Influence of Lipopolysaccharide-Interacting Peptides Fusion with Endolysin LysECD7 and Fatty Acid Derivatization on the Efficacy against Acinetobacter baumannii Infection In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant Fusion Proteins Design
2.2. Plasmid Construction and Bacterial Strains
2.3. Protein Production and Purification
2.4. Bactericidal Activity Assay
2.5. Determination of Minimum Inhibitory Concentration
2.6. Fatty Acid Derivatization
2.7. ELISA
2.8. Pharmacokinetic Studies
2.9. In Vivo Efficacy Evaluation
3. Results
3.1. Production of Fusion Proteins and Determination of Antibacterial Efficiency In Vitro
3.2. Fatty Acid Derivatization
3.3. Pharmacokinetics
3.4. In Vivo Efficacy in a Murine Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2022, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Defraine, V.; Schuermans, J.; Grymonprez, B.; Govers, S.K.; Aertsen, A.; Fauvart, M.; Michiels, J.; Lavigne, R.; Briers, Y. Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Valencia, R.; Arroyo, L.A.; Conde, M.; Aldana, J.M.; Torres, M.J.; Fernández-Cuenca, F.; Garnacho-Montero, J.; Cisneros, J.M.; Ortíz, C.; Pachón, J.; et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect. Control Hosp. Epidemiol. 2009, 30, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, R.; Garcia, E.; Garcia, P. Phage Lysins for Fighting Bacterial Respiratory Infections: A New Generation of Antimicrobials. Front. Immunol. 2018, 9, 2252. [Google Scholar] [CrossRef] [PubMed]
