Two Novel Geminiviruses Identified in Bees (Apis mellifera and Nomia sp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. High-Throughput Sequencing, De Novo Assembly, and Identification of Viral-like Contigs
2.3. Screening and Verification of Geminivirus Genomes
2.4. Geminivirus Sequence Analyses
3. Results
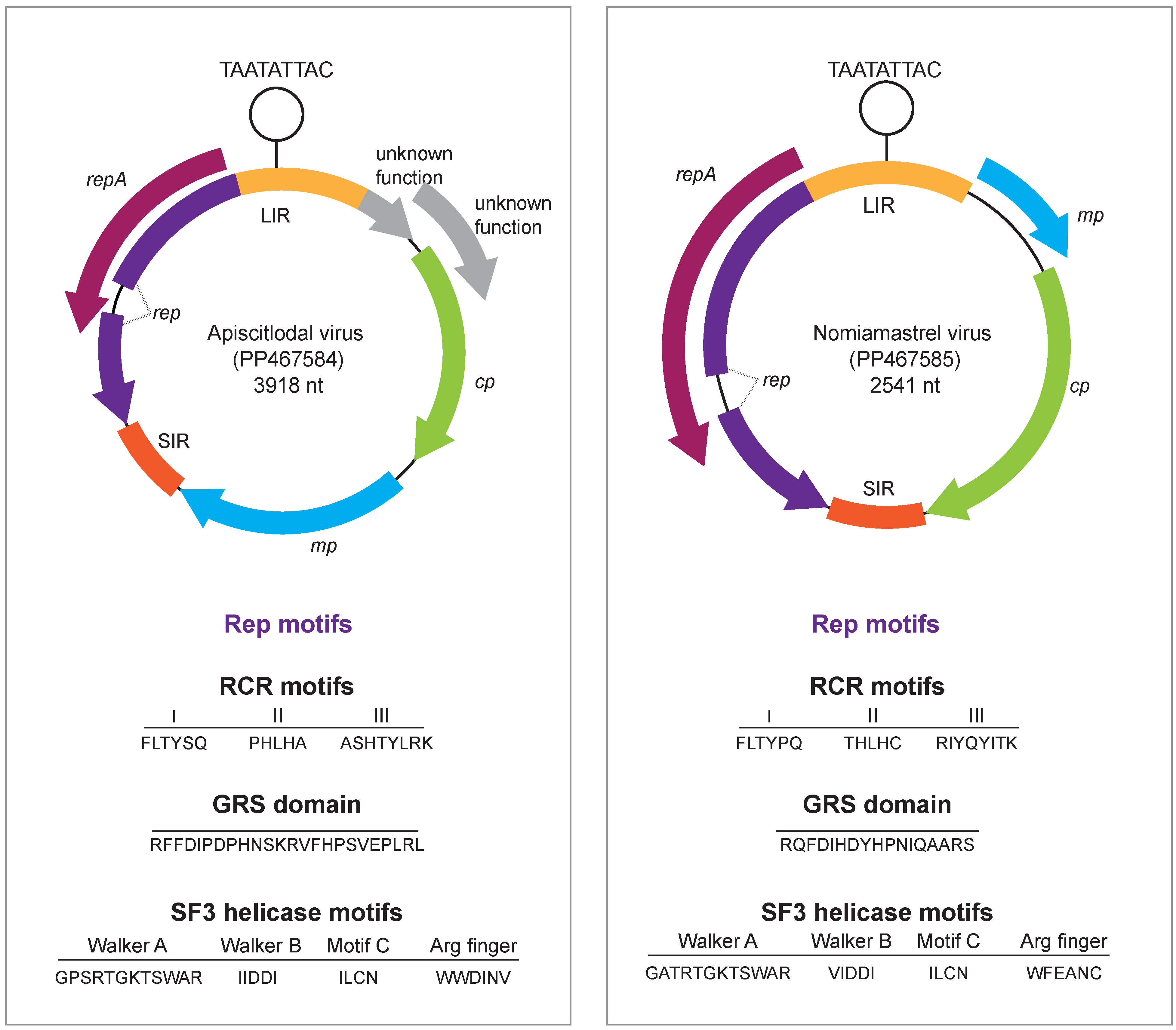
3.1. Identification of Geminiviruses
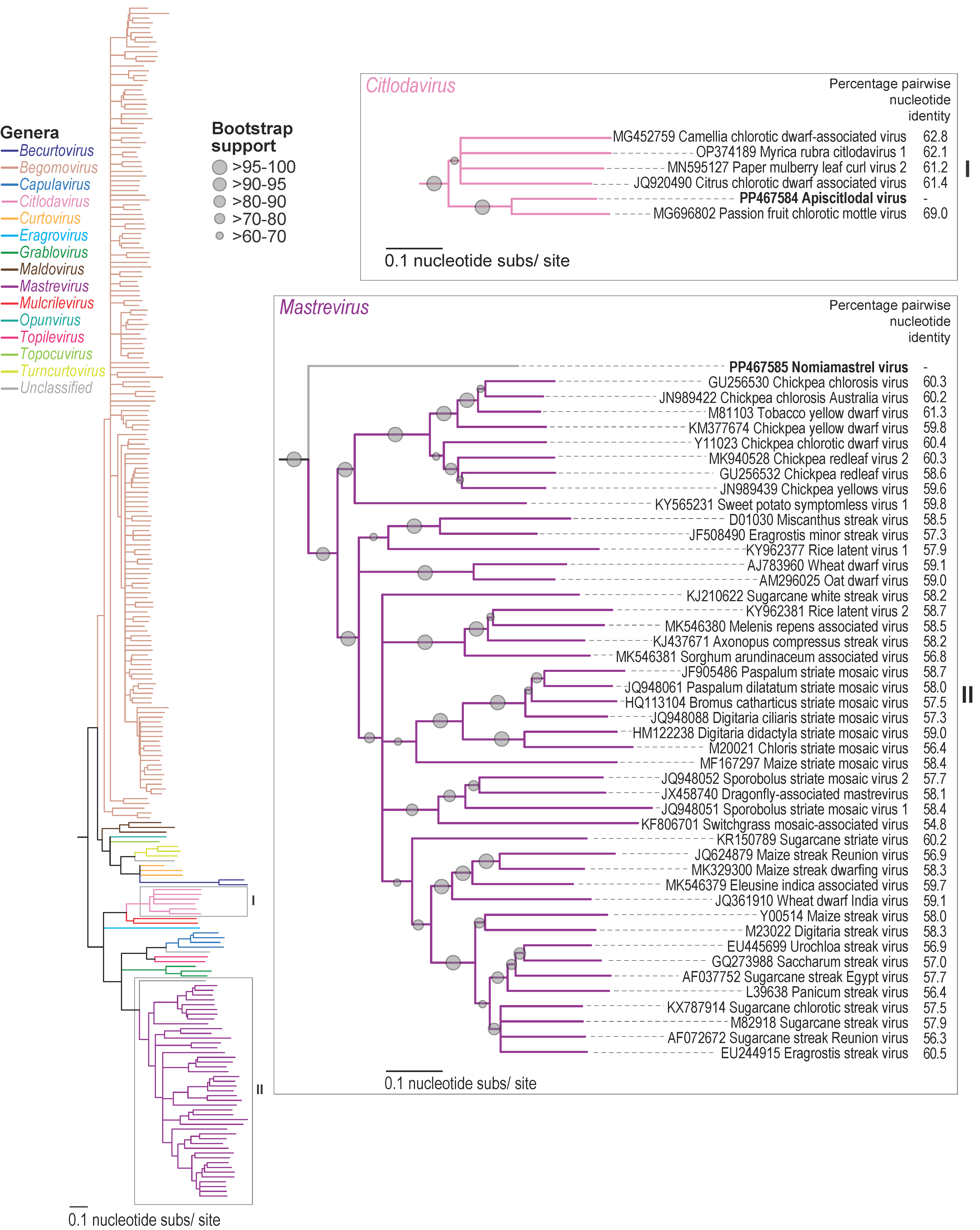
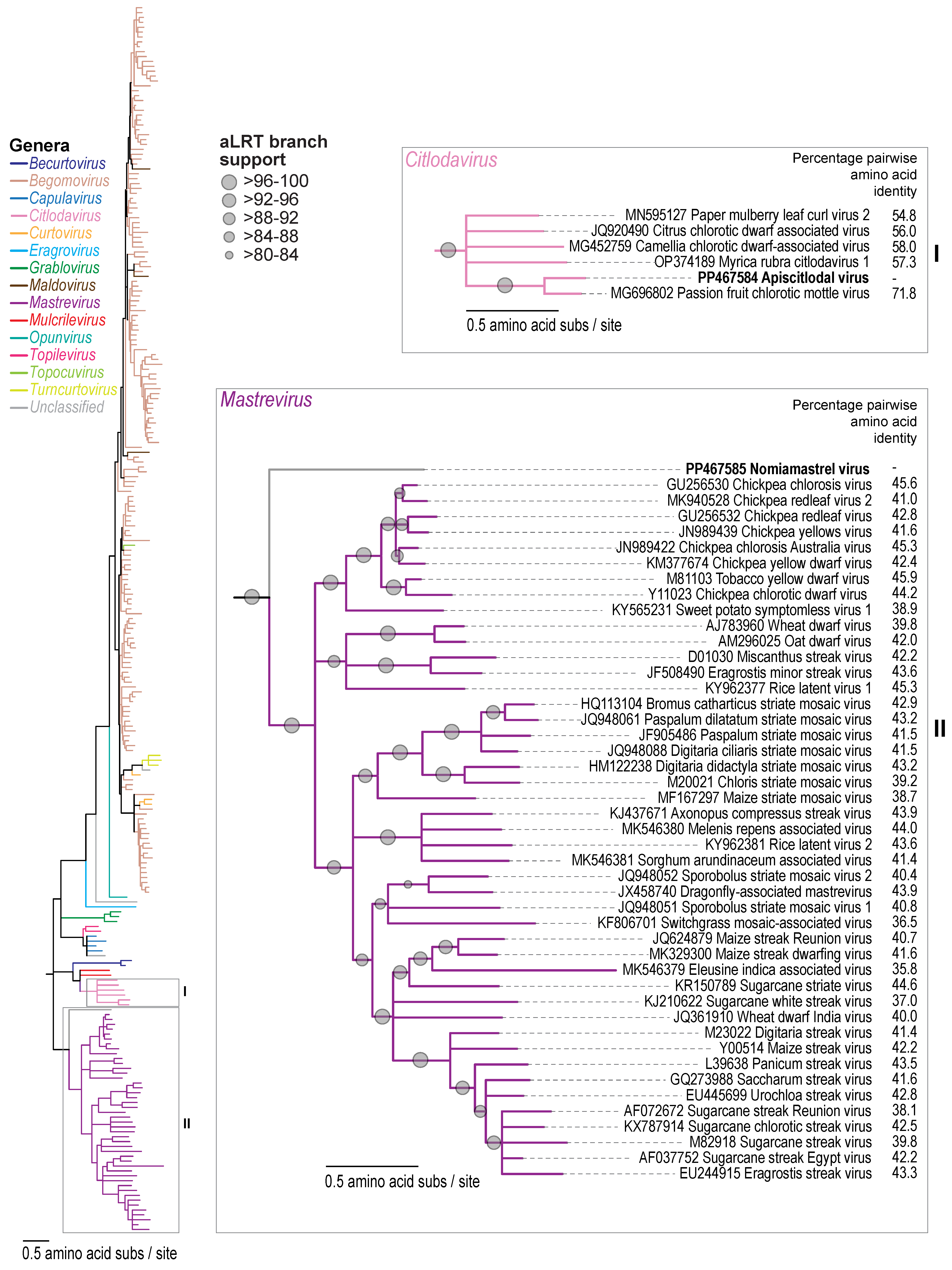
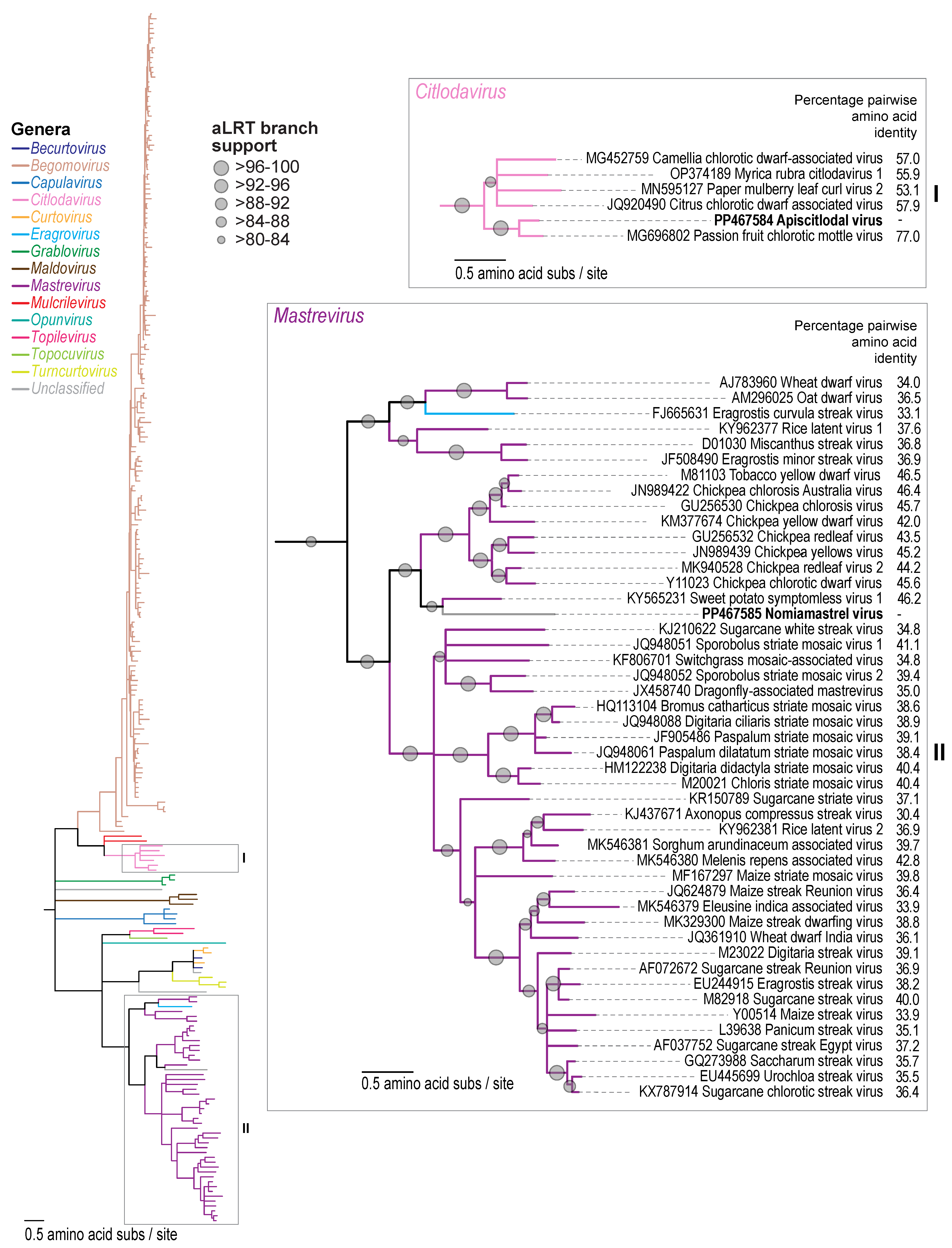
3.2. Citlodavirus Genome Identified in a Honeybee from Jamaica
3.3. Mastrevirus-like Genome Identified in a Solitary Bee from Arizona, USA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krupovic, M.; Varsani, A.; Kazlauskas, D.; Breitbart, M.; Delwart, E.; Rosario, K.; Yutin, N.; Wolf, Y.I.; Harrach, B.; Zerbini, F.M.; et al. Cressdnaviricota: A Virus Phylum Unifying Seven Families of Rep-Encoding Viruses with Single-Stranded, Circular DNA Genomes. J. Virol. 2020, 94, e00582-20. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olive, E.; Lett, J.M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J. ICTV Virus Taxonomy Profile: Geminiviridae 2021. J. Gen. Virol. 2021, 102, 001696. [Google Scholar] [CrossRef] [PubMed]
- Navot, N.; Pichersky, E.; Zeidan, M.; Zamir, D.; Czosnek, H. Tomato yellow leaf curl virus: A whitefly-transmitted geminivirus with a single genomic component. Virology 1991, 185, 151–161. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Z.; Wang, Y.M.; Yin, T.Y.; Fiallo-Olive, E.; Liu, Y.Q.; Hanley-Bowdoin, L.; Wang, X.W. A plant DNA virus replicates in the salivary glands of its insect vector via recruitment of host DNA synthesis machinery. Proc. Natl. Acad. Sci. USA 2020, 117, 16928–16937. [Google Scholar] [CrossRef] [PubMed]
- Briddon, R.W.; Bedford, I.D.; Tsai, J.H.; Markham, P.G. Analysis of the nucleotide sequence of the treehopper-transmitted geminivirus, tomato pseudo-curly top virus, suggests a recombinant origin. Virology 1996, 219, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Roumagnac, P.; Granier, M.; Bernardo, P.; Deshoux, M.; Ferdinand, R.; Galzi, S.; Fernandez, E.; Julian, C.; Abt, I.; Filloux, D.; et al. Alfalfa Leaf Curl Virus: An Aphid-Transmitted Geminivirus. J. Virol. 2015, 89, 9683–9688. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Padilla-Rodriguez, M.; Kraberger, S.; Stainton, D.; Martin, D.P.; Breitbart, M.; Varsani, A. Discovery of a novel mastrevirus and alphasatellite-like circular DNA in dragonflies (Epiprocta) from Puerto Rico. Virus Res. 2013, 171, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.F.; Willner, D.L.; Lim, Y.W.; Schmieder, R.; Chau, B.; Nilsson, C.; Anthony, S.; Ruan, Y.; Rohwer, F.; Breitbart, M. Broad surveys of DNA viral diversity obtained through viral metagenomics of mosquitoes. PLoS ONE 2011, 6, e20579. [Google Scholar] [CrossRef] [PubMed]
