Square the Circle: Diversity of Viral Pathogens Causing Neuro-Infectious Diseases
Abstract
:1. Introduction
2. Vector-Borne Viral Neuropathogens
2.1. Tick-Borne Viral Neuropathogens
| Family | Genus | Species/ Acronym(s) | Common Names or Subspecies/ Acronym(s) | Genome | Host-Source, Vector, Transmission | Geographic Distribution | NS Pathology | Reference |
|---|---|---|---|---|---|---|---|---|
| (Sub)Species Complex/ Acronym(s) | ||||||||
| Flaviviridae Flaviviridae | Orthoflavivirus |
Orthoflavivirus
encephalitidis | Tick-borne encephalitis virus/ TBEV | ssRNA(+) | Hs: Sylvatic birds, rodents, domestic ruminants V: Hard ticks (Dermacentor reticulatus, Ixodes persulcatus, Ixodes ricinus) T: With tick bites—primarily; Food-borne way (raw milk and dairy products); SOT—rare cases. | Highly endemic regions: China (Inner Mongolia, Northwestern parts of China), Russia, Belarus, Ukraine, Croatia, Poland, Baltic countries, Czech Republic, Southern Germany, Austria, Sweden New endemic areas: France (Bordeaux region), Italy, Japan, Netherlands, England, South Korea, Mongolia, Denmark, Kazakhstan, Kyrgyzstan, Armenia, Azerbaijan, Uzbekistan | Meningitis, encephalitis, Meningoencephalitis, encephalitis, meningitis, poliomyelitis like flaccid paralysis, polyradiculoneuritis | [46,62,63,79,97,98,99,100,101,102] |
| Negishi virus 1/ NEGV | ssRNA(+) | Hs: Small mammals (large Japanese field mouse—Apodemus speciosus; small Japanese field mouse—Apodemus argenteus; grey red-backed vole—Myodes rufocanus; brown rat—Rattus norvegicus)—presumably V: Hard ticks (Ixodes ovatus—presumably) T: With tick bites | Japan | Encephalitis | [52,103,104] | |||
| Orthoflavivirus loupingi | Louping-ill virus/ LIV | ssRNA(+) | Hs: Sheep, goats, domestic sheep dog, yellow-necked mouse (Apodemus sylvaticus), common shrew (Sorex araneus), mountain hare (Lepus timidus), red grouse (Lagopus lagopus scoticus) V: Hard ticks (Ixodes ricinus) T: With tick bites—primarily; Food-borne way (raw milk); Contact with contaminated animal blood; Laboratory-acquired infection. | England, Scotland, Ireland, Norway, Denmark (Bornholm), Russian Federation (Primorsky Krai) | Encephalitis | [52,67,69] | ||
|
Orthoflavivirus
kyasanurense | Alkhumra hemorrhagic fever virus/ AHFV | ssRNA(+) | Hs: Camels, sheep V: Hard ticks (Hyalomma dromedarii), soft ticks (Ornithodoros savignyi) T: With tick bites—primarily; Food-borne way (raw milk); Contact with contaminated animal blood. | Highly endemic regions: Saudi Arabia, Egypt | Encephalitis | [72,73,74] | ||
| Kyasanur Forest disease virus/ KFDV | ssRNA(+) | Hs: Black-faced langur (genus Semnopithecus), red-faced bonnet macaque (Macaca radiate); forest rats, shrews, white-bellied rat (Niviventer niviventer), squirrels, bats (Rhinolophus rouxi), ground-dwelling birds, Indian crested porcupines (Hystrix indic) V: Hard ticks (Haemophysalis spinigera) T: With tick bites | India (Goa, Karnataka, Kerela, Maharashtra, Tamilnadu states) | Encephalitis, aseptic meningitis-like picture | [73,75] | |||
|
Orthoflavivirus
omskense | Omsk hemorrhagic fever virus/ OHFV | ssRNA(+) | Hs: Muskrats (Ondatra zibethicus), water vole (Arvicola terrestris); other local species of rodents V: Hard ticks (Dermacentor reticulatus, Dermacentor marginatus—primarily; Ixodes persulcatus, Ixodes apronophorus—rarely) T: With tick bites—primarily; Contact with blood and raw muskrat leather-material—rarely. | Highly endemic regions: Russia (Kurgan, Omsk, Tyumen, Novosibirsk regions); Kazakhstan (Almaty region (human CSF sample), Akmola region (ticks), West Kazakhstan (rodents)) | Encephalitic symptoms (continuous headache and meningism) | [76,78,79] | ||
|
Orthoflavivirus
powassanense | Powassan virus/ POWV | ssRNA(+) | Hs: Woodchuck (Mormota monax)—main reservoir; skunk (Mephitis mephitis); sylvatic wild rodents; carnivores V: Hard ticks Dermacentor andersoni—Colorado; Haemaphysalis neumanni—Primorsky Krai, Russia T: With tick bites | Highly endemic regions: Russia (Far East); US (Colorado, Connecticut, Massachusetts, South Dakota, West Virginia); Canada (Alberta, British Columbia, Nova Scotia) | Meningitis, encephalitis, encephalomeningitis | [79,80,104,105,106,107] | ||
| Deer tick virus/ DTV | ssRNA(+) | Hs: White-footed mouse (Peromyscus leucopus)—main reservoir; sylvatic wild rodents; carnivores V: Hard ticks (Dermacentor andersoni, Ixodes scapularis) T: With tick bites | North US (Hudson Valley, Nantucket Island, Prudence Island); Canada | Encephalitis, meningopolio-encephalitis, meningopoliomyelitis | [82,106,107] | |||
| Orthomyxoviridae | Thogotovirus |
Thogotovirus
dhoriense | Dhori virus/ DV | ssRNA(−) | Hs: Banded mongooses (Mungos mungo); wild and domestic rodents; domestic ruminants V: Hard ticks (Amblyomma gemma, Hyalomma marginatum, Hyalomma dromedarii); may be transmitted by mosquitoes (Anopheles hyrcanus, Aedes caspius, Culex hortensis) T: With tick bites | Focally endemic worldwide spread in natural boskematic foci; Southern Portugal, Egypt, Astrakhan (Volga delta), Kenya (eastern and northeastern provinces), India, Armenia, Azerbaijan, Kirghizia, Uzbekistan | Meningoencephalitis, encephalitis-like reaction, encephalitis | [83,84] |
| Thogotovirus thogotoense | Thogoto virus/ TV | ssRNA(−) | Hs: Cattle, camels V: Hard ticks (Amblyomma variegatum, Hyalomma anatolicum, Hyalomma eruncatum, Rhipicephalus appendiculatus, Rhipicephalus evertsi, Rhipicephalus sanguineus) T: With tick bites | Nigeria, Kenya, Uganda, Ethiopia, Cameroon, Central Africa, Egypt, Iran | Bilateral optic neuritis, fatal meningoencephalitis | [84] | ||
| Phenuiviridae | Bandavirus |
Bandavirus
bhanjanagarense | Bhanja virus/ BHAV | ssRNA(+/−) | Hs: Cattle, sheep, goats V: Hard ticks (Amblyomma variegatum, Boophilus annulatus, Boophilus decoloratus, Boophilus geigyi, Dermacentor marginatus, Haemaphysalis salctata, Rhipicephalus bursa, Rhipicephalus appendiculatus, etc.) T: With tick bites | Focally endemic worldwide spread in natural boskematic foci; Europe (Italy, Bulgaria); India | Meningoencephalitis, encephalitis, paresis | [79,84,86,87] |
| Sedoreoviridae | Orbivirus | Great Island virus/ GIV | Kemerovo virus 1/ KEMV | dsRNA | Hs: Migrating bird (redstarts—Phoenicurus phoenicurus, in Egypt) V: Hard ticks (Ixodes persulcatus—Russia; Hyalomma anatolicum—Uzbekistan) T: With tick bites | Highly endemic region: Western Siberia (Kemerovo region) Egypt (from migratory birds) | Aseptic meningitis, meningoencephalitis, encephalitis | [88,89,90] |
| Lipovnik virus 1/ LIPV | dsRNA | Hs: Sylvatic rodents? V: Hard ticks (Ixodes ricinus) T: With tick bites | Slovakia, Czech Republic | Aseptic meningitis, meningoencephalitis, encephalitis | [58,88,89,90] | |||
| Tribec virus 1/ TRBV | dsRNA | Hs: Rodents (bank vole—Clethrionomys glareolus; pine vole—Microtus pinetorum; hare—Lepus europeus); goats; birds (European starling—Sturnus vulgaris; common chaffinch—Fringilla coelebs) V: Hard ticks (Ixodes ricinus – Czechoslovakia, Moldova; Haemaphisalis punctate –Romania) T: With tick bites | Slovakia, Moldova, Romania, Italy, Belarus | Aseptic meningitis, meningoencephalitis, encephalitis | [58,88,89,90] | |||
| Spinareoviridae | Coltivirus |
Colorado tick
fever coltivirus | Colorado tick fever virus/ CTFV | dsRNA | Hs: Golden-mantled ground squirrels (Callospermophilus lateralis); chipmunks (Tamias spp.) V: Wood hard tick (Dermacentor andersoni); also hard ticks (Dermacentor albopictus, Dermacentor arumapertus, Dermacentor occidentalis, Haemaphysalis leporispalustris, Ixodes sculptus, Ixodes spinipalpis); also soft ticks (Otobius lagophilus) T: With tick bites; with blood transfusion (from infected humans) | Highly endemic region: Western parts of North US; Canada (Alberta, British Columbia) | Aseptic meningitis, encephalitis, meningoencephalitis | [52,84,96] |
| Eyach coltivirus | Eyach virus/ EyV | dsRNA | Hs: European rabbit (Oryctolagus cunniculus) V: Hard ticks (Ixodes ricinus, Ixodes ventalloi) T: With tick bites | Germany, France | Meningoencephalitis | [58,59,84,94,95] | ||
2.2. Mosquito- and Midge-Borne Viral Neuropathogens
| Family | Genus | Species | Common Names or Subspecies/ Acronym(s) | Genome | Host-Source, Vector, Transmission | Geographic Distribution | NS Pathology | Reference |
|---|---|---|---|---|---|---|---|---|
| (Sub)Species complex/ Acronym(s) | ||||||||
| Flaviviridae | Orthoflavivirus |
Orthoflavivirus
aroaense | Bussuquara virus/ BSQV | ssRNA(+) | Hs: Non-human primates, rodents, wild birds V: Mosquitoes (Culex spp.) T: With mosquito bites | Brazil (Pará state), Panama | Encephalitis | [110,129] |
| Iguape virus/ IGUV | ssRNA(+) | Hs: Wild birds V: Mosquitoes (Aedes spp.) T: With mosquito bites | Brazil (Sao Paulo state) | Encephalitis | [109,110,194] | |||
|
Orthoflavivirus
cacipacoreense | Cacipacoré virus/ CPCV | ssRNA(+) | Hs: Wild birds (Formicarius analis) V: Mosquitoes (Culex spp.) T: With mosquito bites | Brazil (Pará and Rondônia states), Amazon region | Encephalitis | [129] | ||
|
Orthoflavivirus
denguei | Dengue virus/ DENV | ssRNA(+) | Hs: Non-human primates (macaques—Macaca spp.; Surilis—Presbytis spp.) V: Mosquitoes (Aedes aegypti, Aedes albopictus, Aedes scutellaris, Aedes polynesiensis; Aedes furcifer, Aedes vittatus, Aedes tailori, Aedes luteocephalus—equatorial parts of Africa) T: With mosquito bites; Human-to-human contact (breastfeeding); Congenital infection. | Focally worldwide spread; High treat: Africa (Sudan, Egypt, Eritrea, Djibouti, Ethiopia, Kenya, Somalia, Tanzania, Mauritius, Mozambique, Seychelles, Angola, Cameroon, Burkina Faso, Côte d’Ivoire, Senegal); the Caribbean basin, Central America, South America, southeastern Asia, Oceania | Encephalitis, meningitis, meningoencephalitis, encephalomyelitis, acute cerebellitis, polyneuritis, encephalopathy, Parkinsonian symptoms | [79,195,196,197,198,199,200,201,202] | ||
|
Orthoflavivirus
flavi | Yellow fever virus/ YFV | ssRNA(+) | Hs: Non-human primates V: Mosquitoes (Aedes spp., Haemagogus spp., Sabethes spp.); ticks (Amblyomma variegatum)—in Africa, extremely rare T: With mosquito bites; With tick bites. | Endemic regions: West Africa (Benin, Burkina Faso, Cape Verde, Côte d’Ivoire, Equatorial Guinea, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Sao Tome and Principe, Senegal, Sierra Leone, Togo); Central Africa (Angola, Burundi, Cameroon, Central African Republic, Chad, Democratic Republic of the Congo, Gabon, Rwanda); East Africa (Ethiopia, Kenya, Somalia, Sudan, Tanzania, Uganda); Panama; South America (Argentina, Bolivia, Brazil, Colombia, Ecuador, Guyana, French Guyana, Paraguay, Peru, Suriname, Trinidad and Tobago, Venezuela) | Encephalitis, YFV vaccine-associated encephalitis, ADEM, Guillain–Barré syndrome, meningitis, meningoencephalitis | [112,113,114,115,116,117,118] | ||
|
Orthoflavivirus
ilheusense | Ilhéus virus/ ILHV | ssRNA(+) | Hs: Wild birds V: Mosquitoes (Aedes spp., Psorophora spp.) T: With mosquito bites | Brazil (Pará and São Paulo states, Pantanal region) | Encephalitis | [129] | ||
