Development and Application of a Duplex RT-RPA Assay for the Simultaneous Detection of Cymbidium mosaic virus and Odontoglossum ringspot virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus and Plant Material
2.1.1. Positive Samples
2.1.2. Samples Collected from the Field for Detecting
2.2. Total RNA Extraction and Reverse Transcription
2.3. Primer Design and Synthesis
2.4. RPA Primer Screening
2.5. Construction of the Recombinant Plasmid Standard
2.6. Optimization of the Duplex RPA Method
2.7. Specificity Analysis
2.8. Sensitivity Analysis
2.9. RPA Reliability for Field Samples
3. Results
3.1. Screening of the Optimal Primer for the RPA Assay
3.2. Optimization of Duplex RPA Detection
3.2.1. Optimization of Primer Proportions and Amounts
3.2.2. Optimization of Amplification Temperature
3.2.3. Optimization of Amplification Time
3.3. Establishment of the Duplex RPA Reaction
3.4. Specific Detection
3.5. Sensitivity Detection
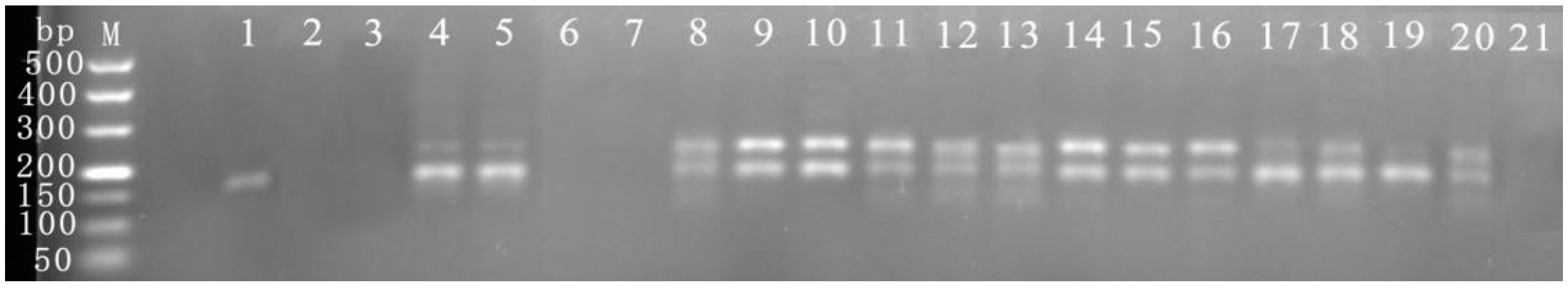
3.6. Sample Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Huang, H.; Luo, H.J.; Du, J.N.; Huang, X.Y.; Chen, Y.; Liang, W.Q.; Hu, S. Establishment of RT-LAMP-HNB method for detection of Cymbidium mosaic Virus and Odontoglossum ringspot Virus. Guangdong Agric. Sci. 2022, 49, 95–101. [Google Scholar]
- Song, Y.Q.; Liu, Z.L.; Willian, S.; Gao, J.Y. Characteristics of the orchid trade at public markets and implications for conservation in Xishuangbanna, Yunnan, China. Biodivers. Sci. 2017, 25, 531–539. [Google Scholar] [CrossRef]
- Chen, J.X.; Wei, Y.Q.; Tang, J.; Zhu, Y.J.; Ma, H.C.; Wu, J.R. Molecular detection and sequence analysis of ORSV and CymMV in cultivated Cymbidium goeringii in Dali, Yunnan. Acta Hortic. Sin. 2022, 49, 655–662. [Google Scholar]
- Lee, C.H.; Zheng, Y.X.; Jan, F.J. The Orchid-Infecting Viruses Found in the 21st Century; World Scientific Publishing: Singapore, 2015; pp. 145–164. [Google Scholar]
- Cheng, X.F.; Dong, J.H.; Fang, Q.; Li, T.T.; Ding, M.; Zhang, Z.K. Detection of a tospovirus infecting phalaenopsis amabilis in Yunnan. Acta Phytopathol. Sin. 2008, 1, 31–34. [Google Scholar]