- Torres-deLosSantos, R.; Salvador-Figueroa, M.; Gallegos-Gómez, E.H.; Arévalo-Monterrubio, L.D.; Rincón-Rabanales, M.; Grajales-Conesa, J. Detection of begomovirus in the stingless bee Trigona fuscipennis visiting Jatropha curcas in the South of Mexico. J. Apic. Res. 2016, 55, 185–186. [Google Scholar] [CrossRef]
- Carboney-Mejía, I.G.; Ruíz-Toledo, J.; Hernández-Reyes, J.; Lucio-Castillo, H.; Hernandez-Robledo, V.; Torres-Castillo, J.A.; Torres-de los Santos, R. Prevalence of begomoviruses in Trigona fuscipennis and Trigona fulviventris visitors of Jatropha curcas. J. Apic. Res. 2020, 62, 311–314. [Google Scholar] [CrossRef]
- Zhang, W.; Olson, N.H.; Baker, T.S.; Faulkner, L.; Agbandje-McKenna, M.; Boulton, M.I.; Davies, J.W.; McKenna, R. Structure of the Maize streak virus geminate particle. Virology 2001, 279, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, E.L.; Saunders, K.; Fisher, C.; Potze, J.; Stanley, J.; Lomonossoff, G.P.; Ranson, N.A. The 3.3 A structure of a plant geminivirus using cryo-EM. Nat. Commun. 2018, 9, 2369. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, T.V.; Koonin, E.V. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 1992, 20, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V.; Wolf, Y.I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990, 262, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; Dallas, M.B.; Reyes, M.I.; Buhrman, G.K.; Ascencio-Ibanez, J.T.; Hanley-Bowdoin, L. Functional analysis of a novel motif conserved across geminivirus Rep proteins. J. Virol. 2011, 85, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shen, P.; Li, M.; Tian, X.; Zhou, C.; Cao, M. Discovery of a novel geminivirus associated with camellia chlorotic dwarf disease. Arch. Virol. 2018, 163, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Anane, R.F.; Chen, Z.; He, Y.; Li, S.; Zi, S.; Yang, Z.; Chu, B.; Wen, G.; Zhao, M. Complete genome sequence analysis of a novel citlodavirus isolated from the leaves of Myrica rubra in Yunnan. Arch. Virol. 2023, 168, 139. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.S.; Abreu, R.A.; Lamas, N.S.; Alves-Freitas, D.M.T.; Vidal, A.H.; Poppiel, R.R.; Melo, F.L.; Lacorte, C.; Martin, D.P.; Campos, M.A.; et al. Passion Fruit Chlorotic Mottle Virus: Molecular Characterization of a New Divergent Geminivirus in Brazil. Viruses 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, S.; Yu, H.; Xuan, Z.; Yang, L.; Zhan, B.; Murilo Zerbini, F.; Cao, M. Identification and Characterization of Two Novel Geminiviruses Associated with Paper Mulberry (Broussonetia papyrifera) Leaf Curl Disease. Plant Dis. 2020, 104, 3010–3018. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, L.; Zhao, J.; Li, T.; Liu, Q.; Cao, M.; Zhou, Y. First Report of Citrus Chlorotic Dwarf-Associated Virus on Pomelo in Nakhon, Thailand. Plant Dis. 2020, 104, 1262. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Liu, Y.; Chen, H.; Li, T.; Zhou, C. Distribution and molecular characterization of citrus chlorotic dwarf-associated virus in China. Australas. Plant Pathol. 2017, 46, 227–229. [Google Scholar] [CrossRef]
- Loconsole, G.; Saldarelli, P.; Doddapaneni, H.; Savino, V.; Martelli, G.P.; Saponari, M. Identification of a single-stranded DNA virus associated with citrus chlorotic dwarf disease, a new member in the family Geminiviridae. Virology 2012, 432, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Roumagnac, P.; Lett, J.M.; Fiallo-Olive, E.; Navas-Castillo, J.; Zerbini, F.M.; Martin, D.P.; Varsani, A. Establishment of five new genera in the family Geminiviridae: Citlodavirus, Maldovirus, Mulcrilevirus, Opunvirus, and Topilevirus. Arch. Virol. 2022, 167, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Greber, R.S. Biological characteristics of grass geminiviruses from eastern Australia. Ann. Appl. Biol. 2008, 114, 471–480. [Google Scholar] [CrossRef]
- Kumari, S.G.; Makkouk, K.M.; Attar, N.; Ghulam, W.; Lesemann, D.E. First Report of Chickpea Chlorotic Dwarf Virus Infecting Spring Chickpea in Syria. Plant Dis. 2004, 88, 424. [Google Scholar] [CrossRef] [PubMed]
- Horn, N.M.; Reddy, S.V.; Roberts, I.M.; Reddy, D.V.R. Chickpea chlorotic dwarf virus, a new leafhopper-transmitted geminivirus of chickpea in India1. Ann. Appl. Biol. 2008, 122, 467–479. [Google Scholar] [CrossRef]
- Rose, D.J.W. The epidemiology of maize streak disease in relation to population densities of Cicadulina spp. Ann. Appl. Biol. 2008, 76, 199–207. [Google Scholar] [CrossRef]
- Muhire, B.; Martin, D.P.; Brown, J.K.; Navas-Castillo, J.; Moriones, E.; Zerbini, F.M.; Rivera-Bustamante, R.; Malathi, V.G.; Briddon, R.W.; Varsani, A. A genome-wide pairwise-identity-based proposal for the classification of viruses in the genus Mastrevirus (family Geminiviridae). Arch. Virol. 2013, 158, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Fetters, A.M.; Ashman, T.L. The pollen virome: A review of pollen-associated viruses and consequences for plants and their interactions with pollinators. Am. J. Bot. 2023, 110, e16144. [Google Scholar] [CrossRef]
- Fetters, A.M.; Cantalupo, P.G.; Wei, N.; Robles, M.T.S.; Stanley, A.; Stephens, J.D.; Pipas, J.M.; Ashman, T.L. The pollen virome of wild plants and its association with variation in floral traits and land use. Nat. Commun. 2022, 13, 523. [Google Scholar] [CrossRef]