- Broendum, S.S.; Buckle, A.M.; McGowan, S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 2018, 110, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Vorob’ev, A.M.; Anurova, M.N.; Aleshkin, A.V.; Gushchin, V.A.; Vasina, D.V.; Antonova, N.P.; Kiseleva, I.A.; Rubalskii, E.O.; Zul’karneev, E.R.; Laishevtsev, A.I.; et al. Determination of Bactericidal Activity Spectrum of Recombinant Endolysins of ECD7, Am24, Ap22, Si3, and St11 Bacteriophages. Bull. Exp. Biol. Med. 2021, 170, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Antonova, N.; Vasina, D.; Lendel, A.; Usachev, E.; Makarov, V.; Gintsburg, A.; Tkachuk, A.; Gushchin, V. Broad Bactericidal Activity of the Myoviridae Bacteriophage Lysins LysAm24, LysECD7, and LysSi3 against Gram-Negative ESKAPE Pathogens. Viruses 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Vasina, D.V.; Antonova, N.P.; Grigoriev, I.V.; Yakimakha, V.S.; Lendel, A.M.; Nikiforova, M.A.; Pochtovyi, A.A.; Remizov, T.A.; Usachev, E.V.; Shevlyagina, N.V.; et al. Discovering the Potentials of Four Phage Endolysins to Combat Gram-Negative Infections. Front. Microbiol. 2021, 12, 748718. [Google Scholar] [CrossRef]
