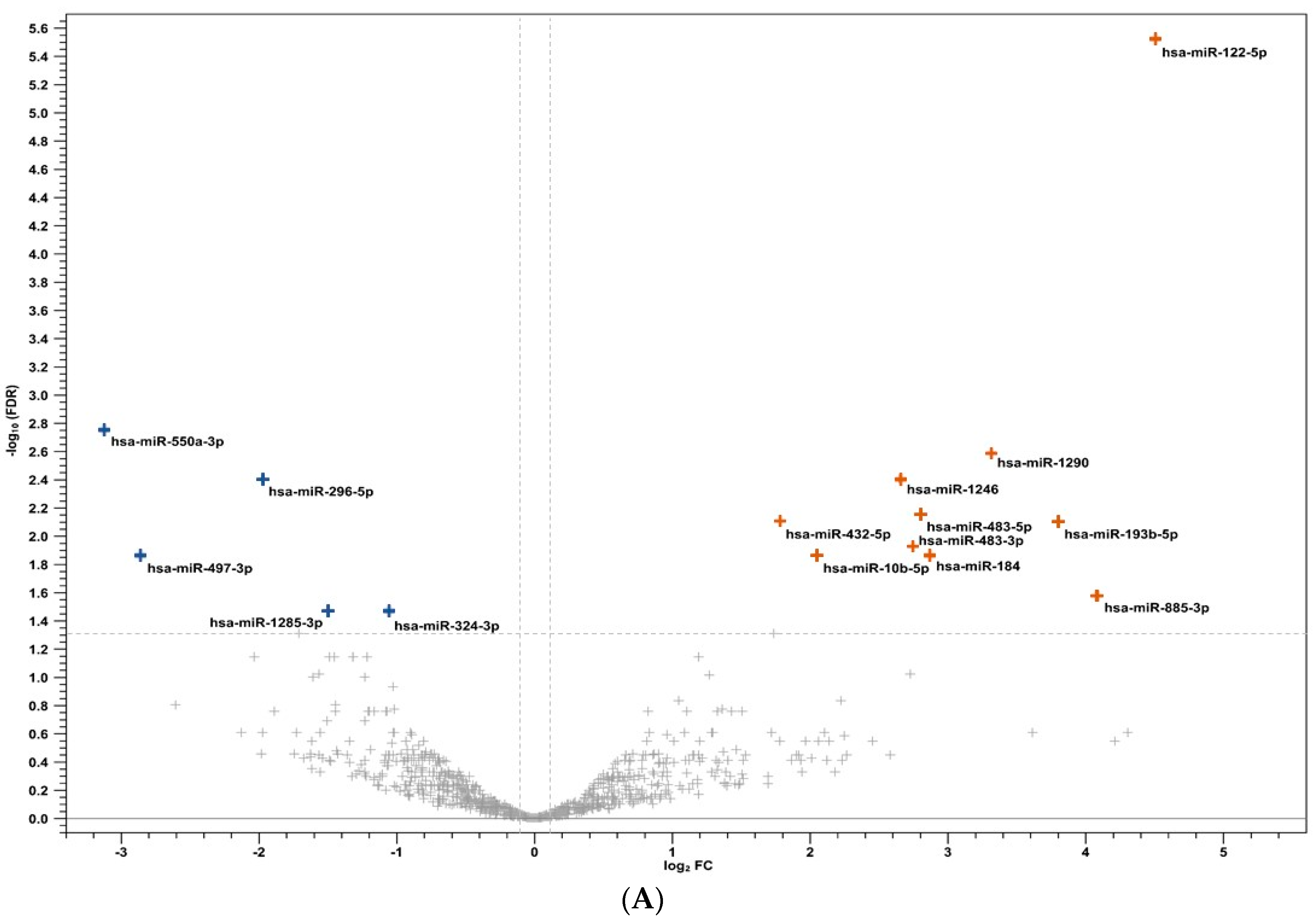
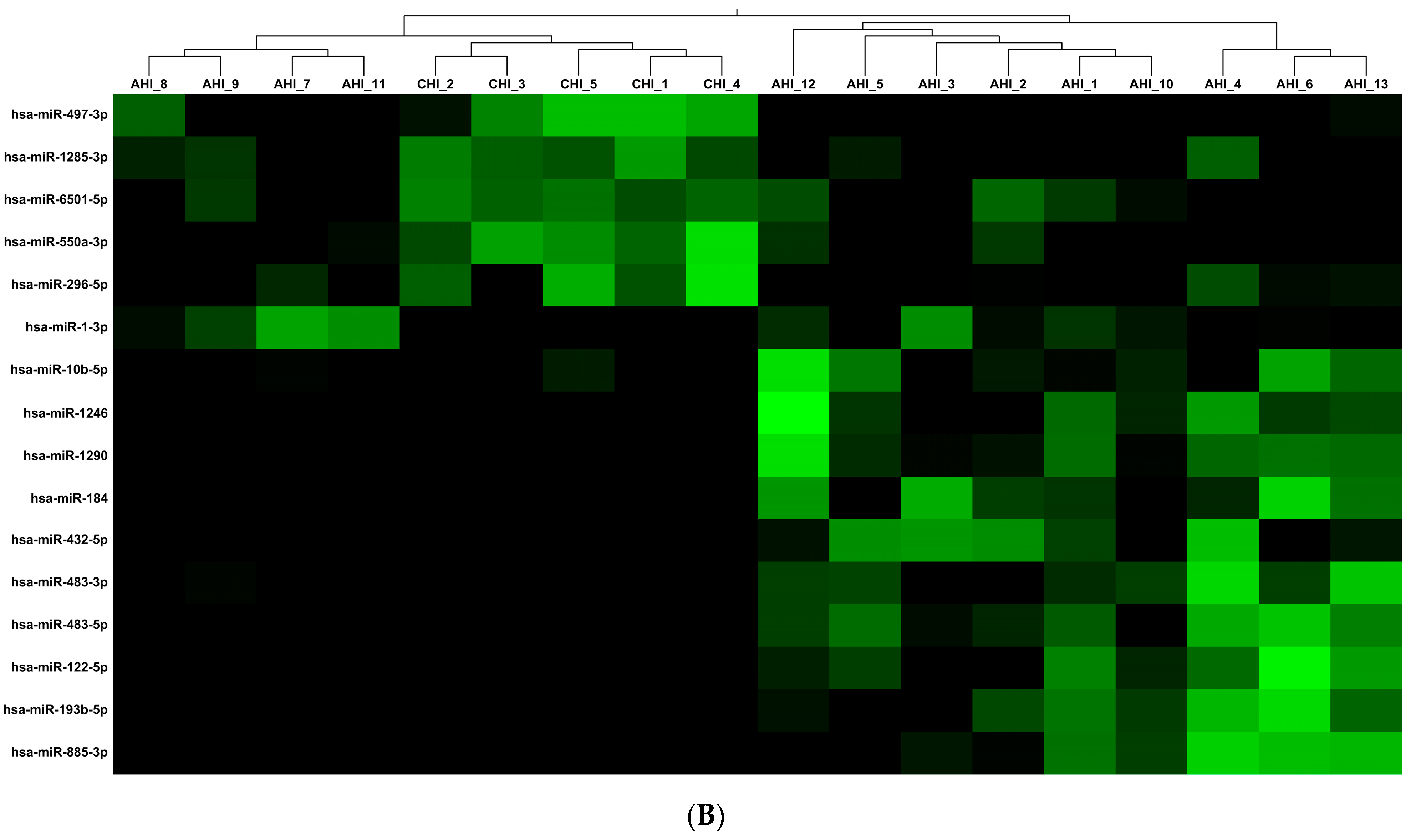
| Human Mature miRNA | AHI Mean % | CHI Mean % |
|---|---|---|
| hsa-miR-16-5p | 8.82 | 10.99 |
| hsa-let-7f-5p | 5.57 | 4.42 |
| hsa-miR-126-3p | 5.41 | 5.19 |
| hsa-let-7i-5p | 4.28 | 4.69 |
| hsa-let-7a-5p | 3.70 | 3.51 |
| hsa-miR-223-3p | 3.44 | 4.33 |
| hsa-miR-122-5p | 3.39 | <1 (0.18) |
| hsa-let-7b-5p | 2.55 | 2.95 |
| hsa-miR-142-3p | 2.30 | 3.09 |
| hsa-miR-21-5p | 2.07 | 2.47 |
| hsa-miR-103a-3p | 2.02 | 1.61 |
| hsa-miR-199a-3p | 2.02 | 1.87 |
| hsa-miR-146a-5p | 2.00 | 2.00 |
| hsa-miR-26a-5p | 1.81 | 2.34 |
| hsa-miR-26b-5p | 1.73 | 1.73 |
| hsa-miR-191-5p | 1.64 | 1.06 |
| hsa-miR-221-3p | 1.02 | 1.37 |
| hsa-miR-92a-3p | 1.5 | 1.04 |
| GO Term | Description | Detected Genes | DE Genes | DE Gene (Names) | MiRNAs (Names) | p-Values |
|---|---|---|---|---|---|---|
| 0030947 | regulation of the VEGF receptor signaling pathway | 4 | 2 | URS00001C3AC1_9606, URS000058760A_9606 | hsa-miR-296-5p, hsa-miR-10b-5p | 0.0045 |
| 0030949 | positive regulation of the VEGF receptor signaling pathway | 4 | 2 | URS00001C3AC1_9606, URS000058760A_9606 | hsa-miR-296-5p, hsa-miR-10b-5p | 0.0046 |
| 0090287 | regulation of cellular response to growth factor stimulus | 20 | 3 | URS00001C3AC1_9606, URS00001DC04F_9606, URS000058760A_9606 | hsa-miR-296-5p, hsa-miR-1-3p, hsa-miR-10b-5p | 0.0150 |
| 0051272 | positive regulation of cellular component movement | 40 | 4 | URS00001C3AC1_9606, URS00001DC04F_9606, URS000043F369_9606, URS000058760A_9606 | hsa-miR-296-5p, hsa-miR-1-3p, hsa-miR-1290, hsa-miR-10b-5p | 0.0166 |