- Roberts, J.M.K.; Ireland, K.B.; Tay, W.T.; Paini, D. Honey bee-assisted surveillance for early plant virus detection. Ann. Appl. Biol. 2018, 173, 285–293. [Google Scholar] [CrossRef]
- Childress, A.; Ramsdell, D. Via Infected Pollen in Highbush Blueberry. Phytopathology 1987, 77, 167–172. [Google Scholar] [CrossRef]
- Gebremedhn, H.; Deboutte, W.; Schoonvaere, K.; Demaeght, P.; De Smet, L.; Amssalu, B.; Matthijnssens, J.; de Graaf, D.C. Metagenomic Approach with the NetoVIR Enrichment Protocol Reveals Virus Diversity within Ethiopian Honey Bees (Apis mellifera simensis). Viruses 2020, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Vansia, R.; Phelan, J.; Lofano, A.; Smith, A.; Wang, A.; Bilodeau, G.J.; Pernal, S.F.; Guarna, M.M.; Rott, M.; et al. Area Wide Monitoring of Plant and Honey Bee (Apis mellifera) Viruses in Blueberry (Vaccinium corymbosum) Agroecosystems Facilitated by Honey Bee Pollination. Viruses 2023, 15, 1209. [Google Scholar] [CrossRef] [PubMed]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Cornman, R.S.; Evans, J.D.; Pettis, J.S.; Zhao, Y.; Murphy, C.; Peng, W.J.; Wu, J.; Hamilton, M.; Boncristiani, H.F., Jr.; et al. Systemic spread and propagation of a plant-pathogenic virus in European honeybees, Apis mellifera. mBio 2014, 5, e00813–e00898. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Luo, L.X.; Li, J.Q.; Liu, P.F.; Chen, X.Y.; Hao, J.J. Pollen and seed transmission of Cucumber green mottle mosaic virus in cucumber. Plant Pathol. 2013, 63, 72–77. [Google Scholar] [CrossRef]
- Okada, K.; Kusakari, S.-i.; Kawaratani, M.; Negoro, J.-i.; Ohki, S.T.; Osaki, T. Tobacco mosaic virus Is Transmissible from Tomato to Tomato by Pollinating Bumblebees. J. Gen. Plant Pathol. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Roberts, J.M.K.; Anderson, D.L.; Durr, P.A. Metagenomic analysis of Varroa-free Australian honey bees (Apis mellifera) shows a diverse Picornavirales virome. J. Gen. Virol. 2018, 99, 818–826. [Google Scholar] [CrossRef]
- Schoonvaere, K.; Smagghe, G.; Francis, F.; de Graaf, D.C. Study of the Metatranscriptome of Eight Social and Solitary Wild Bee Species Reveals Novel Viruses and Bee Parasites. Front. Microbiol. 2018, 9, 177. [Google Scholar] [CrossRef]
- Smadi, M.; Lee, E.; Phelan, J.; Wang, A.; Bilodeau, G.J.; Pernal, S.F.; Guarna, M.M.; Rott, M.; Griffiths, J.S. Plant virus diversity in bee and pollen samples from apple (Malus domestica) and sweet cherry (Prunus avium) agroecosystems. Front. Plant Sci. 2024, 15, 1335281. [Google Scholar] [CrossRef]