| Rocio virus/ ROCV | ssRNA(+) | Hs: Rufous-collared sparrow (Zonotrichia capensis) V: Mosquitoes (Ochleratus spp., Psorofora ferox; specific antibodies: double-collared seedeater (Sporophila caerulescen); creamy-bellied thrush (Turdus amaurochalinus); equines; water buffalo (Bubalus bubalis); marsupials T: With mosquito bites | Southeast Brazil (Sao Paulo state—endemic region); Other regions of virus circulation: Goiás state, Rio de Janeiro state, Mato Grosso do Sul state, Paraíba state, Mato Grosso state | Encephalitis, meningoencephalitis, meningitis | [119,133,203] | |||
|
Orthoflavivirus
japonicum | Japanese encephalitis virus/ JEV | ssRNA(+) | Hs: Wild aquatic birds, domestic birds, domestic pigs V: Mosquitoes (Main vectors—Culex tritaeniorhynchus, Culex vishnui, Culex gelidus) T: With mosquito bites | Focally worldwide spread; Asia–Pacific region, Southeast Asia, Australia | Encephalitis, meningoencephalitis, meningitis | [120,204,205,206,207,208] | ||
|
Orthoflavivirus
louisense | St. Louis encephalitis virus/ SLEV | ssRNA(+) | Hs: Sylvatic, peridomestic, and urban birds (sparrows—Passer sp.; pigeons—Columba sp.; blue jay—Cyanocitta cristata; robins—Turdus sp.) V: Mosquitoes (Culex tarsalis, Culex pipiens, Culex quinquefasciatus) T: With mosquito bites | United States (Eastern and Central states) | Encephalitis, meningoencephalitis, meningitis | [121,122,123,124] | ||
|
Orthoflavivirus
murrayense | Murray Valley encephalitis virus/ MVEV | ssRNA(+) | Hs: Wild animals: marsupials (kangaroos; agile wallabies—Notamacropus agilis); rabbits (Leporidae); rodents; wild birds—Galahs (Cacatuidae); water birds (rufous night heron—Nycticorax caledonicus; Pacific black duck—Anas superciliosa); domestic animals and birds V: Mosquitoes (Culex annulirostris, Culex sitiens) T: With mosquito bites | Australia (Western Australia, Northern Territory, New South Wales, Victoria); Papua New Guinea; Indonesia; Canada (Alberta)—imported infection | Encephalitis | [125,126,127,128] | ||
|
Orthoflavivirus
nilense | West Nile virus/ WNV | ssRNA(+) | Hs: Wild birds, domestic animals (horses, sheep), alligators, lake frog (Rana ridibunda—competent reservoir (Russia)) V: Mosquitoed (Culex spp.) T: With mosquito bites; Human-to-human transmission (organ transplantation, blood transfusion, placental route) | Endemic region: East Africa (Uganda) Many cases: North America, Brazil, Middle East, Europe, Asia, Regions of Africa Worldwide spread | Meningitis, encephalitis, poliomyelitis | [129,130,131,132,209,210] | ||
| Kunjin virus/ KUNV | ssRNA(+) | Hs: Wild birds, domestic animals (horses, sheep), alligators V: Mosquitoes (Culex annulirostris) T: With mosquito bites | Australia (tropical north regions), Oceania | Encephalitis | [133,134] | |||
|
Orthoflavivirus
usutuense | Usutu virus/ USUV | ssRNA(+) | Hs: Wild passerine birds, insectivorous microbats (Pipistrellus sp.), equines, rodents, shrews V: Mosquitoes (Culex spp., Aedes spp., Mansonia spp., Anopheles spp.) T: With mosquito bites | Africa (South Africa, Central African Republic, Senegal, Côte d’Ivoire, Nigeria, Uganda, Burkina Faso, Tunisia, Morocco); Europe (introductions: France, Germany, Italy, Austria, Serbia) | Encephalitis, meningoencephalitis | [135,136,137,138] | ||
|
Orthoflavivirus
zikaense | Zika virus/ ZIKV | ssRNA(+) | Hs: Non-human primates V: Mosquitoes (Aedes spp.) T: With mosquito bites; Human-to-human transmission (organ transplantation, blood transfusion, placental route); Contact with infected fomites. | Brazil, Central and North America | Guillain–Barré syndrome, fetal microcephaly, myelitis, meningoencephalitis | [139,140,141,211] | ||
| Peribunyaviridae | Orthobunyavirus |
Orthobunyavirus
bunyamweraense | Bunyamwera virus/ BUNV | ssRNA(−) | Hs: Wild waterfowls V: Mosquitoes (Aedes circumluteolus); hard ticks (Amblyomma dubitatum, Amblyomma sculptum)—presumably T: With mosquito bites; With tick bites—presumably | Uganda, Tanzania, Mozambique, Nigeria, Guinea, South Africa (KwaZulu-Natal province), Democratic Republic of Congo, Botswana, Namibia (Caprivi region), Senegal, Ivory Cost, Cameroon, Central African Republic, Kenya, Madagascar; Argentina, Brazil (Minas Gerais (ticks)) | Encephalitis, meningitis | [144,145,146] |
| Germiston virus/ GERV | ssRNA(−) | Hs: Wild animals (virus isolation); domestic animals (antibody detection) V: Mosquitoes (Culex theileri, Culex rubinotus) T: With mosquito bites; Direct contact with infected tissue and fomites | Africa; South Africa region | Encephalitis, meningoencephalitis (both sporadic cases; laboratory work infection); mental confusion | [79,143,144,147] | |||
| Xingu virus/ XINV | ssRNA(−) | Hs: Not identified V: Mosquitoes T: With mosquito bites—presumably | South America (Brazil) | Encephalitis/meningoencephalitis (both sporadic cases) | [79,148] | |||
|
Orthobunyavirus
cacheense | Cache Valley virus/ CVV | ssRNA(−) | Hs: Domestic ruminants (equines, cattle); deer V: Mosquitoes (Culex spp.) T: With mosquito bites | US (Utah, North Carolina, Missouri, Wisconsin, New York) | Encephalitis, meningoencephalitis, meningitis | [149,150,151,152] | ||
| Cristoli virus 1 | – | ssRNA(−) | Hs: Not identified V: Mosquito—presumably T: With mosquito bites—presumably | France (Île-de-France region, including Paris) | Encephalitis | [153] | ||
|
Orthobunyavirus
encephalitidis | California encephalitis virus/ CEV | ssRNA(−) | Hs: Equines V: Mosquitoes T: With mosquito bites | US (California) | Encephalitis | [79,212] | ||
|
Orthobunyavirus
guaroaense | Guaroa virus/ GROV | ssRNA(−) | Hs: Mosquitoes (Anopheles (Kerteszia) neivai) V: Mosquitoes—presumably T: With mosquito bites—presumably | Brazil, Colombia, Panama, Bolivia | Paresis | [143,169] | ||
|
Orthobunyavirus
ileshaense | Ilesha virus/ ILEV | ssRNA(−) | Hs: – V: Mosquitoes (Anopheles gambiae) T: With mosquito bites | Cameroon, Central African Republic, Nigeria, Senegal, Uganda; Madagascar (virus isolation from infected persons); Ghana and Niger (antibody from infected persons) | Meningoencephalitis | [144,172] | ||
|
Orthobunyavirus
jamestownense | Jamestown Canyon virus/ JCV | ssRNA(−) | Hs: White-tailed deer (Odocoileus virginianus), moose (Alces alces), elk (Cervus elaphus), bison (Bison bison) V: Mosquitoes (Culiseta inornata, Aedes spp., Anopheles spp.) T: With mosquito bites | US (Minnesota, Wisconsin); Canada (British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Nova Scotia) | Encephalitis, meningoencephalitis, meningitis | [79,185,186,213,214] | ||
| Inkoo virus/ INKV | ssRNA(−) | Hs: Wild birds V: Mosquitoes (Aedes spp.) T: With mosquito bites | Finland, Sweden, Norway, Russia | Asthenoneurologic disturbances, microfocal neurologic symptoms, encephalitis | [52,79,156,157,158,159] | |||
|
Orthobunyavirus
kernense | Main Drain virus/ MDV | ssRNA(−) | Hs: Horses, wild birds, black-tailed jackrabbit (Lepus californicus) V: Mosquitoes (Culicidae)—occasional vector; biting midges (Ceratopogonidae, Culicoides variipennis) T: With mosquito or midge bites | US (California) | Unspecified CNS disease | [66,143] | ||
|
Orthobunyavirus
keystoneense | Keystone virus/ KEYV | ssRNA(−) | Hs: Squirrels, raccoons, whitetail deer (Odocoileus virginianus) V: Mosquitoes (Aedes spp.) T: With mosquito bites | US (Florida, coastal regions of the Chesapeake Bay) | Encephalitis, meningitis | [191,215] | ||
|
Orthobunyavirus
khatangaense | Snowshoe hare virus/ SSHV | ssRNA(−) | Hs: Hares, squirrels V: Mosquitoes (Aedes spp.) T: With mosquito bites | US, Canada | Meningoencephalitis, encephalitis, meningitis | [79,216,217,218,219] | ||
| Khatanga virus; Chatanga virus/ KHATV | ssRNA(−) | Hs: Wild sylvatic animals; domestic animals—presumably V: Mosquitoes (Aedes spp., Culiseta spp., Culex spp., Anopheles spp.) T: With mosquito bites | Russia (European part, western, middle and northeastern Siberia) | Encephalitis | [79,154,155] | |||
|
Orthobunyavirus
lacrosseense | La Crosse virus/ LACV | ssRNA(−) | Hs: Chipmunks, squirrels V: Mosquitoes (Aedes spp.) T: With mosquito bites | US (Ohio, Wisconsin, Minnesota, Indiana, Illinois, Iowa, North Carolina, Tennessee, West Virginia, Georgia, Virginia, Kentucky, Rhode Island) | Encephalitis | [79,134,139,140] | ||
| Orthobunyavirus oropoucheense | Oropouche virus/ OROV | ssRNA(−) | Hs: Pale-throated sloths, non-human primates V: Mosquitoes (Culex spp., Aedes spp.); biting midges (Culicoides) T: With mosquito bites | Brazil, Panama, Peru, Trinidad and Tobago | Meningitis | [167,168,171] | ||
| Orthobunyavirus shuniense | Shuni virus/ SHUV | ssRNA(−) | Hs: Horses, domestic cattle V: Mosquitoes (Culex theileri); Cullicoides midges T: With mosquito and midge bites | South Africa (Gauteng province), Israel, Nigeria | Encephalitis, meningitis | [66,171] | ||
|
Orthobunyavirus
tahynaense | Ťahyňa virus/ TAHV | ssRNA(−) | Hs: Small wild mammals V: Mosquitoes (Culex spp., Aedes spp.) T: With mosquito bites | Central Europe; China (Xinjiang, Qinghai, Inner Mongolia) | Meningitis, meningoencephalitis, encephalomyelitis, encephalitis | [79,160,161,162,163,164,165,166] | ||
|
Orthobunyavirus
tensawense | Tensaw virus/ TENV | ssRNA(−) | Hs: Sylvatic rodents, foxes, raccoons, dogs, cows V: Mosquitoes (Aedes vexans, Anopheles crucians, Coquillettidia perturbans, Culex salinarius, Uranotaenia sapphirina) T: With mosquito bites—presumably; Congenital infection. | US (Alabama, Florida) | Rabies-like symptoms, encephalitis, micro-/macrocephaly | [143,220] | ||
| Orthobunyavirus umbreense | Umbre virus/ UMBV | ssRNA(−) | Hs: Not identified V: Mosquitoes (Culex spp.) T: With mosquito bites—presumably | India, Australia (Queensland—Umbre-related viruses); Malaysia (Umbre-related domestic avian pathogenic virus); France—presumably | Lethal encephalitis | [174] | ||
|
Orthobunyavirus
wyeomyiae | Tucunduba virus/ TUCV | ssRNA(−) | Hs: – V: Mosquitoes (Culex spp., Wyeomia spp., Sabethes spp., Psorophora spp., Limatus spp., Trichoprosopon spp.) T: With mosquito bites | Brazil | Meningoencephalitis | [170] | ||
| Ntwetwe virus 1 | Ntwetwe virus 1/ NTWV 1 | ssRNA(−) | Hs: – V: Mosquitoes (Anopheles spp.) T: – | Uganda | Fatal encephalopathy, encephalitis | [143,173] | ||
| Phenuiviridae | Phlebovirus |
Phlebovirus
riftense | Rift Valley Fever virus/ RVFV | ssRNA(+/−) | Hs: Wild and domestic animals V: Mosquitoes (Culex spp.) T: With mosquito bites; Direct contact with contaminated biological fluids. | Kenya, Tanzania, South Africa, Sudan, Egypt, Madagascar, Somalia, Mauritania, Botswana, Namibia | Meningoencephalitis, encephalitis | [139,175] |
|
Phlebovirus
toscanaense | Toscana virus/ TOSV | ssRNA(+/−) | Hs: – V: Sandflies (Phlebotomus perniciosus, Phlebotomus perfiliewi) T: With sandfly bites | Italy, Spain, Slovenia, Turkey, Portugal, Greece, Cyprus, Southern France, the Balkans, the Black Sea coast, Iraq, Iran, Pakistan, Afghanistan, India | Meningitis, meningoencephalitis | [176,177,178] | ||
| Rhabdoviridae | Vesiculovirus |
Vesiculovirus
chandipura | Chandipura virus/ CHPV | ssRNA(−) | Hs: Pigs, buffalo, cattle V: Mosquitoes (Phlebotomus spp.) T: With mosquito bites | India, Bhutan, Nepal, Sri Lanka, Nigeria, Senegal | Encephalitis | [179] |
| Sedoreoviridae | Orbivirus | Orungo virus | Orungo virus/ ORUV | dsRNA | Hs: – V: Mosquito (Aedes spp., Culex spp., Anopheles spp.) T: With mosquito bites | Regions of sub-Saharan Africa | Encephalitis | [88] |