- Eun, A.J.C.; Huang, L.; Chew, F.T.; Li, S.F.Y.; Wong, S.M. Detection of two orchid viruses using quartz crystal microbalance-based DNA biosensors. Phytopathology 2002, 92, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Xue, M.; Xie, L.; Guo, H.R.; Zhang, Z.S.; Zeng, R.Z. Research progress on technology of virus detection and virus-removing in orchids. J. Anhui Agric. Sci. 2022, 50, 8–11+18. [Google Scholar]
- Mahfut, M.; Daryono, B.; Indrianto, A.; Somowiyarjo, S. Plant-virus interaction on orchids infected Odontoglossum ringspot virus (ORSV) in bogor botanical garden, Indonesia. In Proceedings of the 1st International Conference on Science and Technology, ICOST 2019, Makassar, Indonesia, 2–3 May 2019. [Google Scholar] [CrossRef]
- López-Hernández, M.S.; Palacios-Popo, P.E.; Torre-Almaraz, R.D.L. Detection of Cymbidium mosaic virus (CymMV) and Odontoglossum ringspot virus (ORSV) from orchids in Mexico. Agrociencia 2014, 48, 525–536. [Google Scholar]
- Hang, N.T.M.; Thang, B.V.; Ngoc, P.B.; Ha, C.H. Cloning and sequence analysis of orchids CymMV and ORSV CP gene isolated in Northern of Vietnam. Ciclos En La Hist. La Econ. Y La Soc. 2013, 35, 363–368. [Google Scholar]
- Lakani, I.; Suastika, G.; Mattjik, N.; Damayanti, T.A. Identification and molecular characterization of Odontoglosum ringspot virus (ORSV) from Bogor, Indonesia. Hayati J. Biosci. 2010, 17, 101–104. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Wang, J.H.; Yu, Z.J.; Liang, D.C.; Liu, Z.X.; Mo, R. Symptomatological studies of cymbidium mosaic virus and odontoglossum ringspot virus infections on three tropical orchids. Chin. J. Trop. Agric. 2011, 31, 20–23. [Google Scholar]
- Shen, S.L. Species, detection and prevention of orchid viruses. Plant Quar. 1988, 2, 144–150. [Google Scholar]