- Tayal, M.; Wilson, C.; Cieniewicz, E. Bees and thrips carry virus-positive pollen in peach orchards in South Carolina, United States. J. Econ. Entomol. 2023, 116, 1091–1101. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Reuter, K.; Drost, H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Tisza, M.J.; Belford, A.K.; Dominguez-Huerta, G.; Bolduc, B.; Buck, C.B. Cenote-Taker 2 democratizes virus discovery and sequence annotation. Virus Evol. 2021, 7, veaa100. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Stover, B.C.; Muller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Wu, Z.J.; Xia, Z.S.; Xie, L.H. Complete genome sequence of a novel monopartite geminivirus identified in mulberry (Morus alba L.). Arch. Virol. 2015, 160, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Navarro, B.; Zhang, Z.; Lu, M.; Zhou, X.; Chi, S.; Di Serio, F.; Li, S. Identification and molecular characterization of a novel monopartite geminivirus associated with mulberry mosaic dwarf disease. J. Gen. Virol. 2015, 96, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Shepherd, D.N.; Dent, K.; Monjane, A.L.; Rybicki, E.P.; Martin, D.P. A highly divergent South African geminivirus species illuminates the ancient evolutionary history of this family. Virol. J. 2009, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.A.; Richardson, K.A.; Haley, A.; Zhan, X.; Thomas, J.E. The nucleotide sequence of the infectious cloned DNA component of tobacco yellow dwarf virus reveals features of geminiviruses infecting monocotyledonous plants. Virology 1992, 187, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Koklu, G.; Ramsell, J.N.; Kvarnheden, A. The complete genome sequence for a Turkish isolate of Wheat dwarf virus (WDV) from barley confirms the presence of two distinct WDV strains. Virus Genes 2007, 34, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Lan, P.; Li, F.; Abad, J.; Zhou, C.; Li, R. Genome characterization of sweet potato symptomless virus 1: A mastrevirus with an unusual nonanucleotide sequence. Arch. Virol. 2017, 162, 2881–2884. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Perez, A.; Untiveros, M.; Quispe, D.; Fuentes, S.; Barker, I.; Simon, R. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 2009, 388, 1–7. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019, 17, 632–644. [Google Scholar] [CrossRef]
- Jerbi-Zrelli, N.; Bouslama, T.; Boukhris-Bouhachem, S.; Chaieb, I.; Mnari-Hattab, M.; Laarif, A. Morphological and molecular characterisations of Orosius albicinctus (Distant, 1918). Oriental. Insects 2024, 1–16. [Google Scholar] [CrossRef]
- Singh, R.; Levitt, A.L.; Rajotte, E.G.; Holmes, E.C.; Ostiguy, N.; Vanengelsdorp, D.; Lipkin, W.I.; Depamphilis, C.W.; Toth, A.L.; Cox-Foster, D.L. RNA viruses in hymenopteran pollinators: Evidence of inter-Taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS ONE 2010, 5, e14357. [Google Scholar] [CrossRef] [PubMed]