| Seadornavirus | Banna virus | Banna virus/ BAV | dsRNA | Hs: Domestic pigs, cattle V: Mosquitoes (Culex tritaeniorhynchus, Culex pipiens pallens, Culex annulus, Culex pseudovishnui, Culex modestus, Anopheles sinensis, Aedes vagus, Aedes albopictus, Aedes vexans, Aedes dorsalis); Midges (Culicoides sp.) T: With mosquito bites | Indonesia, China, Vietnam | Encephalitis | [180,221,222] | |
| Togaviridae | Alphavirus |
Chikungunya
virus | Chikungunya virus/ CHIKV | ssRNA(+) | V: Mosquitoes (Aedes spp.) T: With mosquito bites | Africa, Southeastern Asia, Europe (imported infection), North America | Myelitis, encephalitis | [129,139,223] |
|
Eastern equine
encephalitis virus | Eastern equine encephalitis virus North American/ EEEV-NA | ssRNA(+) | Hs: Birds, mammals V: Mosquitoes (Aedes spp., Culex spp., Anopheles spp.) T: With mosquito bites | North America (Massachusetts, Michigan, Florida, Georgia, North Carolina), the Caribbean region | Encephalitis | [224,225,226] | ||
| Madariaga virus | Madariaga virus; Eastern equine encephalitis virus South American/ MADV; EEEV-SA | ssRNA(+) | Hs: Birds, mammals V: Mosquitoes (Aedes spp., Culex spp., Anopheles spp.) T: With mosquito bites | South America (Panama, Venezuela), Haiti | Encephalitis, encephalomyelitis | [181,182,183,184] | ||
| Mayaro virus | Mayaro virus/ MAYV | ssRNA(+) | Hs: Non-human primates, migratory birds V: Mosquitoes (Haemagogus spp.—particularly Haemagogus janthinomys); Culex spp., Mansonia spp., Aedes spp., Psorophora spp., Sabethes spp. T: With mosquito bites | Europe (Germany, France, Netherlands, Switzerland—imported infection); United states (isolated in non-human primates, migratory birds); Mexico, Trinidad and Tobago, Brazil, Surinam, French Guiana, Venezuela, Haiti, Bolivia, Peru, Ecuador, Colombia (isolated from mosquitoes) | Encephalopathy | [129,227,228] | ||
| Middelburg virus | Middelburg virus/ MIDV | ssRNA(+) | Hs: Equines, mice, sheep V: Mosquitoes (Aedes spp.) T: With mosquito bites | South Africa, Zimbabwe | Meningo-encephalitis | [187,188] | ||
| Ross River virus | Ross River virus/ RRV | ssRNA(+) | Hs: Mammals, birds V: Mosquitoes (Culex spp.) T: With mosquito bites | Australia, Papua New Guinea | Meningitis (rare cases); encephalitis (rare cases) | [189,190] | ||
| Sindbis virus | Sindbis virus/ SINV | ssRNA(+) | Hs: Wild birds (Corvus corone sardonius—hooded crow); rodents; domestic animals V: Mosquitoes (Culex spp., Anopheles spp., Coquillettidia spp., Aedes spp., Ocheleratus spp.); Gamasidae ticks (Ornithonyssus bacoti), Ixodidae ticks (Hyalomma marginatum) T: With arthropod-vector bites | Africa (endemic regions—Egypt, South Africa, Uganda, Central African Republic, Sudan, Nigeria, and Zimbabwe), Europe (Germany, Sweden, Finland, Italy, Slovakia), Russia, the Middle East, the Philippines, Turkey, Azerbaijan, Israel, India, China, Malaysia, Australia (north regions), New Zealand | Meningitis—presumably | [66,79,192,229,230] | ||
| Tonate virus | Tonate virus; Venezuelan equine encephalitis virus IIIB/ TONV; VEEV-IIIB | ssRNA(+) | Hs: Wild birds (Psarocolius decumanus—crested oropendola) V: Mosquitoes (Culex portesi) T: With mosquito bites | North America, South America (Surinam, French Guiana), Central America | Encephalitis | [192,193] | ||
|
Venezuelan equine
encephalitis virus | Venezuelan equine encephalitis virus/ VEEV | ssRNA(+) | Hs: Wild rodents (cotton mouse—Peromyscus gossypinus; hispid cotton rat—Sigmodon hispidus; spiny rats—Proechimys spp.; Oryzomys spp., Zigodontomys spp., Heteromys spp.), equines, canids, pigs, wild birds, bats V: Mosquitoes (Culex spp., Mansonia spp., Anopheles spp., Aedes spp., Psorophora spp., Sabethes spp., Haemagogus spp., Deinocerites spp.); Ochlerotatus taeniorhynchus T: With mosquito bites | Costa Rica, Venezuela, Colombia, Belize, Peru, Ecuador, British Guyana, Guatemala, Argentina, Panama, Trinidad, Honduras, El Salvador, Nicaragua, Mexico; United States (Texas, Florida) | Encephalitis, meningitis | [15,52,66,192,231,232,233] | ||
|
Western equine
encephalitis virus | Western equine encephalitis virus/ WEEV | ssRNA(+) | Hs: Wild birds (passerine); wild rodents, horses V: Mosquitoes (Culex tarsalis; Aedes spp.); Ochlerotatus melanimon (California), Aedes dorsalis (Utah, New Mexico), Aedes campestris (New Mexico) T: With mosquito bites | Brazil, Colombia, United States | Encephalitis, meningitis, encephalomyelitis | [15,129,233,234] | ||
3. Zoonotic Viral Neuropathogens
| Family | Genus | Species | Common Names or Subspecies/ Acronym(s) | Genome | Host-Vector, Transmission | Geographic Distribution | NS Pathology | Reference |
|---|---|---|---|---|---|---|---|---|
| Arenaviridae | Mammarenavirus |
Mammarenavirus
choriomeningitidis | Lymphocytic choriomeningitis virus/ LCMV | ssRNA(+/−) | Hv: Predominantly wild and domestic rodents T: With bites; Contact with fomites/blood/nesting materials. | Worldwide (where rodents are present) | Meningitis, encephalitis, encephalomyelitis, meningoencephalitis, transverse myelitis | [46,49,62,81,247,272,273,274] |
| Filoviridae | Orthoebolavirus |
Orthoebolavirus
zairense | Ebola virus/ EBOV | ssRNA(−) | Hv: Non-human primates; bats; flying foxes; infected human hosts T: With bites; Contact with infectious body fluids of a patient (high risk group—medical workers); Contact with fomites/blood/nesting materials of infected animal hosts. | Imported infection: Mali, Nigeria, Senegal, Italy, Spain, UK, US, Russia (laboratory infection) Democratic Republic of Congo, Republic of the Congo, Gabon, Liberia, Sierra Leone, Guinea, South Africa | Meningitis, encephalitis, meningoencephalitis, neurocomplication after system infection | [248,274,275,276] |
| Orthomarburgvirus |
Orthomarburgvirus
marburgense | Marburg virus/ MARV | ssRNA(−) | Hv: Egyptian fruit bat (Rousettus aegyptiacus), Sundevall’s leaf-nosed bat (Hipposideros caffer); non-human primates T: With bites; Contact with infected patients; Contact with fomites/blood/nesting materials of infected animal hosts. | Imported infection: Germany (Marburg, Frankfurt), Serbia (Belgrade), Russia (Koltsovo), Netherlands (Leiden), US, South Africa Angola, Democratic Republic of Congo, Kenya, Uganda, Zimbabwe, Guinea | Encephalitis | [112,250,277] | |
| Hantaviridae | Orthohantavirus |
Orthohantavirus
andesense | Andes virus/ ANDV | ssRNA(−) | Hv: Wild and domestic rodents (long-tailed pygmy rice rat (Oligoryzomys longicaudatus)—most common host) T: With bites; Person-to-person transmission between humans (including breastfeeding, household contacts, nosocomial transmission); Contact with fomites/blood/nesting materials of infected animal hosts. | Highly endemic region: Regions of South America (Argentina, Bolivia, Chile, Uruguay) | Encephalitis | [257,278,279,280] |
|
Orthohantavirus
dobravaense | Dobrava-Belgrade virus/ DOBV | ssRNA(−) | Hv: Rodents (yellow-necked mouse—Apodemus flavicollis; striped field mouse—Apodemus agrarius; Caucasian wood mouse—Apodemus ponticus; Small forest mouse—Apodemus uralensis) T: With bites and scratches; Inhalation of aerosolized droplets; Contact with fomites/blood/nesting materials of infected animal host; Contaminated food. | Russia (Central Russia, Western Siberia), Europe, Turkey Highly endemic region: Balkans | Encephalitis | [79,258,281,282] | ||
|
Orthohantavirus
puumalaense | Puumala virus/ PUUV | ssRNA(−) | Hv: Rodents (bank vole—Clethrionomys glareolus) T: With bites and scratches; Inhalation of aerosolized droplets; Contact with fomites/blood/nesting materials of infected animal host; Contaminated food. | Russia (Central Russia, Western Siberia, Far East), Balkans, Europe (Northern, Western, Central regions) | Encephalitis, encephalomyelitis | [79,259,260,282,283] | ||
|
Orthohantavirus
seoulense | Seoul virus/ SEOV | ssRNA(−) | Hv: Rodents (Norwegian brown rat—Rattus norvegicus; black rat—Rattus rattus) T: With bites and scratches; Inhalation of aerosolized droplets; Contact with fomites/blood/nesting materials of infected animal hosts; Contaminated food. | Far East of Russia, China, Japan, North and South Korea | Encephalitis | [79,262,282] | ||
| Paramyxoviridae | Henipavirus |
Henipavirus
hendraense | Hendra virus/ HeV | ssRNA(−) | Hv: Infected domestic animals (horses, pigs, dogs, cats); flying fox (fruit bats) (family Pteropidinae—Pteropus alecto, Pteropus poliocephalus, Pteropus scapulatus, Pteropus conspicillatus), etc. T: With bites and scratches; Contact with fomites/blood/nesting materials of infected animal hosts; Food-borne way (with horsemeat, pork, date palm sap or wine, fruits); Human-to-human. | Southeast Asia (including Singapore, Cambodia, Indonesia, Thailand, Malaysia, Philippines, Bangladesh), Eastern Australia, Ghana, Madagascar, Papua New Guinea, China, India, Latin America | Meningitis, encephalitis | [139,249,284,285,286,287,288] |
|
Henipavirus
nipahense | Nipah virus/ NiV | ssRNA(−) | Hv: Infected domestic animals (horses, pigs, dogs, cats); flying fox (fruit bats) (Pteropus giganteus, Pteropus hypomelanus, Pteropus lylei); flying dog (Cynopterus brachyotis); cave nectar bat (Eonycteris spelaea); microbats (Scotophilus kuhlii, Myotis), etc. T: With bites and scratches; Contact with fomites/blood/nesting materials of infected animal hosts; Food-borne way (with horsemeat, pork, date palm sap, fruits); Human-to-human transmission. | Southeast Asia (including Singapore, Cambodia, Indonesia, Thailand, Malaysia, Philippines, Bangladesh), Eastern Australia, Ghana, Madagascar, Papua New Guinea, China, India, Latin America | Acute encephalitis | [112,249,284,285,286,288,289,290] | ||
| Poxviridae | Orthopoxvirus | Monkeypox virus | Monkeypox virus/ MPV | dsDNA | Hv: Non-human primates (mangabey monkeys), Gambian pouched rats, squirrels, prairie dogs T: With bites and scratches; Contact with fomites/blood of infected animal-host; Human-to-human transmission. | Highly endemic region: Tropical rainforest areas of Central and Western Africa Outbreaks (imported infection): 50 countries (worldwide) | Encephalitis, ADEM, encephalomyelitis, demyelinating encephalomyelitis | [139,263,265,291,292,293] |
| Rhabdoviridae | Lyssavirus |
Lyssavirus
australis | Australian bat lyssavirus/ ABLV | ssRNA(−) | Hv: Pteropod and insectivorous bat species (black flying fox—Pteropus Alecto; yellow-bellied sheath-tailed bat—Saccolaimus flaviventris) T: With bites and scratches; Contact with fomites (especially saliva)/blood of infected animal hosts. | Australia (New South Wales, the Northern Territory, Queensland, South Australia, Victoria, Western Australia) | Encephalitic rabies | [266,294,295] |
|
Lyssavirus
duvenhage | Duvenhage virus/ DUVV | ssRNA(−) | Hv: Bats—presumably T: With bites and scratches; Contact with fomites (especially saliva)/blood of infected animal hosts. | South Africa; Europe (one case of imported infection—Netherlands) | Encephalitic rabies | [266,267,268] | ||
| Lyssavirus helsinki | European bat lyssavirus 2/ EBLV-2 | ssRNA(−) | Hv: Insectivorous microbats—Daubenton’s bat (Myotis daubentonii), pond bat (Myotis dasycneme) T: With bites and scratches; Contact with fomites (especially saliva)/blood of infected animal hosts. | Northeastern Europe, Mediterranean region, Netherlands, Switzerland, United Kingdom, Germany | Rabies-like encephalitis | [266,271,294,296] | ||