- Sun, A.Q.; Wang, L.H.; Zhang, Y.P.; Yang, X.M.; Wei, Y.; Yang, D.; Li, W.H.; Wu, X.W. Establishment of a triplex TaqMan quantitative real-time PCR assay for simultaneous detection of Cymbidium mosaic virus, Odontoglossum ringspot virus and Cymbidium ringspot virus. Front. Microbiol. 2023, 14, 1129259. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.R.; Duan, L.F.; Yang, L.; Ma, Y.X.; Wang, Q.; Zhai, Y.Y.; Zhou, L.Y.; Zhang, S.; Ming, S.S.; Yang, G.Y.; et al. Study on replicationpPatterns of pseudorabies virus in cell lines with NF-kB family p65 gene knocked out. China Anim. Husb. Vet. Med. 2021, 48, 83–92. [Google Scholar]
- Jiang, Y.H.; Wu, Y.X. CRISPR/Cas9, a new era of genome editing, A brief introduction to the Nobel Prize in Chemistry 2020. Chin. J. Nat. 2020, 42, 456–462. [Google Scholar]
- Xie, L.N.; Su, M.Y.; Zhu, M.M.; Gong, H.T.; Zhu, L.M.; Xu, M.; Zhang, B.; Gan, L.M.; Luo, F.X. ELISA detection of two viruses in different Phalaenopsis species and their symptoms. Jiangsu Agric. Sci. 2017, 45, 80–83. [Google Scholar]
- Kim, S.M.; Choi, S.H. Simultaneous detection of Cymbidium mosaic virus and Odontoglossum ringspot virus in orchids using multiplex RT-PCR. Virus Genes 2015, 51, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.R.; Chen, Y.; Luo, X.C.; Du, Z.C.; Hao, K.Q.; An, M.N.; Xia, Z.H.; Wu, Y.H. Recombinase polymerase amplification assay for simultaneous detection of Maize Chlorotic Mottle Virus and Sugarcane Mosaic Virus in maize. ACS Omega 2021, 6, 18008–18013. [Google Scholar] [CrossRef]
- Prendeville, H.R.; Ye, X.; Morris, T.J.; Pilson, D. Virus infections in wild plant populations are both frequent and often unapparent. Am. J. Bot. 2012, 99, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, K.; Bardani, E.; Kallemi, P.; Kalantidis, K. Viral detection, Past, present, and future. Bioessays 2019, 41, e1900049. [Google Scholar] [CrossRef]
- Thamizhvanan, S.; Sivakumar, S.; Kumar, S.S.; Kumar, D.V.; Suryakodi, S.; Balaji, K.; Rajkumar, T.; Vimal, S.; Majeed, A.S.; Taju, G.; et al. Multiple infections caused by white spot syndrome virus and enterocytozoon hepatopenaei in pond-reared penaeus vannamei in India and multiplex PCR for their simultaneous detection. J. Fish Dis. 2019, 42, 447–454. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Euler, M.; Wang, Y.; Otto, P.; Tomaso, H.; Escudero, R.; Anda, P.; Hufert, F.T.; Weidmann, M. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J. Clin. Microbiol. 2012, 50, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.J.; Zang, Y.X.; Liu, H.B.; Li, P.; Wang, S. Recombinase polymerase amplification combined with lateral flow strip for listeria monocytogenes detection in food. J. Food Sci. 2018, 83, 1041–1047. [Google Scholar]
- Ma, B.; Fang, J.H.; Wang, Y.; He, H.Z.; Dai, M.Y.; Lin, W.; Su, W.; Zhang, M.Z. Isothermal method of a recombinase polymerase amplification assay for the detection of most common high-risk human papillomavirus type 16 and type 18 DNA. Clin. Lab. 2017, 63, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J.; Von-Stetten, F. Review, A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2018, 144, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Kim, S.M.; Jeong, R.D. Reverse transcription recombinase polymerase amplification assay for rapid and sensitive detection of barley yellow dwarf virus in oat. Plant Pathol. J. 2020, 36, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Qiao, N.; Sun, X.H.; Zhang, X.P.; Zhao, D.; Li, J.T.; Zhu, X.P. Reverse transcription recombinase polymerase amplification assay for rapid detection of the cucurbit chlorotic yellows virus. J. Virol. Methods 2022, 300, 114388. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Go, S.M.; Jeong, R.D. Rapid and specific detection of apple chlorotic leaf spot virus in pear by reverse-transcription recombinase polymerase amplification. Acta Virol. 2021, 65, 237–241. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Nie, X.; Zhong, Z.; Li, C.; Li, K.; Huang, W.; Fu, X.Y.; Liu, J.; Nie, B.H. Rapid and sensitive detection of potato virus Y by isothermal reverse transcription-recombinase polymerase amplification assay in potato. Mol. Cell. Probes 2020, 50, 101505. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, C.; Li, J.; Gu, Y.; Xia, C.; Xie, Q.; Xie, Y.B.; An, M.N.; Xia, Z.H.; Wu, Y.H. Characterization and a RT-RPA assay for rapid detection of chilli veinal mottle virus (ChiVMV) in tobacco. Virol. J. 2020, 17, 33. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Zhang, T.Y.; Lei, J.J.; Wang, Z.Q.; Liu, P.; Zhong, K.L.; Chen, J.P.; Liu, J.Q. Rapid, sensitive and simultaneous detection of two wheat RNA viruses using reverse transcription recombinase polymerase amplification (RT-RPA). Life 2022, 12, 1952. [Google Scholar] [CrossRef]
- Ma, B.; Li, J.; Chen, K.; Yu, X.; Sun, C.; Zhang, M. Multiplex recombinase polymerase amplification assay for the simultaneous detection of three foodborne pathogens in seafood. Food 2020, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pollak, N.M.; Macdonald, J. Multiplex detection of nucleic acids using recombinase polymerase amplification and a molecular colorimetric 7-segment display. ACS Omega 2019, 4, 11388–11396. [Google Scholar] [CrossRef] [PubMed]
- Kersting, S.; Rausch, V.; Bier, F.F.; von Nickisch-Rosenegk, M. Multiplex isothermal solid-phase recombinase polymerase amplification for the specific and fast DNA-based detection of three bacterial pathogens. Microchim. Acta 2014, 181, 1715–1723. [Google Scholar] [CrossRef]
- Lei, R.; Wang, X.; Zhang, D.; Liu, Y.; Chen, Q.; Jiang, N. Rapid isothermal duplex real-time recombinase polymerase amplification (RPA) assay for the diagnosis of equine piroplasmosis. Sci. Rep. 2020, 10, 4096. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Chen, X. Single-copy sensitive, field-deployable, and simultaneous dual-gene detection of SARS-COV-2 RNA via modified RT-RPA. Cell Discov. 2020, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J. Development of a multiplex real-time recombinase polymerase amplification (RPA) assay for rapid quantitative detection of Campylobacter coli and Jejuni from eggs and chicken products. Food Control 2016, 73, 1247–1255. [Google Scholar] [CrossRef]
- Song, H.C.; Zhang, Y.; Xing, B.; Xie, Y.C.; Liu, F.X.; Shi, X.Q. ELISA Detection of two viruses in tropical orchid. J. South China Univ. Trop. Agric. 2011, 2, 148–152. [Google Scholar]