| 1903672 | positive regulation of sprouting angiogenesis | 22 | 3 | URS00001C3AC1_9606, URS00001DC04F_9606, URS000058760A_9606 | hsa-miR-296-5p, hsa-miR-1-3p, hsa-miR-10b-5p | 0.0198 |
| Viral miRNA | Virus | AHI Positive/Total Subjects | CHI Positive/Total Subjects | Total Counts |
|---|---|---|---|---|
| ebv-miR-BART11-3p | EBV | 0/13 | 1/5 | 1 |
| ebv-miR-BART7-3p | 1/13 | 3/5 | 11 | |
| ebv-miR-BART9-3p | 0/13 | 1/5 | 1 | |
| ebv-miR-BART2-5p | 0/13 | 2/5 | 2 | |
| ebv-miR-BART6-3p | 0/13 | 1/5 | 1 | |
| ebv-miR-BART15 | 0/13 | 1/5 | 2 | |
| ebv-miR-BART8-5p | 0/13 | 2/5 | 3 | |
| ebv-miR-BART12 | 0/13 | 1/5 | 1 | |
| ebv-miR-BART19-3p | 0/13 | 2/5 | 2 | |
| ebv-miR-BART3-5p | 0/13 | 1/5 | 2 | |
| ebv-miR-BART17-5p | 0/13 | 1/5 | 1 | |
| ebv-miR-BART10-3p | 1/13 | 0/5 | 1 | |
| kshv-miR-K12-6-3p | Kaposi’s Sarcoma-associated Herpesvirus (HHV8) | 3/13 | 1/5 | 7 |
| kshv-miR-K12-8-3p | 1/13 | 1/5 | 2 | |
| kshv-miR-K12-10a-3p | 2/13 | 1/5 | 6 | |
| kshv-miR-K12-4-3p | 2/13 | 0/5 | 1 | |
| kshv-miR-K12-5-3p | 1/13 | 0/5 | 1 | |
| kshv-miR-K12-12-3p | 1/13 | 0/5 | 1 | |
| kshv-miR-K12-11-3p | 1/13 | 0/5 | 1 | |
| hcmv-miR-US25-2-5p | CMV | 2/13 | 1/5 | 80 |
| hcmv-miR-US25-2-3p | 2/13 | 0/5 | 63 | |
| hcmv-miR-US25-1-5p | 1/13 | 0/5 | 115 | |
| hcmv-miR-UL112-3p | 1/13 | 0/5 | 3 | |
| hcmv-miR-US29-3p | 1/13 | 0/5 | 1 | |
| hcmv-miR-US33-5p | 1/13 | 0/5 | 13 | |
| hcmv-miR-UL59 | 1/13 | 0/5 | 2 | |
| hcmv-miR-UL22A-5p | 1/13 | 0/5 | 53 | |