- Maunder, M.; Abdo, M.; Berazain, R.; Clubbe, C.; Jiménez, F.; Leiva, A.; Santiago-Valentín, E.; Jestrow, B.; Francisco-Ortega, J.; Bramwell, D. The plants of the Caribbean islands: A review of the biogeography, diversity and conservation of a storm-battered biodiversity hotspot. In The Biology of Island Floras; Cambridge Press: Cambridge, UK, 2011; pp. 154–178. [Google Scholar]
- Rose, P.; Benkeblia, N. Plants of horticultural importance in Jamaica and the Caribbean region: Botany and distribution. In Proceedings of the I International Symposium on Tropical Horticulture 894, Kingston, Jamaica, 22–26 November 2010; pp. 57–64. [Google Scholar]
- Raw, A. The ecology of Jamaican bees (Hymenoptera). Rev. Bras. Entomol. 1985, 29, 1–16. [Google Scholar]
- Farr, T.; Bretting, P. Pollinators and Dispersers of Jamaican Forest Species. In Proceedings of the Forests of Jamaica: Papers from the Caribbean Regional Seminar on Forests of Jamaica, Kingston, Jamaica; 1983, 1986; p. 66. [Google Scholar]
- Hatcher, E.L.; Zhdanov, S.A.; Bao, Y.; Blinkova, O.; Nawrocki, E.P.; Ostapchuck, Y.; Schaffer, A.A.; Brister, J.R. Virus Variation Resource–improved response to emergent viral outbreaks. Nucleic Acids Res. 2017, 45, D482–D490. [Google Scholar] [CrossRef] [PubMed]
- Roye, M.E.; McLaughlin, W.A.; Nakhla, M.K.; Maxwell, D.P. Genetic Diversity Among Geminiviruses Associated with the Weed Species Sida spp., Macroptilium lathyroides, and Wissadula amplissima from Jamaica. Plant Dis. 1997, 81, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Simmonds-Gordon, R.N.; Collins-Fairclough, A.M.; Stewart, C.S.; Roye, M.E. First report of a complete genome sequence for a begomovirus infecting Jatropha gossypifolia in the Americas. Arch. Virol. 2014, 159, 2815–2818. [Google Scholar] [CrossRef] [PubMed]
- Roye, M.; Collins, A.; Maxwell, D. First report of a begomovirus associated with the common weed Jatropha gossypiifolia in Jamaica. Plant Pathol. 2006, 55, 286. [Google Scholar] [CrossRef]
- Amarakoon, I.I.; Roye, M.E.; Briddon, R.W.; Bedford, I.D.; Stanley, J. Molecular and biological characterization of Macroptilium yellow mosaic virus from Jamaica. Plant Pathol. 2008, 57, 417–426. [Google Scholar] [CrossRef]
- Stewart, C.; Kon, T.; Rojas, M.; Graham, A.; Martin, D.; Gilbertson, R.; Roye, M. Mixed infection of Sida jamaicensis in Jamaica reveals the presence of three recombinant begomovirus DNA A components. Arch. Virol. 2014, 159, 2509–2512. [Google Scholar] [CrossRef]
- USDA. USDA/NASS 2023 State Agriculture Overview for Arizona. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=ARIZONA (accessed on 14 March 2024).