- Antonova, N.; Vasina, D.; Rubalskii, E.; Fursov, M.; Savinova, A.; Grigoriev, I.; Usachev, E.; Shevlyagina, N.; Zhukhovitsky, V.; Balabanyan, V.; et al. Modulation of Endolysin LysECD7 Bactericidal Activity by Different Peptide Tag Fusion. Biomolecules 2020, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Das, R.; Chavan, B.; Bajpai, U.; Hanif, S.; Ahmed, S. Beyond antibiotics: Phage-encoded lysins against Gram-negative pathogens. Front. Microbiol. 2023, 14, 1170418. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Schmelcher, M.; Loessner, M.J.; Hendrix, J.; Engelborghs, Y.; Volckaert, G.; Lavigne, R. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem. Biophys. Res. Commun. 2009, 383, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.N.; Bhunia, A.; Ramamoorthy, A.; Bhattacharjya, S. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: Role of the helical hairpin conformation in outer-membrane permeabilization. J. Am. Chem. Soc. 2010, 132, 18417–18428. [Google Scholar] [CrossRef] [PubMed]
- Gottler, L.M.; Ramamoorthy, A. Structure, membrane orientation, mechanism, and function of pexiganan--a highly potent antimicrobial peptide designed from magainin. Biochim. Biophys. Acta 2009, 1788, 1680–1686. [Google Scholar] [CrossRef]
- Matsumoto, M.; Horiuchi, Y.; Yamamoto, A.; Ochiai, M.; Niwa, M.; Takagi, T.; Omi, H.; Kobayashi, T.; Suzuki, M.M. Lipopolysaccaride-binding peptides obtained by phage display method. J. Microbiol. Methods 2010, 82, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, A.M.; Huemer, M.; Zinsli, L.V.; Meile, S.; Keller, A.P.; Röhrig, C.; Eichenseher, F.; Shen, Y.; Zinkernagel, A.S.; Loessner, M.J.; et al. Engineering of Long-Circulating Peptidoglycan Hydrolases Enables Efficient Treatment of Systemic Staphylococcus aureus Infection. mBio 2020, 11, e01781-20. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.M.; Djurkovic, S.; Fischetti, V.A. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 2003, 71, 6199–6204. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Shah, A.; Mond, J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother. 2003, 47, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Youm, S.Y.; Han, H.Y.; Lee, J.H.; Kang, S.H. Pharmacokinetics of the phage endolysin-based candidate drug SAL200 in monkeys and its appropriate intravenous dosing period. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1013–1016. [Google Scholar] [CrossRef]
- Resch, G.; Moreillon, P.; Fischetti, V.A. PEGylating a bacteriophage endolysin inhibits its bactericidal activity. AMB Express 2011, 1, 29. [Google Scholar] [CrossRef]
- De Maesschalck, V.; Gutiérrez, D.; Paeshuyse, J.; Lavigne, R.; Briers, Y. Advanced engineering of third-generation lysins and formulation strategies for clinical applications. Crit. Rev. Microbiol. 2020, 46, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Resch, G.; Moreillon, P.; Fischetti, V.A. A stable phage lysin (Cpl-1) dimer with increased antipneumococcal activity and decreased plasma clearance. Int. J. Antimicrob. Agents 2011, 38, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Grishin, A.V.; Lavrova, N.V.; Lyashchuk, A.M.; Strukova, N.V.; Generalova, M.S.; Ryazanova, A.V.; Shestak, N.V.; Boksha, I.S.; Polyakov, N.B.; Galushkina, Z.M.; et al. The Influence of Dimerization on the Pharmacokinetics and Activity of an Antibacterial Enzyme Lysostaphin. Molecules 2019, 24, 1879. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, P.; Ostergaard, S.; Nishimura, E.; Kjeldsen, T. Derivatization with fatty acids in peptide and protein drug discovery. Nat. Rev. Drug Discov. 2023, 22, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Fothergill, A.; Ghannoum, M.; Manavathu, E.; Ostrosky-Zeichner, L.; Pfaller, M.; Rinaldi, M.; Schell, W.; Walsh, T. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M 38-A document) for amphotericin B, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 2005, 43, 5243–5246. [Google Scholar] [CrossRef]
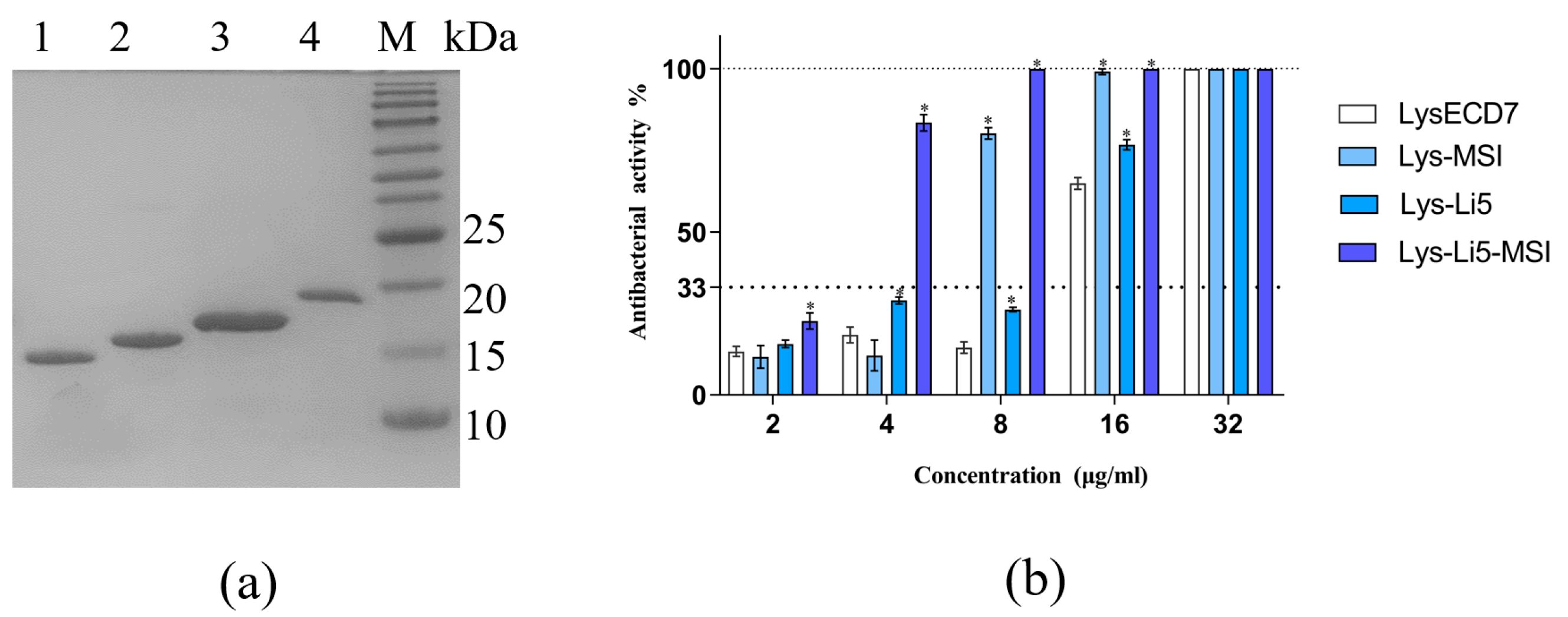
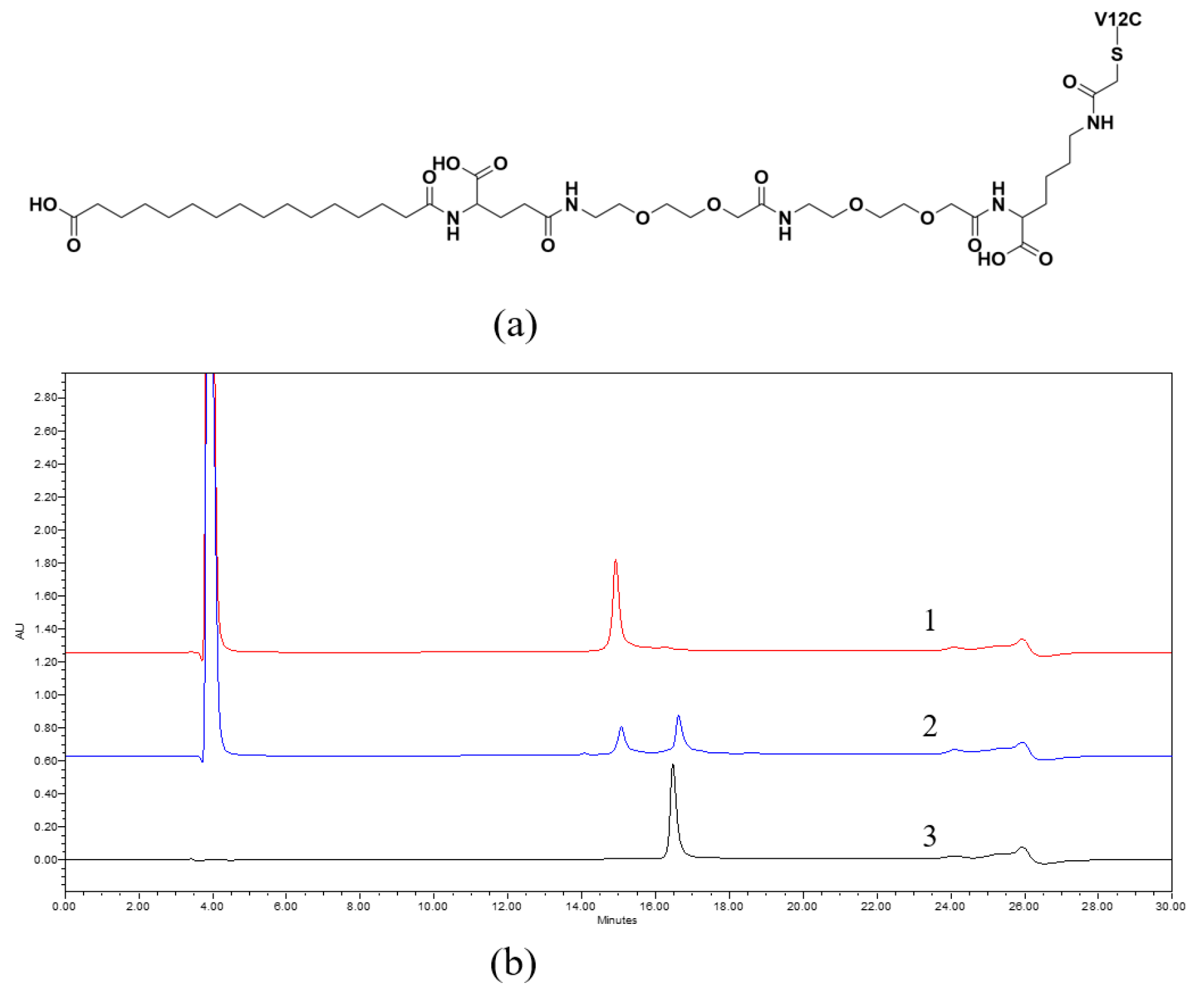

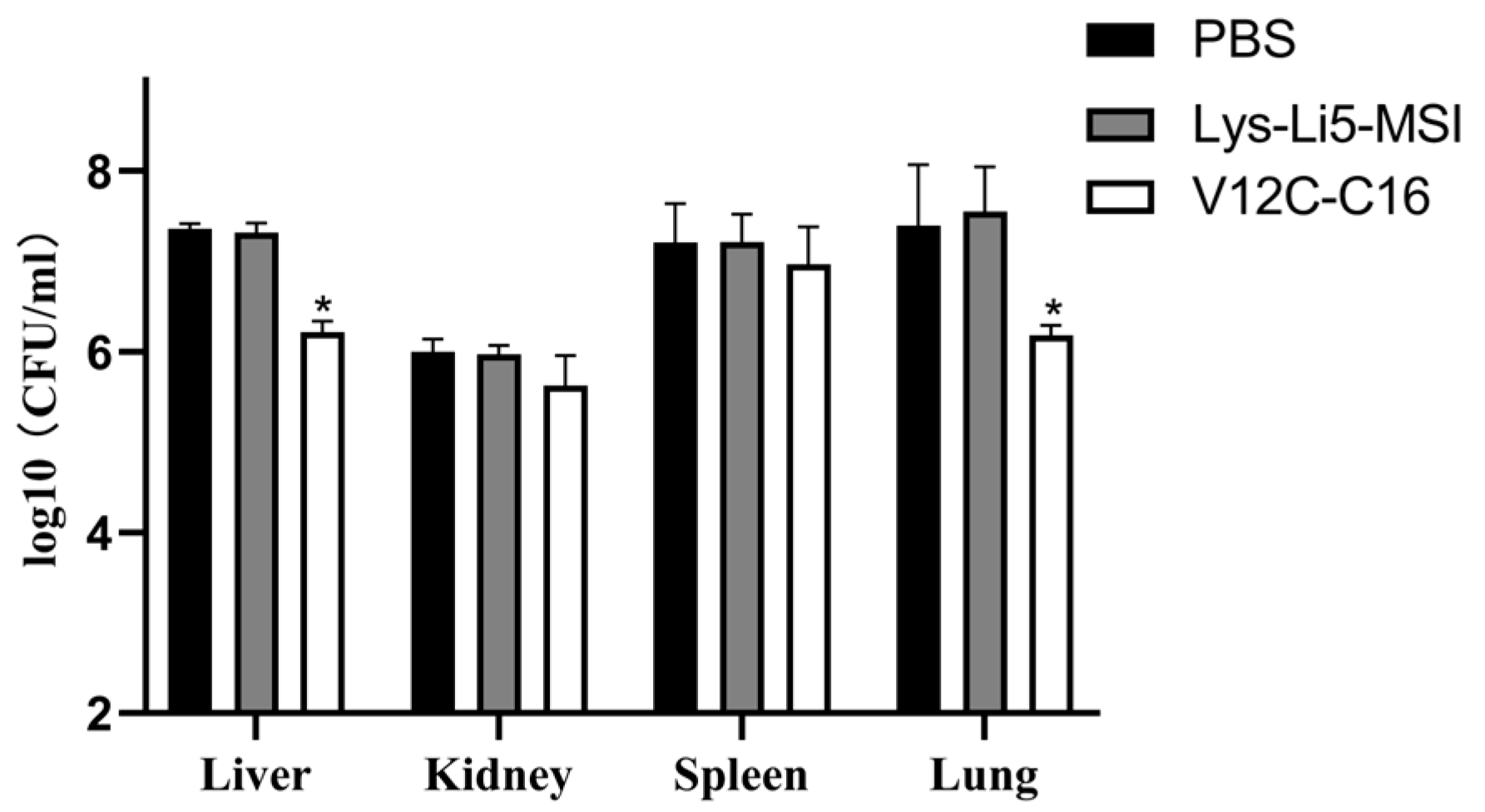
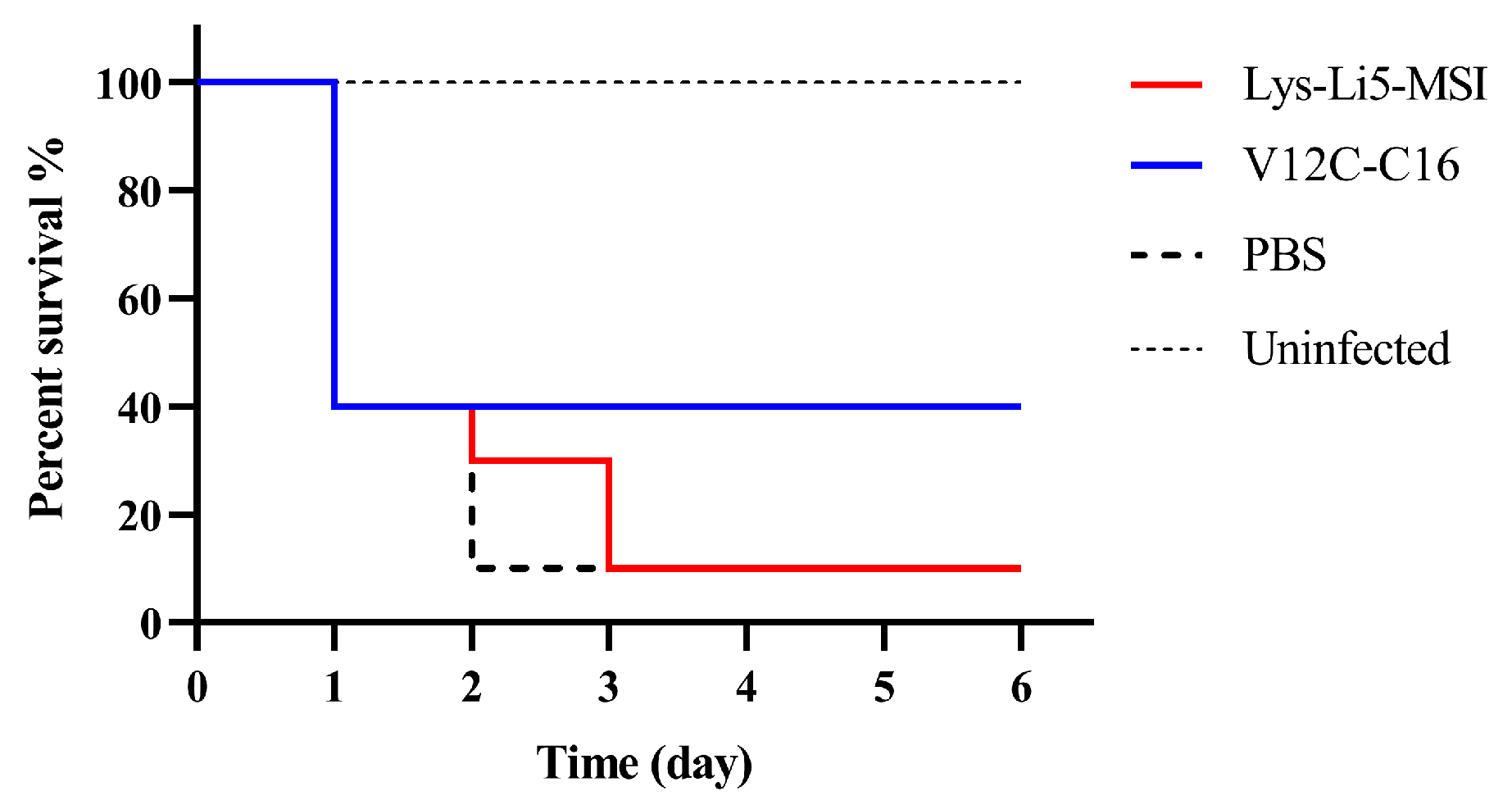
| Sample | MIC (μg/mL) |
|---|---|
| LysECD7 | >1024 |
| Lys-Li5 | >1024 |
| Lys-MSI | 16 |
| Lys-Li5-MSI | 16 |
| Lys-Li5-MSI V12C | 16 |
| V12C-C16 | 16 |
| Sample | Half-Life (h) | Vd (L kg−1) | CL (L h−1 kg−1) | AUC (0–∞) (μg L−1 h−1) |
|---|---|---|---|---|
| Lys-Li5-MSI | 0.397 | 0.021 | 1.034 | 14,512 |