| hcmv-miR-UL22A-3p | 1/13 | 0/5 | 8 | |
| hcmv-miR-US33-3p | 1/13 | 0/5 | 2 | |
| hcmv-miR-US25-1-3p | 1/13 | 0/5 | 13 | |
| hcmv-miR-UL36-5p | 1/13 | 0/5 | 50 | |
| hcmv-miR-US5-1 | 1/13 | 0/5 | 9 | |
| hcmv-miR-US4-5p | 1/13 | 0/5 | 12 | |
| hcmv-miR-US29-5p | 1/13 | 0/5 | 9 | |
| hcmv-miR-US22-5p | 1/13 | 0/5 | 7 | |
| hcmv-miR-UL36-3p | 1/13 | 0/5 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, E.; Rozera, G.; Gagliardini, R.; Esvan, R.; Mondi, A.; Mazzotta, V.; Camici, M.; Girardi, E.; Antinori, A.; Maggi, F.; et al. Human and Viral microRNA Expression in Acute and Chronic HIV Infections. Viruses 2024, 16, 496. https://doi.org/10.3390/v16040496
Lazzari E, Rozera G, Gagliardini R, Esvan R, Mondi A, Mazzotta V, Camici M, Girardi E, Antinori A, Maggi F, et al. Human and Viral microRNA Expression in Acute and Chronic HIV Infections. Viruses. 2024; 16(4):496. https://doi.org/10.3390/v16040496
Chicago/Turabian StyleLazzari, Elisabetta, Gabriella Rozera, Roberta Gagliardini, Rozenn Esvan, Annalisa Mondi, Valentina Mazzotta, Marta Camici, Enrico Girardi, Andrea Antinori, Fabrizio Maggi, and et al. 2024. "Human and Viral microRNA Expression in Acute and Chronic HIV Infections" Viruses 16, no. 4: 496. https://doi.org/10.3390/v16040496
APA StyleLazzari, E., Rozera, G., Gagliardini, R., Esvan, R., Mondi, A., Mazzotta, V., Camici, M., Girardi, E., Antinori, A., Maggi, F., & Abbate, I. (2024). Human and Viral microRNA Expression in Acute and Chronic HIV Infections. Viruses, 16(4), 496. https://doi.org/10.3390/v16040496