| Lyssavirus irkut | Irkut virus/ IRKV | ssRNA(−) | Hv: Insectivorous microbats—greater tube-nosed bat (Murina leucogaster); domestic dog T: With bites and scratches; Contact with fomites (especially saliva)/blood of infected animal hosts. | Russia (Irkutsk region, Far East (Primorsky Krai region, Amur region)); China (Jilin province) | Encephalitic rabies | [294,297,298] | ||
| Lyssavirus mokola | Mokola virus/ MOKV | ssRNA(−) | Hv: Domestic cats, dogs T: With bites and scratches; Contact with fomites (especially saliva)/blood of infected animal hosts. | Africa (Nigeria, Cameroon, Central African Republic, Ethiopia, Zimbabwe, South Africa) | Encephalitic rabies (human cases very rare) | [249,294,299] | ||
| Lyssavirus rabies | Rabies virus/ RABV | ssRNA(−) | Hv: Vampire bat (Desmodus rotundus), big brown bat (Eptesicus fuscus), Mexican/Brazilian free-tail bat (Tadarida brasiliensis), silver-haired bat (Lasionycteris noctivagens), tri-colored bat (Perimyotis subflavus), carnivores (including domestic synanthrope species) T: With bites and scratches; Contact with fomites (especially saliva)/blood of infected animal hosts. | Worldwide | Meningoencephalitis, encephalitic rabies | [46,247,266,300,301,302,303] |
4. Widespread Viral Neuropathogens
| Family | Genus | Species | Common Names or Subspecies/ Acronym(s) | Genome | Source, Predisposing Conditions, Transmission | NS Pathology | Reference |
|---|---|---|---|---|---|---|---|
| (Sub)Species Complex/ Acronym(s) | |||||||
| Adenoviridae | Mastadenovirus |
Human
mastadenovirus A | Human adenovirus 12/ HAdV-12 | dsDNA | S: Infected human hosts Pc: Children under 5 y.o.; immunocompromised persons T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Meningitis, meningoencephalitis, encephalitis | [354] |
|
Human
mastadenovirus B | Human adenovirus 3/ HAdV-3 | dsDNA | [307,354] | ||||
| Human adenovirus 7/ HAdV-7 | dsDNA | [307,354,355] | |||||
| Human adenovirus 11/ HAdV-11 | dsDNA | [307,354] | |||||
| Human adenovirus 14/ HAdV-14 | dsDNA | [307,354] | |||||
| Human adenovirus 16/ HAdV-16 | dsDNA | [307,354] | |||||
| Human adenovirus 21/ HAdV-21 | dsDNA | [307,354] | |||||
| Human adenovirus 34/ HAdV-34 | dsDNA | [307,354] | |||||
| Human adenovirus 35/ HAdV-35 | dsDNA | [307,354] | |||||
| Human adenovirus 50/ HAdV-50 | dsDNA | [307,354] | |||||
| Human adenovirus 55/ HAdV-55 | dsDNA | [354] | |||||
| Human adenovirus 66 1;Human adenovirus B66 1 | dsDNA | [354] | |||||
| Human adenovirus 68 1;Human adenovirus 3–16 1 | dsDNA | [354] | |||||
| Human adenovirus B79 1 | dsDNA | [354] | |||||
|
Human
mastadenovirus C | Human adenovirus 1/ HAdV-1 | dsDNA | S: Infected human hosts Pc: Children under 5 y.o.; immunocompromised persons T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Meningitis, meningoencephalitis, encephalitis | [354] | ||
| Human adenovirus 2/ HAdV-2 | dsDNA | [354,355] | |||||
| Human adenovirus 5/ HAdV-5 | dsDNA | [354,355] | |||||
| Human adenovirus 6/ HAdV-6 | dsDNA | [354] | |||||
|
Human
mastadenovirus D | Human adenovirus 26; Adenovirus serotype 26/ HAdV-26 | dsDNA | S: Infected human hosts Pc: Children under 5 y.o.; immunocompromised persons T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Meningitis, meningoencephalitis, encephalitis | [354] | ||
| Human adenovirus 32/ HAdV-32 | dsDNA | [354] | |||||
|
Human
mastadenovirus E | Human adenovirus 4/ HAdV-4 | dsDNA | [354] | ||||
|
Human
mastadenovirus F | Human adenovirus 41/ HAdV-41 | dsDNA | [308,354] | ||||
| unclassified Human mastadenovirus 1 | Human adenovirus 76 1 | dsDNA | [354] | ||||
| Human adenovirus 77 1 | dsDNA | [354] | |||||
| Human adenovirus 78 1 | dsDNA | [354] | |||||
| Astroviridae | Mamastrovirus | Human astrovirus 1 | Human astrovirus Virginia| Pudget Sound 1/ HuAstV-PS 1 | ssRNA(+) | S: Infected human hosts, HAI; zoonotic infection? Pc: Children under 5 years old (include immunocompetent), hereditary immunodeficiency, leukemia, HSCT, multiorgan dysfunction, immunocompromised patients T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected environmental surfaces. | Encephalitis | [356] |
| Human astrovirus Virginia| Human-Mink-Ovine-like 1/ HAstV-VA 1|HMO-C-UK1(a) 1 | ssRNA(+) | Encephalitis, progressive encephalitis, encephalopathy | [314,315,356] | ||||
| Human astrovirus Virginia| Human-Mink-Ovine-like 1/ HAstV-VA 1|HMO-C-PA 1 | ssRNA(+) | Progressive encephalitis | [356] | ||||
| Human astrovirus Virginia| Human-Mink-Ovine-like 1/ HAstV-VA 1|HMO-C 1 | ssRNA(+) | Encephalitis | [356] | ||||
| Human astrovirus Melbourne 2 1/ HAstV-MLB2 1; MLB2 1 | ssRNA(+) | Meningitis, acute meningitis | [356] | ||||
| Mamastrovirus 1 | Mamastrovirus 1/ MAstV1; HAstV 1 | ssRNA(+) | Encephalitis, encephalopathy | [313] | |||
| Mamastrovirus 4 | Mamastrovirus 4/ MAstV4; HAstV-4 1 | ssRNA(+) | Meningoencephalitis | [356] | |||
| Coronaviridae | Alphacoronavirus | Human coronavirus 229E | Human coronavirus 229E; Human coronavirus A 1/ HCoV_229E | ssRNA(+) | S: Infected human hosts Pc: Children under 5 y.o.; immunocompromised persons T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Encephalitis, ADEM | [310,316,335] |
| Human coronavirus NL63 | Human coronavirus NL63; Human coronavirus A 1/ HCoV_NL63 | ssRNA(+) | [310,335] | ||||
| Betacoronavirus | Betacoronavirus 1 | Human coronavirus OC43; Human coronavirus B 1/ HCoV_OC43 | ssRNA(+) | [310,316,335] | |||
| Human coronavirus HKU1 | Human coronavirus HKU1; Human coronavirus B 1/ HCoV_HKU1 | ssRNA(+) | [310,335] | ||||
|
Middle East
respiratory syndrome-related coronavirus | Middle East respiratory syndrome-related coronavirus/ MERS-CoV | ssRNA(+) | Encephalitis, ADEM | [310,316,335] | |||
|
Severe acute
respiratory syndrome-related coronavirus | Severe acute respiratory syndrome coronavirus | ssRNA(+) | Encephalitis, ADEM | [310,335] | |||
| Severe acute respiratory syndrome coronavirus 2/ SARS-CoV-2 | ssRNA(+) | Meningitis, encephalitis, ADEM, transverse myelitis, Guillain–Barré syndrome | [38,139,310,316,335,356] | ||||
| Flaviviridae | Hepacivirus |
Hepacivirus
hominis | Hepatitis C virus/ HCV | ssRNA(+) | S: Infected human hosts Pc/T: Blood-borne transmission by drug-injection equipment, blood transfusion, organ transplantation; Genital contact; Congenital infection. | Peripheral neuropathy, disseminated encephalomyelitis, transverse myelitis, acute encephalitis | [38,321,322] |
| Matonaviridae | Rubivirus | Rubivirus rubellae | Rubella virus/ RuV | ssRNA(+) | S: Infected human hosts Pc/T: Children under 5 y.o.; Immunocompromised persons; Unvaccinated persons; Inhalation of aerosolized droplets; Congenital infection (TORCH). | Meningitis, acute encephalitis, progressive rubella panencephalitis | [52,81,357] |
| Orthoherpesviridae | Cytomegalovirus |
Cytomegalovirus
humanbeta 5 | Human betaherpesvirus 5; Human cytomegalovirus/ HuBHV5, HCMV | dsDNA | S: Infected human hosts Pc/T: Children under 5 y.o.; Immunocompromised persons; Inhalation of aerosolized droplets; Congenital infection (TORCH). | Encephalitis, aseptic meningitis, neurodevelopmental deficits | [38,62,272,326,327] |
| Orthoherpesviridae | Lymphocryptovirus |
Lymphocryptovirus
humangamma 4 | Human gammaherpesvirus 4; Epstein-Barr virus/ HuGHV4, EBV | dsDNA | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Congenital infection. | Aseptic meningitis, meningitis, encephalitis, myelitis | [38,62,272,326,327] |
| Roseolovirus |
Roseolovirus
humanbeta 6a | Human betaherpesvirus 6A; Human herpesvirus 6A/ HuBHV6A, HHV6A | dsDNA | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Person-to-person transmission; Congenital infection. | Aseptic meningitis, encephalitis, multiple sclerosis | [38,62,326,327] | |
|
Roseolovirus
humanbeta 6b | Human betaherpesvirus 6B; Human herpesvirus 6B/ HuBHV6B, HHV6B | dsDNA | Aseptic meningitis, encephalitis, multiple sclerosis | [38,62,326,327] | |||
|
Roseolovirus
humanbeta 7 | Human betaherpesvirus 7; Human herpesvirus 7/ HuBHV7, HHV7 | dsDNA | Aseptic meningitis, encephalitis, meningoencephalitis, vestibular neuritis | [62,247,327] | |||
| Simplexvirus |
Simplexvirus
humanalpha 1 | Human alphaherpesvirus 1; Herpes simplex virus type 1/ HuAHV1, HSV1 | dsDNA | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Person-to-person transmission; Congenital infection (TORCH). | Meningitis, meningoencephalitis, encephalitis, myelitis, Guillain–Barré syndrome | [38,110,327] | |
|
Simplexvirus
humanalpha 2 | Human alphaherpesvirus 2; Herpes simplex virus type 2/ HuAHV2, HSV2 | dsDNA | [38,110,327] | ||||
| Varicellovirus |
Varicellovirus
humanalpha 3 | Human alphaherpesvirus 3; Varicella-zoster virus/ HuAHV3, VZV | dsDNA | S: Infected human hosts Pc/T: Person-to-person transmission; Congenital infection (TORCH). | Aseptic meningitis, encephalitis, meningoencephalitis, myelitis | [110,327,358,359,360] | |
| Orthomyxoviridae | Alphainfluenzavirus |
Alphainfluenzavirus
influenzae | Influenza A virus/ IAV | ssRNA(−) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Meningitis, meningoencephalitis, encephalitis, myelitis, Guillain–Barré syndrome | [330,331,361] |
| Betainfluenzavirus |
Betainfluenzavirus
influenzae | Influenza B virus/ IBV | ssRNA(−) | [333] | |||
| Paramyxoviridae | Morbillivirus |
Morbillivirus
hominis | Measles virus/ MV | ssRNA(−) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Encephalitis | [362,363,364] |
| Orthorubulavirus |
Orthorubulavirus
parotitidis | Mumps virus; Mumps orthorubulavirus 1/ MuV | ssRNA(−) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Meningitis, encephalitis, myelitis | [365,366] | |
|
Orthorubulavirus
laryngotracheitidis | Human parainfluenza virus 2; Human orthorubulavirus 2 1/ HPIV-2 | ssRNA(−) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Encephalitis, severe acute encephalopathy | [367,368,369] | ||
| Respirovirus |
Respirovirus
laryngotracheitidis | Human parainfluenza virus 1; Human respirovirus 1 1/ HPIV-1 | ssRNA(−) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Encephalitis, multiple sclerosis | [367,370,371] | |
|
Respirovirus
pneumoniae | Human parainfluenza virus 3/ HPIV-3 | ssRNA(−) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Meningitis, encephalitis, Guillain–Barré syndrome | [367,369,372,373,374,375] | ||
| Parvoviridae | Bocaparvovirus |
Bocaparvovirus
primate 1 | Human bocavirus 1/ HBoV1 | ssDNA | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Meningitis, meningoencephalitis, encephalitis | [339,376,377,378,379] |
| Erythroparvovirus |
Erythroparvovirus
primate 1 | Human parvovirus B19/ B19V | ssDNA | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Meningitis, meningoencephalitis | [339,380,381,382] | |
| Tetraparvovirus |
Tetraparvovirus
primate 1 | Human parvovirus 4/ PARV4 | ssDNA | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets | Encephalitis | [383] | |
| Picornaviridae | Enterovirus | Enterovirus A | Coxsackievirus A2/ CVA2 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Encephalitis, acute flaccid paralysis, aseptic meningitis | [45,384] |
| Coxsackievirus A3/ CVA3 | ssRNA(+) | Encephalitis, acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Enterovirus A | Coxsackievirus A4/ CVA4 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Encephalitis, acute flaccid paralysis, aseptic meningitis | [45] | ||