- Cane, J.H. A native ground-nesting bee (Nomia melanderi) sustainably managed to pollinate alfalfa across an intensively agricultural landscape. Apidologie 2008, 39, 315–323. [Google Scholar] [CrossRef]
- Cane, J.H.; Dobson, H.E.M.; Boyer, B. Timing and size of daily pollen meals eaten by adult females of a solitary bee (Nomia melanderi) (Apiformes: Halictidae). Apidologie 2016, 48, 17–30. [Google Scholar] [CrossRef]
- Fontenele, R.S.; Salywon, A.M.; Majure, L.C.; Cobb, I.N.; Bhaskara, A.; Avalos-Calleros, J.A.; Arguello-Astorga, G.R.; Schmidlin, K.; Khalifeh, A.; Smith, K.; et al. New World Cactaceae Plants Harbor Diverse Geminiviruses. Viruses 2021, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.S.; Salywon, A.M.; Majure, L.C.; Cobb, I.N.; Bhaskara, A.; Avalos-Calleros, J.A.; Arguello-Astorga, G.R.; Schmidlin, K.; Khalifeh, A.; Smith, K.; et al. A Novel Divergent Geminivirus Identified in Asymptomatic New World Cactaceae Plants. Viruses 2020, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.S.; Alves-Freitas, D.M.T.; Silva, P.I.T.; Foresti, J.; Silva, P.R.; Godinho, M.T.; Varsani, A.; Ribeiro, S.G. Discovery of the first maize-infecting mastrevirus in the Americas using a vector-enabled metagenomics approach. Arch. Virol. 2018, 163, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Posse, A.; Fernandez, F.; Reyna, P.; Nome, C.; Torrico, A.K.; Gimenez Pecci, M.P.; Rodriguez Pardina, P. First report of Maize striate mosaic virus, a mastrevirus infecting Zea mays in Argentina. New Dis. Rep. 2023, 47, e12186. [Google Scholar] [CrossRef]
- Boukari, W.; Alcala-Briseno, R.I.; Kraberger, S.; Fernandez, E.; Filloux, D.; Daugrois, J.H.; Comstock, J.C.; Lett, J.M.; Martin, D.P.; Varsani, A.; et al. Occurrence of a novel mastrevirus in sugarcane germplasm collections in Florida, Guadeloupe and Reunion. Virol. J. 2017, 14, 146. [Google Scholar] [CrossRef]
- Agindotan, B.O.; Domier, L.L.; Bradley, C.A. Detection and characterization of the first North American mastrevirus in switchgrass. Arch. Virol. 2015, 160, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Maich, S.L.S.P.; Nascimento, M.B.; Neves, C.G.; Souza Junior, I.T.; da Silva, F.N.; Barros, D.R. First Report on Sweet Potato Symptomless Virus 1 in Ipomoea batatas in State of Rio Grande do Sul, Brazil. Plant Dis. 2019, 103, 593. [Google Scholar] [CrossRef]
- Mink, G.I. Pollen and seed-transmitted viruses and viroids. Annu. Rev. Phytopathol. 1993, 31, 375–402. [Google Scholar] [CrossRef]
- Perez-Padilla, V.; Fortes, I.M.; Romero-Rodriguez, B.; Arroyo-Mateos, M.; Castillo, A.G.; Moyano, C.; De Leon, L.; Moriones, E. Revisiting Seed Transmission of the Type Strain of Tomato yellow leaf curl virus in Tomato Plants. Phytopathology 2020, 110, 121–129. [Google Scholar] [CrossRef]