- Kim, D.H.; Jeong, R.D.; Choi, S.; Ju, H.J.; Yoon, J.Y. Application of rapid and reliable detection of Cymbidium mosaic virus by reverse transcription recombinase polymerase amplification combined with lateral flow immunoassay. Plant Pathol. J. 2022, 38, 665–672. [Google Scholar] [CrossRef]
- Castellanos-Gonzalez, A.; White, A.C.; Melby, P.; Travi, B. Molecular diagnosis of protozoan parasites by recombinase polymerase amplification. Acta Trop. 2018, 182, 4–11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wang, J.; Sun, X.; Yuan, W. Recombinase polymerase amplification assay-a simple, fast and cost-effective alternative to real time PCR for specific detection of feline herpesvirus-1. PLoS ONE 2017, 12, e0166903. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.D.; Zhou, D.H.; Zhang, L.X.; Zheng, W.B.; Ma, J.G.; Wang, M.; Zhu, X.Q.; Xu, M.J. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for equipment-free detection of Cryptosporidium spp. oocysts in dairy cattle feces. Parasitol. Res. 2016, 115, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, B.; Li, J.; Chen, E.; Xu, Y.; Yu, X.; Sun, C.X.; Zhang, M.Z. A rapid and sensitive europium nanoparticle-based lateral flow immunoassay combined with recombinase polymerase amplification for simultaneous detection of three food-borne pathogens. Int. J. Environ. Res. Public Health 2021, 18, 4574. [Google Scholar] [CrossRef] [PubMed]
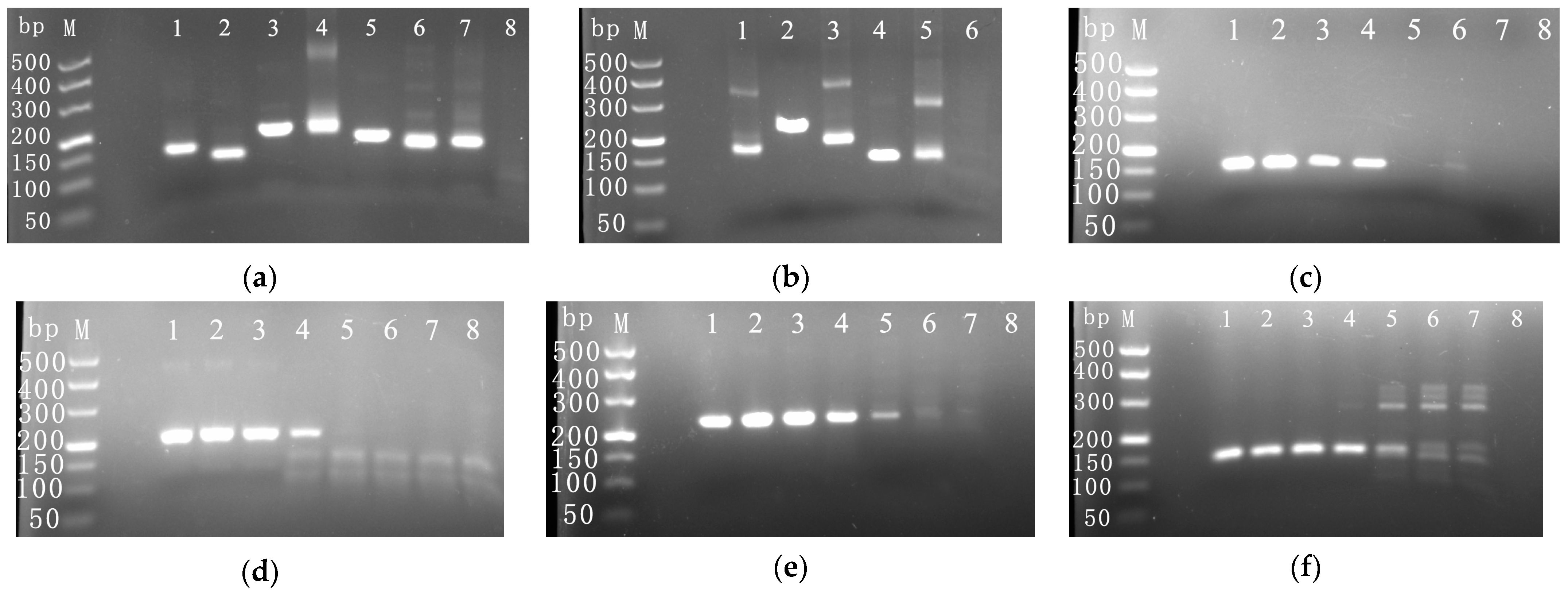
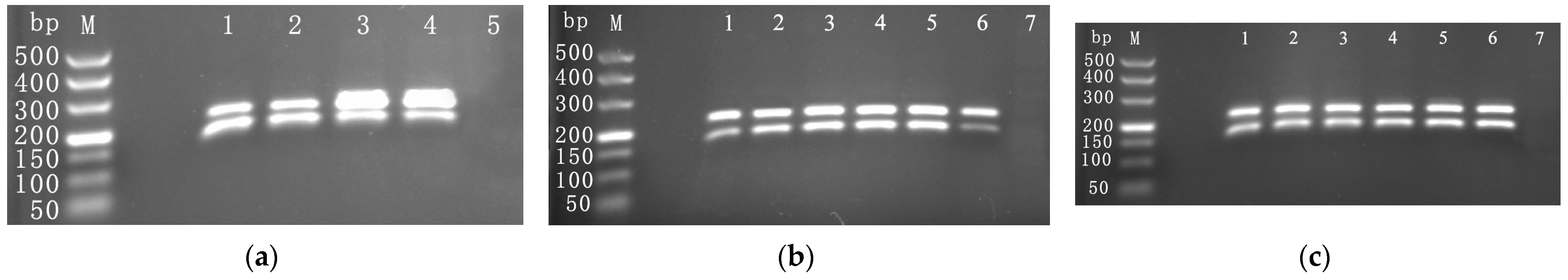
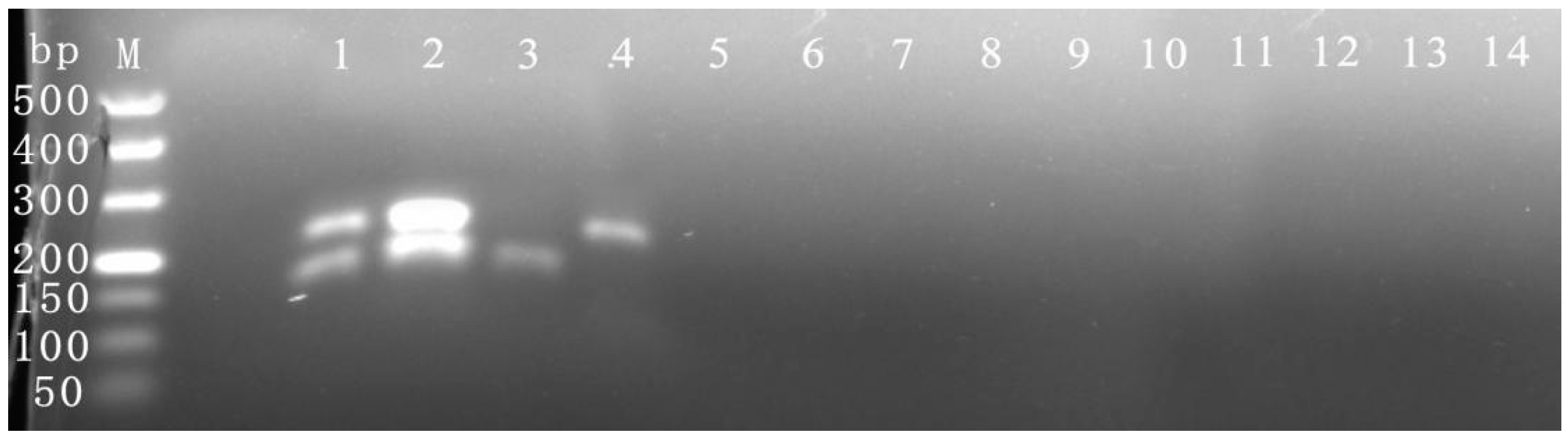
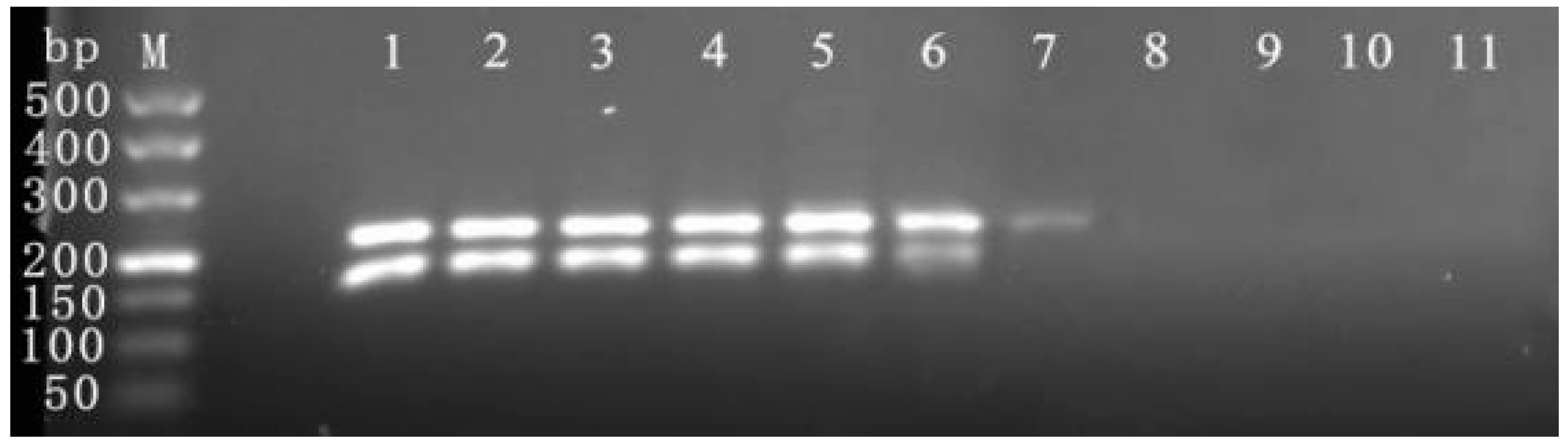
| Primer Name | Sequences (5′–3′) | Fragment Length (bp) |
|---|---|---|
| CymMV-F1 | AATCTGATGCTGGCCACTAACGATCCGCCCGC | 173 |
| CymMV-R1 | ATCGAGTGCGCAGCACGTTCACGGTCAGTAGGG | |
| CymMV-F2 | AATTGTGGGTTAACAACCTTGGCCTCCCCGCCG | 153 |
| CymMV-R2 | TAGAGCGGCGCGACGGACGTCAGGTTTCGTAGGG | |
| CymMV-F3 | AAACCTGACGTCCGTCGCGCCGCTCTAGCC | 226 |
| CymMV-R3 | ATTCAGCAGGTTCCAGTGCGGCAGTGGAATCG | |