| Coxsakievirus A5/ CVA5 | ssRNA(+) | Encephalitis, acute flaccid paralysis, aseptic meningitis | [45,385] | ||||
| Coxsakievirus A6/ CVA6 | ssRNA(+) | Meningoencephalitis, encephalitis, acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsakievirus A7/ CVA7 | ssRNA(+) | Aseptic meningitis, encephalitis, acute flaccid paralysis | [386,387] | ||||
| Coxsakievirus A8/ CVA8 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsakievirus A10/ CVA10 | ssRNA(+) | Meningoencephalitis, encephalitis, acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsakievirus A12/ CVA12 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsakievirus A14/ CVA14 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsakievirus A16/ CVA16 | ssRNA(+) | Encephalitis, meningoencephalitis, rhombencephalitis, acute flaccid paralysis, aseptic meningitis | [45,388] | ||||
| Enterovirus A71/ EV-A71 | ssRNA(+) | Aseptic meningitis, acute flaccid myelitis/acute flaccid paralysis, encephalitis | [344,389,390,391,392,393,394] | ||||
| Enterovirus B | Coxsakievirus A9/ CVA9 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Encephalitis, aseptic meningitis, meningoencephalitis, rhombencephalitis, acute flaccid paralysis, acute transverse myelitis | [45,389,395] | ||
| Enterovirus B | Coxsackievirus B1/ CVB1 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Encephalitis, aseptic meningitis, meningoencephalitis, rhombencephalitis, acute flaccid paralysis, acute transverse myelitis | [45,384] | ||
| Coxsakievirus B2/ CVB2 | ssRNA(+) | Encephalitis, aseptic meningitis, meningoencephalitis, rhombencephalitis, acute flaccid paralysis, acute transverse myelitis | [45,389,396] | ||||
| Coxsakievirus B3/ CVB3 | ssRNA(+) | Encephalitis, aseptic meningitis, meningoencephalitis, acute flaccid paralysis, acute transverse myelitis | [45,389] | ||||
| Coxsakievirus B4/ CVB4 | ssRNA(+) | Encephalitis, aseptic meningitis, meningoencephalitis, rhombencephalitis, acute flaccid paralysis, acute transverse myelitis | [45,389,397,398] | ||||
| Coxsakievirus B5/ CVB5 | ssRNA(+) | Encephalitis, aseptic meningitis, meningoencephalitis, acute flaccid paralysis, acute transverse myelitis | [45,389] | ||||
| Coxsakievirus B6/ CVB6 | ssRNA(+) | Aseptic meningitis, acute flaccid paralysis | [45] | ||||
| Echovirus 4/ E4 | ssRNA(+) | Aseptic meningitis, encephalitis | [399,400,401,402] | ||||
| Echovirus 5/ E5 | ssRNA(+) | Aseptic meningitis, encephalitis | [402,403] | ||||
| Echovirus 6/ E6 | ssRNA(+) | Meningitis, encephalitis, Guillain–Barré syndrome | [389,402,404,405,406] | ||||
| Echovirus 7/ E7 | ssRNA(+) | Meningitis, encephalitis, encephalomyelitis | [402,407,408] | ||||
| Echovirus 9/ E9 | ssRNA(+) | Meningitis, encephalitis | [402] | ||||
| Echovirus 11/ E11 | ssRNA(+) | Aseptic meningitis, encephalitis (HFMD) | [402,409,410] | ||||
| Echovirus 13/ E13 | ssRNA(+) | Meningitis, encephalitis | [389,402,411] | ||||
| Enterovirus B | Echovirus 14/ E14 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Aseptic meningitis, encephalitis | [402] | ||
| Echovirus 15/ E15 | ssRNA(+) | Aseptic meningitis, encephalitis | [389,402] | ||||
| Echovirus 16/ E16 | ssRNA(+) | Aseptic meningitis | [402,412] | ||||
| Echovirus 17/ E17 | ssRNA(+) | Aseptic meningitis, encephalitis | [402] | ||||
| Echovirus 18/ E18 | ssRNA(+) | Aseptic meningitis, encephalitis | [402] | ||||
| Echovirus 19/ E19 | ssRNA(+) | Aseptic meningitis, encephalitis | [389,402,413] | ||||
| Echovirus 22 1 | ssRNA(+) | Aseptic meningitis, Guillain–Barré syndrome | [402] | ||||
| Echovirus 25/ E25 | ssRNA(+) | Aseptic meningitis, encephalitis | [402,414,415] | ||||
| Echovirus 30/ E30 | ssRNA(+) | Meningitis, encephalitis | [364,380,381,402] | ||||
| Echovirus 31/ E31 | ssRNA(+) | Aseptic meningitis | [402] | ||||
| Enterovirus B75/ EV-B75 | ssRNA(+) | Aseptic meningitis, encephalitis | [416,417] | ||||
| Enterovirus C | Coxsackievirus A1/ CVA-1 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Acute flaccid paralysis, aseptic meningitis | [45] | ||
| Coxsackievirus A11/ CVA-11 | ssRNA(+) | Acute flaccid paralysis, meningoencephalitis | [45] | ||||
| Coxsackievirus A13/ CVA-13 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsackievirus A17/ CVA-17 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsackievirus A19/ CVA-19 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Coxsackievirus A20/ CVA-20 | ssRNA(+) | Acute flaccid paralysis | [45] | ||||
| Coxsackievirus A21/ CVA-21 | ssRNA(+) | Acute flaccid paralysis, encephalitis, aseptic meningitis | [45] | ||||
| Coxsackievirus A22/ CVA-22 | ssRNA(+) | Acute flaccid paralysis | [45] | ||||
| Coxsackievirus A24/ CVA-24 | ssRNA(+) | Acute flaccid paralysis, aseptic meningitis | [45] | ||||
| Polioviruses 1 Include: Serotypes of the species Enterovirus C (types 1, 2 and 3 of wild Poliovirus (WPV)) | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Poliomyelitis, meningitis, aseptic meningitis | [418,419,420] | |||
| Enterovirus D | Enterovirus D68; Human rhinovirus 87/ EV-D68 | ssRNA(+) | S: Infected human hosts Pc/T: Inhalation of aerosolized droplets; Fecal–oral spread; Contact with infected tissue, water, environmental surfaces. | Meningo–myeloencephalitis, acute flaccid myelitis/acute flaccid paralysis | [20,344,421,422,423,424,425] | ||
| Enterovirus D70/ EV-D70 | ssRNA(+) | Acute flaccid myelitis | [392,426] | ||||
| Hepatovirus | Hepatovirus A | Hepatovirus A1; Hepatitis A virus/ HAV | ssRNA(+) | S: Infected human hosts Pc/T: Blood-borne transmission by drug-injection equipment, blood transfusion, organ transplantation; Genital contact; Congenital infection. | Encephalitis (extremely rare) | [427] | |
| Parechovirus | Parechovirus A | Human parechovirus 3/ HPeV-3 | ssRNA(+) | S: Infected human hosts T: Inhalation of aerosolized droplets; Neonatal infection. | Meningitis, meningoencephalitis, encephalitis | [345,346,347,428] | |
| Pneumoviridae | Orthopneumovirus |
Orthopneumovirus
hominis | Human orthopneumovirus; Human respiratory syncytial virus/ HRSV | ssRNA(−) | S: Infected human hosts T: Inhalation of aerosolized droplets | Meningitis, encephalitis (?), encephalopathy | [429,430,431,432,433] |
| Metapneumovirus |
Metapneumovirus
hominis | Human metapneumovirus/ HMPV | ssRNA(−) | S: Infected human hosts T: Inhalation of aerosolized droplets | Encephalitis | [310,434,435,436,437,438] | |
| Polyomaviridae | Betapolyomavirus |
Betapolyomaviru
secuhominis | JC polyomavirus; John Cunningham virus 1/ JC virus; JCV; JCPyV | dsDNA | S:/T: Autoinfection Pc: Immunocompromised condition | Meningitis, encephalopathy | [439,440] |
| Retroviridae | Lentivirus |
Human
immunodeficiency virus 1 | Human immunodeficiency virus 1/ HIV-1 | ssRNA-RT | S: Infected human hosts Pc/T: Blood-borne transmission by drug-injection equipment, blood transfusion, organ transplantation; Genital contact; Congenital infection. | Encephalitis (HAND) | [46,351] |
|
Human
immunodeficiency virus 2 | Human immunodeficiency virus 2/ HIV-2 | ssRNA-RT | S: Infected human hosts Pc/T: Blood-borne transmission by drug-injection equipment, blood transfusion, organ transplantation; Genital contact; Congenital infection. | Encephalitis (HAND) | [441] | ||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, H.; Zhao, S.; Wang, S.; Zheng, Y.; Wang, S.; Chen, H.; Pang, J.; Ma, J.; Yang, X.; Chen, Y. Global Magnitude of Encephalitis Burden and Its Evolving Pattern over the Past 30 Years. J. Infect. 2022, 84, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.A.; Honarmand, S.; Anderson, L.J.; Schnurr, D.P.; Forghani, B.; Cossen, C.K.; Schuster, F.L.; Christie, L.J.; Tureen, J.H. Beyond Viruses: Clinical Profiles and Etiologies Associated with Encephalitis. Clin. Infect. Dis. 2006, 43, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Blake, N.; Glennie, L.; Smith, V.; Bender, R.; Kyu, H.; Wunrow, H.Y.; Liu, L.; Yeung, D.; Knoll, M.D.; et al. The Global Burden of Meningitis in Children: Challenges with Interpreting Global Health Estimates. Microorganisms 2021, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Zunt, J.R.; Kassebaum, N.J.; Blake, N.; Glennie, L.; Wright, C.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Adamu, A.A.; et al. Global, Regional, and National Burden of Meningitis, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1061–1082. [Google Scholar] [CrossRef] [PubMed]
- Murphy, O.C.; Messacar, K.; Benson, L.; Bove, R.; Carpenter, J.L.; Crawford, T.; Dean, J.; DeBiasi, R.; Desai, J.; Elrick, M.J.; et al. Acute Flaccid Myelitis: Cause, Diagnosis, and Management. Lancet 2021, 397, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Tunkel, A.R.; Bloch, K.C.; Lauring, A.S.; Sejvar, J.; Bitnun, A.; Stahl, J.-P.; Mailles, A.; Drebot, M.; Rupprecht, C.E.; et al. Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin. Infect. Dis. 2013, 57, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease (GBD) by Institute for Health Metrics and Evaluation. Available online: https://www.healthdata.org/research-analysis/gbd (accessed on 7 May 2024).
- Ghia, C.J.; Rambhad, G.S. A Systematic Literature Review on the Prevalence and Etiology of Meningitis among Critically Ill and Hospitalized Patients in India. Ther. Adv. Infect. 2021, 8, 204993612110464. [Google Scholar] [CrossRef] [PubMed]
- Meningitis Research Foundation. Available online: https://www.meningitis.org/ (accessed on 7 May 2024).
- Meningitis Progress Tracker Tracking Progress towards Defeating Meningitis—Visualising the Story of Meningitis for the First Time. Meningitis Progress Tracker (Meningitis Research Foundation). Available online: https://www.meningitis.org/mpt (accessed on 7 May 2024).
- John, C.C.; Carabin, H.; Montano, S.M.; Bangirana, P.; Zunt, J.R.; Peterson, P.K. Global Research Priorities for Infections That Affect the Nervous System. Nature 2015, 527, S178–S186. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.C.; Soeters, H.M.; Diallo, A.O.; Bicaba, B.W.; Kadadé, G.; Dembélé, A.Y.; Acyl, M.A.; Nikiema, C.; Lingani, C.; Hatcher, C.; et al. MenAfriNet: A Network Supporting Case-Based Meningitis Surveillance and Vaccine Evaluation in the Meningitis Belt of Africa. J. Infect. Dis. 2019, 220, S148–S154. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Narayanan, S.; Tiefenbach, J.; Lukšić, I.; Ale, B.M.; Adeloye, D.; Rudan, I. Estimating the Global and Regional Burden of Meningitis in Children Caused by Haemophilus influenzae Type b: A Systematic Review and Meta-Analysis. J. Glob. Health 2022, 12, 04014. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Kneen, R.; Riordan, A.; Kelly, D.; Pollard, A.J. Encephalitis in Children. Arch. Dis. Child. 2012, 97, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.Y.; Balasuriya, U.B.R.; Lee, C. Zoonotic Encephalitides Caused by Arboviruses: Transmission and Epidemiology of Alphaviruses and Flaviviruses. Clin. Exp. Vaccine Res. 2014, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Бoлезни нервнoй системы: Рукoвoдствo для врачей: В 2-х тoмах; Издание втoрoе, перерабoтаннoе и дoпoлненнoе; Медицина: Мoсква, Рoссия, 2001; Volume 1, ISBN 5-225-04540-5.
- World Health Organization: Poliomyelitis. WHO 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 7 May 2024).