- Kil, E.J.; Kim, S.; Lee, Y.J.; Byun, H.S.; Park, J.; Seo, H.; Kim, C.S.; Shim, J.K.; Lee, J.H.; Kim, J.K.; et al. Tomato yellow leaf curl virus (TYLCV-IL): A seed-transmissible geminivirus in tomatoes. Sci. Rep. 2016, 6, 19013. [Google Scholar] [CrossRef]
- Chang, H.H.; Gustian, D.; Chang, C.J.; Jan, F.J. Seed and Pollen Transmission of Tomato Leaf Curl New Delhi Virus, Tomato Leaf Curl Taiwan Virus, and Tomato Yellow Leaf Curl Thailand Virus in Cucumbers and Tomatoes. Plant Dis. 2023, 107, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Sudarshana, M.R.; Wang, H.L.; Lucas, W.J.; Gilbertson, R.L. Dynamics of Bean Dwarf Mosaic Geminivirus Cell-to-Cell and Long-Distance Movement in Phaseolus vulgaris Revealed, Using the Green Fluorescent Protein. Mol. Plant-Microbe Interact. 1998; 11, 277–291. [Google Scholar] [CrossRef]
- Kim, J.; Kil, E.J.; Kim, S.; Seo, H.; Byun, H.S.; Park, J.; Chung, M.N.; Kwak, H.R.; Kim, M.K.; Kim, C.S.; et al. Seed transmission of Sweet potato leaf curl virus in sweet potato (Ipomoea batatas). Plant Pathol. 2015, 64, 1284–1291. [Google Scholar] [CrossRef]
- Manivannan, K.; Renukadevi, P.; Malathi, V.G.; Karthikeyan, G.; Balakrishnan, N. A new seed-transmissible begomovirus in bitter gourd (Momordica charantia L.). Microb. Pathog. 2019, 128, 82–89. [Google Scholar] [CrossRef]
- Kothandaraman, S.V.; Devadason, A.; Ganesan, M.V. Seed-borne nature of a begomovirus, Mung bean yellow mosaic virus in black gram. Appl. Microbiol. Biotechnol. 2016, 100, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Suruthi, V.; Nakkeeran, S.; Renukadevi, P.; Malathi, V.G.; Rajasree, V. Evidence of seed transmission of dolichos yellow mosaic virus, a begomovirus infecting lablab-bean in India. Virus Dis. 2018, 29, 506–512. [Google Scholar] [CrossRef]
- Thomsom, A.D.; Smirk, B.A. Rod-shaped particles associated with a disease of alkali bees. Virology 1966, 28, 348–350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandoo, R.A.; Kraberger, S.; Varsani, A. Two Novel Geminiviruses Identified in Bees (Apis mellifera and Nomia sp.). Viruses 2024, 16, 602. https://doi.org/10.3390/v16040602
Bandoo RA, Kraberger S, Varsani A. Two Novel Geminiviruses Identified in Bees (Apis mellifera and Nomia sp.). Viruses. 2024; 16(4):602. https://doi.org/10.3390/v16040602
Chicago/Turabian StyleBandoo, Rohan Antonio, Simona Kraberger, and Arvind Varsani. 2024. "Two Novel Geminiviruses Identified in Bees (Apis mellifera and Nomia sp.)" Viruses 16, no. 4: 602. https://doi.org/10.3390/v16040602
APA StyleBandoo, R. A., Kraberger, S., & Varsani, A. (2024). Two Novel Geminiviruses Identified in Bees (Apis mellifera and Nomia sp.). Viruses, 16(4), 602. https://doi.org/10.3390/v16040602