| CymMV-F4 | ACACAGTAGGTACCGCGGCCATTGACCTGGC | 212 |
| CymMV-R4 | ATCGTTAGTGGCCAGCATCAGATTCCACACC | |
| CymMV-F5 | AAGAGTGCTACCCTGCTCGGTTTCTGCCCTACG | 200 |
| CymMV-R5 | AACCGAGTATCCTCCTGGAAACCAGCCTTGGC | |
| CymMV-F6 | TTCCAGGAGGATACTCGGTTTGCCGCCTTTGAC | 173 |
| CymMV-R6 | ATGAGGTTGCCGTTTTGGATACGCTGACGG | |
| CymMV-F7 | TTCCACTGCCGCACTGGAACCTGCTGAATGGC | 178 |
| CymMV-R7 | ATAGAGGGTGTTGGTGGAGCCAAGATGGCC | |
| ORSV-F1 | TTCCTACTTTGACCAGTAGGTTCCCTGCAGGC | 167 |
| ORSV-R1 | TAATGTTTCCGTAGTTGTCGGATTCTGCGG | |
| ORSV-F2 | AATCAGTTCCAAACACAACAAGCTCGAACAACTG | 224 |
| ORSV-R2 | TAGTTGTCGGATTCTGCGGATTTTCTACCTCG | |
| ORSV-F3 | TGCAGGCGCTGGTTACTTCAGAGTTTATCGC | 180 |
| ORSV-R3 | ATTGCTACAGTTGCATCATCAACTCTACGAG | |
| ORSV-F4 | TAGAAAATCCGCAGAATCCGACAACTACGG | 145 |
| ORSV-R4 | AATGAGACTTGATTGTACATACCAGTTCCC | |
| ORSV-F5 | ATGCAACTCGTAGAGTTGATGATGCAACTGTAGC | 142 |
| ORSV-R5 | TAGGAAGAGGTCCAAGTAAGTCCAGACATCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, A.; Wang, L.; Zhang, Y.; Yang, X.; Su, Y.; Wu, X. Development and Application of a Duplex RT-RPA Assay for the Simultaneous Detection of Cymbidium mosaic virus and Odontoglossum ringspot virus. Viruses 2024, 16, 543. https://doi.org/10.3390/v16040543
Sun A, Wang L, Zhang Y, Yang X, Su Y, Wu X. Development and Application of a Duplex RT-RPA Assay for the Simultaneous Detection of Cymbidium mosaic virus and Odontoglossum ringspot virus. Viruses. 2024; 16(4):543. https://doi.org/10.3390/v16040543
Chicago/Turabian StyleSun, Aiqing, Lihua Wang, Yiping Zhang, Xiumei Yang, Yan Su, and Xuewei Wu. 2024. "Development and Application of a Duplex RT-RPA Assay for the Simultaneous Detection of Cymbidium mosaic virus and Odontoglossum ringspot virus" Viruses 16, no. 4: 543. https://doi.org/10.3390/v16040543
APA StyleSun, A., Wang, L., Zhang, Y., Yang, X., Su, Y., & Wu, X. (2024). Development and Application of a Duplex RT-RPA Assay for the Simultaneous Detection of Cymbidium mosaic virus and Odontoglossum ringspot virus. Viruses, 16(4), 543. https://doi.org/10.3390/v16040543