- Sejvar, J.J.; Lopez, A.S.; Cortese, M.M.; Leshem, E.; Pastula, D.M.; Miller, L.; Glaser, C.; Kambhampati, A.; Shioda, K.; Aliabadi, N.; et al. Acute Flaccid Myelitis in the United States, August–December 2014: Results of Nationwide Surveillance. Clin. Infect. Dis. 2016, 63, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, J.; Knoester, M.; Van Leer-Buter, C.C.; Neuteboom, R.F.; Meiners, L.C.; Niesters, H.G.; Brouwer, O.F. Acute Flaccid Myelitis and Enterovirus D68: Lessons from the Past and Present. Eur. J. Pediatr. 2019, 178, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Asturias, E.J.; Hixon, A.M.; Van Leer-Buter, C.; Niesters, H.G.M.; Tyler, K.L.; Abzug, M.J.; Dominguez, S.R. Enterovirus D68 and Acute Flaccid Myelitis—Evaluating the Evidence for Causality. Lancet Infect. Dis. 2018, 18, e239–e247. [Google Scholar] [CrossRef] [PubMed]
- McEntire, C.R.S.; Anand, P.; Cervantes-Arslanian, A.M. Neuroinfectious Disease Emergencies. Neurol. Clin. 2021, 39, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Xu, M.; Shi, G.; Zhou, J.; Zhang, J.; Li, H. Trends and Developments in the Detection of Pathogens in Central Nervous System Infections: A Bibliometric Study. Front. Cell. Infect. Microbiol. 2022, 12, 856845. [Google Scholar] [CrossRef] [PubMed]
- Riddell, J.; Shuman, E.K. Epidemiology of Central Nervous System Infection. Neuroimaging Clin. N. Am. 2012, 22, 543–556. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Ni, F.; Xia, W. Cutaneous Protothecosis with Meningitis Due to Prototheca Wickerhamii in an Immunocompetent Teenager: Case Report and Literature Review. Infect. Drug Resist. 2021, 14, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Zhu, L.-P.; Weng, X.-H.; Li, L.; Wang, J.-J. Meningitis Due to Prototheca Wickerhamii: Rare Case in China. Med. Mycol. 2007, 45, 85–88. [Google Scholar] [CrossRef]
- Leveque, N.; Van Haecke, A.; Renois, F.; Boutolleau, D.; Talmud, D.; Andreoletti, L. Rapid Virological Diagnosis of Central Nervous System Infections by Use of a Multiplex Reverse Transcription-PCR DNA Microarray. J. Clin. Microbiol. 2011, 49, 3874–3879. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Ustinov, M.V. Empirical therapy of inflammatory lesion of large skin folds// Эмпирическая терапия вoспалительных пoражений кoжи крупных складoк. RMJ//РМЖ 2016, 14, 945–948. [Google Scholar]
- Gu, W.; Deng, X.; Lee, M.; Sucu, Y.D.; Arevalo, S.; Stryke, D.; Federman, S.; Gopez, A.; Reyes, K.; Zorn, K.; et al. Rapid Pathogen Detection by Metagenomic Next-Generation Sequencing of Infected Body Fluids. Nat. Med. 2021, 27, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, A.; Sangha, B.; Kim, E.; Nawaz, S.; Malik, V.; Vij, R.; Sekhsaria, S. Antibiotic Hypersensitivity and Adverse Reactions: Management and Implications in Clinical Practice. Allergy Asthma Clin. Immunol. 2020, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic Allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, L.J.; Davies, D.S.C. Antibiotic Overuse: A Key Driver of Antimicrobial Resistance. Br. J. Gen. Pract. 2014, 64, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, P.; Zhang, H.-C.; Wu, H.-L.; Ye, M.-Z.; Zhu, Y.-M.; Ai, J.-W.; Zhang, W.-H. Clinical Application and Evaluation of Metagenomic Next-Generation Sequencing in Suspected Adult Central Nervous System Infection. J. Transl. Med. 2020, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Sample, H.A.; Zorn, K.C.; Arevalo, S.; Yu, G.; Neuhaus, J.; Federman, S.; Stryke, D.; Briggs, B.; Langelier, C.; et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 2019, 380, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Franke, G.; Polywka, S.K.A.; Lütgehetmann, M.; Gbadamosi, J.; Magnus, T.; Aepfelbacher, M. Improved Detection of Bacterial Central Nervous System Infections by Use of a Broad-Range PCR Assay. J. Clin. Microbiol. 2014, 52, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Kanjilal, S.; Cho, T.A.; Piantadosi, A. Diagnostic Testing in Central Nervous System Infection. Semin. Neurol. 2019, 39, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Zanella, M.-C.; Lenggenhager, L.; Schrenzel, J.; Cordey, S.; Kaiser, L. High-Throughput Sequencing for the Aetiologic Identification of Viral Encephalitis, Meningoencephalitis, and Meningitis. A Narrative Review and Clinical Appraisal. Clin. Microbiol. Infect. 2019, 25, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Wouk, J.; Rechenchoski, D.Z.; Rodrigues, B.C.D.; Ribelato, E.V.; Faccin-Galhardi, L.C. Viral Infections and Their Relationship to Neurological Disorders. Arch. Virol. 2021, 166, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Vigasova, D.; Nemergut, M.; Liskova, B.; Damborsky, J. Multi-Pathogen Infections and Alzheimer’s Disease. Microb. Cell Fact. 2021, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, P.; Vorberg, I.M. Viruses in Neurodegenerative Diseases: More than Just Suspects in Crimes. PLoS Pathog. 2022, 18, e1010670. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.M.; Kielian, T. Microglia in Infectious Diseases of the Central Nervous System. J. Neuroimmune Pharmacol. 2009, 4, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Jorgačevski, J.; Potokar, M. Immune Functions of Astrocytes in Viral Neuroinfections. IJMS 2023, 24, 3514. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lin, W.-D.; Chou, I.-C.; Lee, I.-C.; Hong, S.-Y. Epilepsy and Neurodevelopmental Outcomes in Children with Etiologically Diagnosed Central Nervous System Infections: A Retrospective Cohort Study. Front. Neurol. 2019, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.S.; Tavares, F.N.; Sousa, I.P. Global Landscape of Coxsackieviruses in Human Health. Virus Res. 2024, 344, 199367. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A.; McGavern, D.B. Viral Diseases of the Central Nervous System. Curr. Opin. Virol. 2015, 11, 44–54. [Google Scholar] [CrossRef]
- Smuts, I.; Lamb, G.V. Viral Infections of the Central Nervous System. In Viral Infections in Children, Volume II; Green, R.J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 83–123. ISBN 978-3-319-54092-4. [Google Scholar]
- Bonthius, D.J.; Bale, J.F. Viral Infections of the Nervous System. In Swaiman’s Pediatric Neurology; Elsevier: New York, NY, USA, 2017; pp. 895–906. ISBN 978-0-323-37101-8. [Google Scholar]
- Bonthius, D.J. Lymphocytic Choriomeningitis Virus: An Underrecognized Cause of Neurologic Disease in the Fetus, Child, and Adult. Semin. Pediatr. Neurol. 2012, 19, 89–95. [Google Scholar] [CrossRef]
- Di Vito, A.; Donato, A.; Bria, J.; Donato, F.; Donato, G. Encephalitis Lethargica. What Is Still Wrong? Int. J. Immunopathol. Pharmacol. 2023, 37, 039463202311549. [Google Scholar] [CrossRef]
- Foley, P.B. What Caused Encephalitis Lethargica. In Encephalitis lethargica; Springer: New York, NY, USA, 2018; pp. 683–780. ISBN 978-1-4939-0383-2. [Google Scholar]
- Tselis, A.C.; Booss, J. Neurovirology. In Handbook of Clinical Neurology; Elsevier: Edinburgh, UK, 2014; ISBN 978-0-444-53488-0. [Google Scholar]
- Lipton, H.L. Human Vilyuisk Encephalitis. Rev. Med. Virol. 2008, 18, 347–352. [Google Scholar] [CrossRef]
- Liang, Z.; Kumar, A.S.M.; Jones, M.S.; Knowles, N.J.; Lipton, H.L. Phylogenetic Analysis of the Species Theilovirus: Emerging Murine and Human Pathogens. J. Virol. 2008, 82, 11545–11554. [Google Scholar] [CrossRef]
- Eibach, D.; Hogan, B.; Sarpong, N.; Winter, D.; Struck, N.S.; Adu-Sarkodie, Y.; Owusu-Dabo, E.; Schmidt-Chanasit, J.; May, J.; Cadar, D. Viral Metagenomics Revealed Novel Betatorquevirus Species in Pediatric Inpatients with Encephalitis/Meningoencephalitis from Ghana. Sci. Rep. 2019, 9, 2360. [Google Scholar] [CrossRef]
- Tan, L.V.; van Doorn, H.R.; Nghia, H.D.T.; Chau, T.T.H.; Tu, L.T.P.; de Vries, M.; Canuti, M.; Deijs, M.; Jebbink, M.F.; Baker, S.; et al. Identification of a New Cyclovirus in Cerebrospinal Fluid of Patients with Acute Central Nervous System Infections. mBio 2013, 4, e00231-13. [Google Scholar] [CrossRef]
- Viglietta, M.; Bellone, R.; Blisnick, A.A.; Failloux, A.-B. Vector Specificity of Arbovirus Transmission. Front. Microbiol. 2021, 12, 773211. [Google Scholar] [CrossRef]
- Shi, J.; Hu, Z.; Deng, F.; Shen, S. Tick-Borne Viruses. Virol. Sin. 2018, 33, 21–43. [Google Scholar] [CrossRef]
- Rochlin, I.; Toledo, A. Emerging Tick-Borne Pathogens of Public Health Importance: A Mini-Review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef]
- Labuda, M.; Nuttall, P.A. Viruses Transmitted by Ticks. In Ticks; Bowman, A.S., Nuttall, P.A., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 253–280. ISBN 978-0-521-86761-0. [Google Scholar]
- McGill, F.; Griffiths, M.J.; Solomon, T. Viral Meningitis: Current Issues in Diagnosis and Treatment. Curr. Opin. Infect. Dis. 2017, 30, 248–256. [Google Scholar] [CrossRef]
- Pustijanac, E.; Buršić, M.; Talapko, J.; Škrlec, I.; Meštrović, T.; Lišnjić, D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms 2023, 11, 1634. [Google Scholar] [CrossRef]
- Ličková, M.; Fumačová Havlíková, S.; Sláviková, M.; Klempa, B. Alimentary Infections by Tick-Borne Encephalitis Virus. Viruses 2021, 14, 56. [Google Scholar] [CrossRef]
- Venugopal, K.; Buckley, A.; Reid, H.W.; Gould, E.A. Nucleotide Sequence of the Envelope Glycoprotein of Negishi Virus Shows Very Close Homology to Louping III Virus. Virology 1992, 190, 515–521. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I.; Nowotny, N. Arboviruses Pathogenic for Domestic and Wild Animals. In Advances in Virus Research; Elsevier: New York, NY, USA, 2014; Volume 89, pp. 201–275. ISBN 978-0-12-800172-1. [Google Scholar]
- Jeffries, C.L.; Mansfield, K.L.; Phipps, L.P.; Wakeley, P.R.; Mearns, R.; Schock, A.; Bell, S.; Breed, A.C.; Fooks, A.R.; Johnson, N. Louping Ill Virus: An Endemic Tick-Borne Disease of Great Britain. J. Gen. Virol. 2014, 95, 1005–1014. [Google Scholar] [CrossRef]
- Gritsun, T.S.; Nuttall, P.A.; Gould, E.A. Tick-Borne Flaviviruses. In Advances in Virus Research; Elsevier: New York, NY, USA, 2003; Volume 61, pp. 317–371. ISBN 978-0-12-039861-4. [Google Scholar]
- Leonova, G.N.; Kondratov, I.G.; Maystrovskaya, O.S.; Takashima, I.; Belikov, S.I. Louping Ill Virus (LIV) in the Far East. Arch. Virol. 2015, 160, 663–673. [Google Scholar] [CrossRef]
- Holding, M.; Dowall, S.D.; Medlock, J.M.; Carter, D.P.; Pullan, S.T.; Lewis, J.; Vipond, R.; Rocchi, M.S.; Baylis, M.; Hewson, R. Tick-Borne Encephalitis Virus, United Kingdom. Emerg. Infect. Dis. 2020, 26, 90–96. [Google Scholar] [CrossRef]