| V12C-C16 | 1.544 | 0.452 | 0.411 | 32,754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Shangguan, W.; Yang, X.; Hu, X.; Li, Y.; Zhao, W.; Feng, M.; Feng, J. Influence of Lipopolysaccharide-Interacting Peptides Fusion with Endolysin LysECD7 and Fatty Acid Derivatization on the Efficacy against Acinetobacter baumannii Infection In Vitro and In Vivo. Viruses 2024, 16, 760. https://doi.org/10.3390/v16050760
Li X, Shangguan W, Yang X, Hu X, Li Y, Zhao W, Feng M, Feng J. Influence of Lipopolysaccharide-Interacting Peptides Fusion with Endolysin LysECD7 and Fatty Acid Derivatization on the Efficacy against Acinetobacter baumannii Infection In Vitro and In Vivo. Viruses. 2024; 16(5):760. https://doi.org/10.3390/v16050760
Chicago/Turabian StyleLi, Xiaowan, Wenwen Shangguan, Xiaoqian Yang, Xiaoyue Hu, Yanan Li, Wenjie Zhao, Meiqing Feng, and Jun Feng. 2024. "Influence of Lipopolysaccharide-Interacting Peptides Fusion with Endolysin LysECD7 and Fatty Acid Derivatization on the Efficacy against Acinetobacter baumannii Infection In Vitro and In Vivo" Viruses 16, no. 5: 760. https://doi.org/10.3390/v16050760
APA StyleLi, X., Shangguan, W., Yang, X., Hu, X., Li, Y., Zhao, W., Feng, M., & Feng, J. (2024). Influence of Lipopolysaccharide-Interacting Peptides Fusion with Endolysin LysECD7 and Fatty Acid Derivatization on the Efficacy against Acinetobacter baumannii Infection In Vitro and In Vivo. Viruses, 16(5), 760. https://doi.org/10.3390/v16050760