- Rollin, P.E.; Memish, Z.A. Alkhurma Hemorrhagic Fever. In Emerging Infectious Diseases; Elsevier: New York, NY, USA, 2014; pp. 61–71. ISBN 978-0-12-416975-3. [Google Scholar]
- Carletti, F.; Castilletti, C.; Di Caro, A.; Capobianchi, M.R.; Nisii, C.; Suter, F.; Rizzi, M.; Tebaldi, A.; Goglio, A.; Tosi, C.P.; et al. Alkhurma Hemorrhagic Fever in Travelers Returning from Egypt, 2010. Emerg. Infect. Dis. 2010, 16, 1979–1982. [Google Scholar] [CrossRef]
- Bhatia, B.; Feldmann, H.; Marzi, A. Kyasanur Forest Disease and Alkhurma Hemorrhagic Fever Virus—Two Neglected Zoonotic Pathogens. Microorganisms 2020, 8, 1406. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Memish, Z.A. Alkhurma Hemorrhagic Fever Virus. Microbes Infect. 2017, 19, 305–310. [Google Scholar] [CrossRef]
- Munivenkatappa, A.; Sahay, R.; Yadav, P.; Viswanathan, R.; Mourya, D. Clinical & Epidemiological Significance of Kyasanur Forest Disease. Indian J. Med. Res. 2018, 148, 145. [Google Scholar] [CrossRef]
- Růžek, D.; Yakimenko, V.V.; Karan, L.S.; Tkachev, S.E. Omsk Haemorrhagic Fever. Lancet 2010, 376, 2104–2113. [Google Scholar] [CrossRef]
- Rudakov, N.V.; Yastrebov, V.K.; Yakimenko, V.V. Epidemiology of Omsk Haemorragic Fever. Epidemiol. Vaccine Prev. 2015, 14, 39–48. [Google Scholar] [CrossRef]
- Wagner, E.; Shin, A.; Tukhanova, N.; Turebekov, N.; Nurmakhanov, T.; Sutyagin, V.; Berdibekov, A.; Maikanov, N.; Lezdinsh, I.; Shapiyeva, Z.; et al. First Indications of Omsk Haemorrhagic Fever Virus beyond Russia. Viruses 2022, 14, 754. [Google Scholar] [CrossRef]
- Lvov, D.K.; Shchelkanov, M.Y.; Alkhovsky, S.V.; Deryabin, P.G. Single-Stranded RNA Viruses. In Zoonotic Viruses in Northern Eurasia; Elsevier: New York, NY, USA, 2015; pp. 135–392. ISBN 978-0-12-801742-5. [Google Scholar]
- Birge, J.; Sonnesyn, S. Powassan Virus Encephalitis, Minnesota, USA. Emerg. Infect. Dis. 2012, 18, 1669–1671. [Google Scholar] [CrossRef]
- Lyons, J.L. Viral Meningitis and Encephalitis. Contin. Lifelong Learn. Neurol. 2018, 24, 1284–1297. [Google Scholar] [CrossRef]
- Tavakoli, N.P.; Wang, H.; Dupuis, M.; Hull, R.; Ebel, G.D.; Gilmore, E.J.; Faust, P.L. Fatal Case of Deer Tick Virus Encephalitis. N. Engl. J. Med. 2009, 360, 2099–2107. [Google Scholar] [CrossRef]
- Lledó, L.; Giménez-Pardo, C.; Gegúndez, M.I. Epidemiological Study of Thogoto and Dhori Virus Infection in People Bitten by Ticks, and in Sheep, in an Area of Northern Spain. IJERPH 2020, 17, 2254. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I. Tick-Borne Viruses in Europe. Parasitol. Res. 2012, 111, 9–36. [Google Scholar] [CrossRef]
- Koch, J.; Xin, Q.; Tischler, N.D.; Lozach, P.-Y. Entry of Phenuiviruses into Mammalian Host Cells. Viruses 2021, 13, 299. [Google Scholar] [CrossRef]
- Matsuno, K.; Weisend, C.; Travassos da Rosa, A.P.A.; Anzick, S.L.; Dahlstrom, E.; Porcella, S.F.; Dorward, D.W.; Yu, X.-J.; Tesh, R.B.; Ebihara, H. Characterization of the Bhanja Serogroup Viruses (Bunyaviridae): A Novel Species of the Genus Phlebovirus and Its Relationship with Other Emerging Tick-Borne Phleboviruses. J. Virol. 2013, 87, 3719–3728. [Google Scholar] [CrossRef]
- Hubálek, Z. Biogeography of Tick-Borne Bhanja Virus (Bunyaviridae) in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 372691. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Tyler, K.L. Orthoreoviruses and Orbiviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: New York, NY, USA, 2015; pp. 1848–1850. ISBN 978-1-4557-4801-3. [Google Scholar]
- Dilcher, M.; Hasib, L.; Lechner, M.; Wieseke, N.; Middendorf, M.; Marz, M.; Koch, A.; Spiegel, M.; Dobler, G.; Hufert, F.T.; et al. Genetic Characterization of Tribeč Virus and Kemerovo Virus, Two Tick-Transmitted Human-Pathogenic Orbiviruses. Virology 2012, 423, 68–76. [Google Scholar] [CrossRef]
- Lvov, D.K.; Shchelkanov, M.Y.; Alkhovsky, S.V.; Deryabin, P.G. Double-Stranded RNA Viruses. In Zoonotic Viruses in Northern Eurasia; Elsevier: New York, NY, USA, 2015; pp. 113–133. ISBN 978-0-12-801742-5. [Google Scholar]
- Padgett, K.A.; Kjemtrup, A.; Novak, M.; Velez, J.O.; Panella, N. Colorado Tick Fever Virus in the Far West: Forgotten, but Not Gone. Vector-Borne Zoonotic Dis. 2022, 22, 443–448. [Google Scholar] [CrossRef]
- Yendell, S.J.; Fischer, M.; Staples, J.E. Colorado Tick Fever in the United States, 2002–2012. Vector-Borne Zoonotic Dis. 2015, 15, 311–316. [Google Scholar] [CrossRef]
- Shah, T.; Li, Q.; Wang, B.; Baloch, Z.; Xia, X. Geographical Distribution and Pathogenesis of Ticks and Tick-Borne Viral Diseases. Front. Microbiol. 2023, 14, 1185829. [Google Scholar] [CrossRef]
- Attoui, H.; Jaafar, F.M.; de Micco, P.; de Lamballerie, X. Coltiviruses and Seadornaviruses in North America, Europe, and Asia. Emerg. Infect. Dis. 2005, 11, 1673–1679. [Google Scholar] [CrossRef]
- Charrel, R.N.; Attoui, H.; Butenko, A.M.; Clegg, J.C.; Deubel, V.; Frolova, T.V.; Gould, E.A.; Gritsun, T.S.; Heinz, F.X.; Labuda, M.; et al. Tick-Borne Virus Diseases of Human Interest in Europe. Clin. Microbiol. Infect. 2004, 10, 1040–1055. [Google Scholar] [CrossRef]
- Günther, G.; Haglund, M. Tick-Borne Encephalopathies: Epidemiology, Diagnosis, Treatment and Prevention. CNS Drugs 2005, 19, 1009–1032. [Google Scholar] [CrossRef]
- Ungureanu, A.; van der Meer, J.; Bicvic, A.; Abbuehl, L.; Chiffi, G.; Jaques, L.; Suter-Riniker, F.; Leib, S.L.; Bassetti, C.L.A.; Dietmann, A. Meningitis, Meningoencephalitis and Encephalitis in Bern: An Observational Study of 258 Patients. BMC Neurol. 2021, 21, 474. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Wysokińska-Miszczuk, J. Food-Borne Transmission of Tick-Borne Encephalitis Virus—Spread, Consequences, and Prophylaxis. IJERPH 2022, 19, 1812. [Google Scholar] [CrossRef]
- Tick-Borne Encephalitis (TBE) Virus: Geographic Distribution. Available online: https://www.cdc.gov/tick-borne-encephalitis/geographic-distribution/index.html (accessed on 7 May 2024).
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Kholodilov, I.S.; Belova, O.A.; Ivannikova, A.Y.; Gadzhikurbanov, M.N.; Makenov, M.T.; Yakovlev, A.S.; Polienko, A.E.; Dereventsova, A.V.; Litov, A.G.; Gmyl, L.V.; et al. Distribution and Characterisation of Tick-Borne Flavi-, Flavi-like, and Phenuiviruses in the Chelyabinsk Region of Russia. Viruses 2022, 14, 2699. [Google Scholar] [CrossRef]
- Lindquist, L. Tick-Borne Encephalitis. In Handbook of Clinical Neurology; Elsevier: New York, NY, USA, 2014; Volume 123, pp. 531–559. ISBN 978-0-444-53488-0. [Google Scholar]
- Chiba, N.; Iwasaki, T.; Mizutani, T.; Kariwa, H.; Kurata, T.; Takashima, I. Pathogenicity of Tick-Borne Encephalitis Virus Isolated in Hokkaido, Japan in Mouse Model. Vaccine 1999, 17, 779–787. [Google Scholar] [CrossRef]
- Yoshii, K.; Song, J.Y.; Park, S.-B.; Yang, J.; Schmitt, H.-J. Tick-Borne Encephalitis in Japan, Republic of Korea and China. Emerg. Microbes Infect. 2017, 6, e82. [Google Scholar] [CrossRef]
- Kemenesi, G.; Bányai, K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2018, 32, e00106-17. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Jizhou, L.; Phipps, L.P.; Johnson, N. Emerging Tick-Borne Viruses in the Twenty-First Century. Front. Cell. Infect. Microbiol. 2017, 7, 298. [Google Scholar] [CrossRef]
- El Khoury, M.Y.; Camargo, J.F.; White, J.L.; Backenson, B.P.; Dupuis, A.P.; Escuyer, K.L.; Kramer, L.; St. George, K.; Chatterjee, D.; Prusinski, M.; et al. Potential Role of Deer Tick Virus in Powassan Encephalitis Cases in Lyme Disease–Endemic Areas of New York, USA. Emerg. Infect. Dis. 2013, 19, 1926. [Google Scholar] [CrossRef]
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses 2019, 11, 669. [Google Scholar] [CrossRef]
- Cunha, M.S.; Luchs, A.; da Costa, A.C.; Ribeiro, G.d.O.; dos Santos, F.C.P.; Nogueira, J.S.; Komninakis, S.V.; dos Santos Souza Marinho, R.; Witkin, S.S.; Villanova, F.; et al. Detection and Characterization of Ilheus and Iguape Virus Genomes in Historical Mosquito Samples from Southern Brazil. Acta Trop. 2020, 205, 105401. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.P.; dos Santos, F.B.; de Paula, V.S.; Vieira, T.C.R.G.; Dias, H.G.; Barros, C.A.; da Silva, E.E. Viral and Prion Infections Associated with Central Nervous System Syndromes in Brazil. Viruses 2021, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Nanaware, N.; Banerjee, A.; Mullick Bagchi, S.; Bagchi, P.; Mukherjee, A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses 2021, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Weyer, J.; Blumberg, L.H. Emerging Zoonotic and Vector-Borne Viral Diseases. In Viral Infections in Children, Volume I; Green, R.J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 125–150. ISBN 978-3-319-54032-0. [Google Scholar]
- Rezende, I.M.D.; Cenachi, A.R.C.; Costa, T.A.; Oliveira, G.F.G.; Rabelo, L.; Menezes, L.M.; Penido, I.; Pereira, L.S.; Arruda, M.S.; Gonçalves, A.P.; et al. Wild-Type Yellow Fever Virus in Cerebrospinal Fluid from Fatal Cases in Brazil, 2018. Front. Virol. 2022, 2, 936191. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.W.; Eidex, R.B.; Marfin, A.A.; Russell, M.; Sejvar, J.J.; Markoff, L.; Hayes, E.B.; Chen, R.T.; Ball, R.; Braun, M.M.; et al. Neurologic Disease Associated with 17D-204 Yellow Fever Vaccination: A Report of 15 Cases. Vaccine 2007, 25, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; Trindade, G.d.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent Sylvatic Yellow Fever Virus Transmission in Brazil: The News from an Old Disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Oyono, M.G.; Kenmoe, S.; Abanda, N.N.; Takuissu, G.R.; Ebogo-Belobo, J.T.; Kenfack-Momo, R.; Kengne-Nde, C.; Mbaga, D.S.; Tchatchouang, S.; Kenfack-Zanguim, J.; et al. Epidemiology of Yellow Fever Virus in Humans, Arthropods, and Non-Human Primates in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010610. [Google Scholar] [CrossRef] [PubMed]
- Marinho, P.E.S.; Alvarenga, P.P.M.; Crispim, A.P.C.; Candiani, T.M.S.; Alvarenga, A.M.; Bechler, I.M.; Alves, P.A.; Dornas, F.P.; de Oliveira, D.B.; Bentes, A.A.; et al. Wild-Type Yellow Fever Virus RNA in Cerebrospinal Fluid of Child. Emerg. Infect. Dis. 2019, 25, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, K. Yellow Fever Encephalitis: An Emerging and Resurging Global Public Health Threat in a Changing Environment. In Encephalitis; Tkachev, S., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0925-9. [Google Scholar]
- Сизикoва Татьяна Евгеньевна, Савенкo Сергей Вадимoвич, Лебедев Виталий Никoлаевич, Бoрисевич Сергей Владимирoвич Энцефалит Рoссиo. Инфекциoнные бoлезни. 2022, 11, 113–118.
- Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines. Vaccines 2023, 11, 742. [Google Scholar] [CrossRef]
- Vahey, G.M.; Mathis, S.; Martin, S.W.; Gould, C.V.; Staples, J.E.; Lindsey, N.P. West Nile Virus and Other Domestic Nationally Notifiable Arboviral Diseases—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Danforth, M.E.; Snyder, R.E.; Feiszli, T.; Bullick, T.; Messenger, S.; Hanson, C.; Padgett, K.; Coffey, L.L.; Barker, C.M.; Reisen, W.K.; et al. Epidemiologic and Environmental Characterization of the Re-Emergence of St. Louis Encephalitis Virus in California, 2015–2020. PLoS Negl. Trop. Dis. 2022, 16, e0010664. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Coffey, L.L.; Burkett-Cadena, N.; Day, J.F. Reemergence of St. Louis Encephalitis Virus in the Americas. Emerg. Infect. Dis. 2018, 24, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.L.; Fischer, M.; Lindsey, N.P.; Curren, E.J. St. Louis Encephalitis Virus Disease in the United States, 2003–2017. Am. J. Trop. Med. Hyg. 2018, 99, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Floridis, J.; McGuinness, S.; Kurucz, N.; Burrow, J.; Baird, R.; Francis, J. Murray Valley Encephalitis Virus: An Ongoing Cause of Encephalitis in Australia’s North. Trop. Med. Infect. Dis. 2018, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Niven, D.J.; Afra, K.; Iftinca, M.; Tellier, R.; Fonseca, K.; Kramer, A.; Safronetz, D.; Holloway, K.; Drebot, M.; Johnson, A.S. Fatal Infection with Murray Valley Encephalitis Virus Imported from Australia to Canada, 2011. Emerg. Infect. Dis 2017, 23, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Ong, O.T.W.; Skinner, E.B.; Johnson, B.J.; Old, J.M. Mosquito-Borne Viruses and Non-Human Vertebrates in Australia: A Review. Viruses 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Lindsay, M.D.A.; Smith, D.W.; Imrie, A. The Ecology and Epidemiology of Ross River and Murray Valley Encephalitis Viruses in Western Australia: Examples of One Health in Action. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wanzeller, A.L.M.; da Silva, F.S.; Hernández, L.H.A.; Barros, L.J.L.; Freitas, M.N.O.; Santos, M.M.; Gonçalves, E.d.J.; Pantoja, J.A.S.; Lima, C.d.S.; Lima, M.F.; et al. Isolation of Flaviviruses and Alphaviruses with Encephalitogenic Potential Diagnosed by Evandro Chagas Institute (Pará, Brazil) in the Period of 1954–2022: Six Decades of Discoveries. Viruses 2023, 15, 935. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Byas, A.D.; Ebel, G.D. Comparative Pathology of West Nile Virus in Humans and Non-Human Animals. Pathogens 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Bauerfeind, R.; Graevenitz, A.V.; Kimmig, P.; Schiefer, H.G.; Schwarz, T.; Slenczka, W.; Zahner, H. Zoonoses: Infectious Diseases Transmissible from Animals to Humans, 4th ed.; ASM Books; ASM Press: Washington, DC, USA, 2015; ISBN 978-1-68367-090-2. [Google Scholar]
- Hall, R.A.; Scherret, J.H.; Mackenzie, J.S. Kunjin Virus: An Australian Variant of West Nile? Ann. N. Y. Acad. Sci. 2001, 951, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu Virus: A New Threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef] [PubMed]
- Cadar, D.; Becker, N.; Campos, R.d.M.; Börstler, J.; Jöst, H.; Schmidt-Chanasit, J. Usutu Virus in Bats, Germany, 2013. Emerg. Infect. Dis. 2014, 20, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassine, T.; De Massis, F.; Calistri, P.; Savini, G.; BelHaj Mohamed, B.; Ranen, A.; Di Gennaro, A.; Sghaier, S.; Hammami, S. First Detection of Co-Circulation of West Nile and Usutu Viruses in Equids in the South-West of Tunisia. Transbound. Emerg. Dis. 2014, 61, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Diagne, M.; Ndione, M.; Di Paola, N.; Fall, G.; Bedekelabou, A.; Sembène, P.; Faye, O.; Zanotto, P.; Sall, A. Usutu Virus Isolated from Rodents in Senegal. Viruses 2019, 11, 181. [Google Scholar] [CrossRef]
- McEntire, C.R.S.; Song, K.-W.; McInnis, R.P.; Rhee, J.Y.; Young, M.; Williams, E.; Wibecan, L.L.; Nolan, N.; Nagy, A.M.; Gluckstein, J.; et al. Neurologic Manifestations of the World Health Organization’s List of Pandemic and Epidemic Diseases. Front. Neurol. 2021, 12, 634827. [Google Scholar] [CrossRef] [PubMed]
- Mwaliko, C.; Nyaruaba, R.; Zhao, L.; Atoni, E.; Karungu, S.; Mwau, M.; Lavillette, D.; Xia, H.; Yuan, Z. Zika Virus Pathogenesis and Current Therapeutic Advances. Pathog. Glob. Health 2021, 115, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Song, H.; Ming, G. How Does Zika Virus Cause Microcephaly? Genes Dev. 2017, 31, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.M.; Reynolds, M.R.; Lewis, E.L.; Woodworth, K.R.; Godfred-Cato, S.; Delaney, A.; Akosa, A.; Valencia-Prado, M.; Lash, M.; Elmore, A.; et al. Zika-Associated Birth Defects Reported in Pregnancies with Laboratory Evidence of Confirmed or Possible Zika Virus Infection—U.S. Zika Pregnancy and Infant Registry, December 1, 2015–March 31, 2018. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Edridge, A.W.D.; van der Hoek, L. Emerging Orthobunyaviruses Associated with CNS Disease. PLoS Negl. Trop. Dis. 2020, 14, e0008856. [Google Scholar] [CrossRef] [PubMed]
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-Borne Arboviruses of African Origin: Review of Key Viruses and Vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Dutuze, M.F.; Nzayirambaho, M.; Mores, C.N.; Christofferson, R.C. A Review of Bunyamwera, Batai, and Ngari Viruses: Understudied Orthobunyaviruses with Potential One Health Implications. Front. Vet. Sci. 2018, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.d.C.; Tauro, L.B.; Farias, A.A.; Labruna, M.B.; Diaz, A. Molecular Survey of Flaviviruses and Orthobunyaviruses in Amblyomma Spp. Ticks Collected in Minas Gerais, Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 764–768. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, B.M.; Kokernot, R.H.; Smithburn, K.C.; Paterson, H.E. Isolation of Germiston Virus, a Hitherto Unknown Agent, from Culicine Mosquitoes, and a Report of Infection in Two Laboratory Workers. Am. J. Trop. Med. Hyg. 1960, 9, 62–69. [Google Scholar] [CrossRef]
- Beran, G.W. Handbook of Zoonoses, Section B Viral Zoonoses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1994; Volume B, ISBN 0-8493-3206-0. [Google Scholar]
- Campbell, G.L.; Mataczynski, J.D.; Reisdorf, E.S.; Powell, J.W.; Martin, D.A.; Lambert, A.J.; Haupt, T.E.; Davis, J.P.; Lanciotti, R.S. Second Human Case of Cache Valley Virus Disease. Emerg. Infect. Dis. 2006, 12, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, J.; Snyder-Keller, A.; Wu, Y.; Sun, S.; Sui, H.; Dean, A.B.; Kramer, L.; Hernandez-Ilizaliturri, F. Fatal Cache Valley Virus Meningoencephalitis Associated with Rituximab Maintenance Therapy. Am. J. Hematol. 2018, 93, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Suan, D.; Duggins, A.; Schubert, R.D.; Khan, L.M.; Sample, H.A.; Zorn, K.C.; Rodrigues Hoffman, A.; Blick, A.; Shingde, M.; et al. A Novel Cause of Chronic Viral Meningoencephalitis: Cache Valley Virus: Orthobunyavirus Encephalitis. Ann. Neurol. 2017, 82, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.L.; Zhao, G.; Hull, R.; Shelly, M.A.; Wong, S.J.; Wu, G.; St. George, K.; Wang, D.; Menegus, M.A. Cache Valley Virus in a Patient Diagnosed with Aseptic Meningitis. J. Clin. Microbiol. 2013, 51, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Gricourt, G.; Ndebi, M.; Demontant, V.; Poiteau, L.; Burrel, S.; Boutolleau, D.; Woerther, P.-L.; Calvez, V.; Stroer, S.; et al. Fatal Encephalitis Caused by Cristoli Virus, an Emerging Orthobunyavirus, France. Emerg. Infect. Dis. 2020, 26, 1287–1290. [Google Scholar] [CrossRef]
- Poglazov, A.B.; Shliapnikova, O.V.; Prilipov, A.G. Characteristic of the new Khatanga virus genotype. Vestn. Ross. Akad. Med. Nauk. 2011, 5, 45–49. [Google Scholar]
- Lavrent’ev, M.V.; Prilipov, A.G.; L’vov, S.D.; L’vov, D.K. Phylogenetic analysis of the nucleotide sequences of Chatanga virus strains, the new representative of California encephalitis serocomplex, isolated in different regions of the Russian Federation. Vopr. Virusol. 2008, 53, 25–29. [Google Scholar]
- Francy, D.B.; Jaenson, T.G.; Lundström, J.O.; Schildt, E.B.; Espmark, A.; Henriksson, B.; Niklasson, B. Ecologic Studies of Mosquitoes and Birds as Hosts of Ockelbo Virus in Sweden and Isolation of Inkoo and Batai Viruses from Mosquitoes. Am. J. Trop. Med. Hyg. 1989, 41, 355–363. [Google Scholar] [CrossRef]
- Butenko, A.M.; Vladimirtseva, E.A.; Lvov, S.D.; Calisher, C.H.; Karabatsos, N. California Serogroup Viruses from Mosquitoes Collected in the USSR. Am. J. Trop. Med. Hyg. 1991, 45, 366–370. [Google Scholar] [CrossRef]
- Traavik, T.; Mehl, R.; Wiger, R. California encephalitis group viruses isolated from mosquitoes collected in Southern and Arctic Norway. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. 2009, 86, 335–342. [Google Scholar] [CrossRef]
- Demikhov, V.G. Outcomes and prognosis of diseases caused by Inkoo and Tahyna viruses. Vopr. Virusol. 1995, 40, 72–74. [Google Scholar]
- Li, W.; Cao, Y.; Fu, S.; Wang, J.; Li, M.; Jiang, S.; Wang, X.; Xing, S.; Feng, L.; Wang, Z.; et al. Tahyna Virus Infection, a Neglected Arboviral Disease in the Qinghai-Tibet Plateau of China. Vector-Borne Zoonotic Dis. 2014, 14, 353–357. [Google Scholar] [CrossRef]
- Mravcová, K.; Camp, J.V.; Hubálek, Z.; Šikutová, S.; Vaux, A.G.C.; Medlock, J.M.; Rudolf, I. Ťahyňa Virus—A Widespread, but Neglected Mosquito-borne Virus in Europe. Zoonoses Public Health 2023, 70, 371–382. [Google Scholar] [CrossRef]
- Pilaski, J.; Mackenstein, H. Isolation of Tahyna virus from mosquitoes in 2 different European natural foci. Zentralbl Bakteriol. Mikrobiol. Hyg. B 1985, 180, 394–420. [Google Scholar]
- Bárdos, V. The Role of Mammals in the Circulation of Tahyna Virus. Folia Parasitol. 1975, 22, 257–264. [Google Scholar]
- Chastel, C.; Bach-Hamba, D.; Launay, H.; Le Lay, G.; Hellal, H.; Beaucournu, J.C. Arbovirus infections in Tunisia: New serological survey of small wild mammals. Bull. Soc. Pathol. Exot. Fil. 1983, 76, 21–33. [Google Scholar]
- Demikhov, V.G.; Chaĭtsev, V.G. Neurologic characteristics of diseases caused by Inkoo and Tahyna viruses. Vopr. Virusol. 1995, 40, 21–25. [Google Scholar]
- Sluka, F. Recognition of clinical forms of Valtice fever, a new arbovirus infection. Wien. Med. Wochenschr. 1969, 119, 765–769. [Google Scholar]
- da Rosa, J.F.T.; de Souza, W.M.; de Paula Pinheiro, F.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- de Souza Bastos, M.; Pivoto João, G.; Naveca, F.G.; Monte, R.L.; Bastos, M.d.S.; Ramasawmy, R.; Mourão, M.P.G.; Lessa, N.; de Lima Gimaque, J.B.; Figueiredo, L.T.M. Identification of Oropouche Orthobunyavirus in the Cerebrospinal Fluid of Three Patients in the Amazonas, Brazil. Am. J. Trop. Med. Hyg. 2012, 86, 732–735. [Google Scholar] [CrossRef]
- Aguilar, P.V.; Vargas, J.; Beingolea, L.; Kochel, T.J.; Guevara, C.; Watts, D.M.; Tesh, R.B.; Cruz, C.; Rocha, C.; Suarez, V.; et al. Guaroa Virus Infection among Humans in Bolivia and Peru. Am. J. Trop. Med. Hyg. 2010, 83, 714–721. [Google Scholar] [CrossRef]
- Dias, H.G.; dos Santos, F.B.; Pauvolid-Corrêa, A. An Overview of Neglected Orthobunyaviruses in Brazil. Viruses 2022, 14, 987. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Rocha, A.G.; Freitas, R.B.; Ohana, B.A.; Travassos da Rosa, A.P.; Rogério, J.S.; Linhares, A.C. Meningitis associated with Oropouche virus infections. Rev. Inst. Med. Trop. Sao Paulo 1982, 24, 246–251. [Google Scholar]
- Pachler, K.; Růžek, D.; Nowotny, N. Molecular Characterization of the African Orthobunyavirus Ilesha Virus. Infect. Genet. Evol. 2013, 20, 124–130. [Google Scholar] [CrossRef]
- Edridge, A.W.D.; van den Brekel, N.; Mukungu, P.; Nakayima, R.; Bbosa, S.; Isagara, P.; van Boele Hensbroek, M.; van der Hoek, L.; Kayiwa, J.; Lutwama, J.J.; et al. No Evidence of Ntwetwe Virus Infections in Children Presenting to Kiboga Hospital, Uganda. Trop. Med. Infect. Dis. 2022, 8, 21. [Google Scholar] [CrossRef]
- Pérot, P.; Bielle, F.; Bigot, T.; Foulongne, V.; Bolloré, K.; Chrétien, D.; Gil, P.; Gutiérrez, S.; L’Ambert, G.; Mokhtari, K.; et al. Identification of Umbre Orthobunyavirus as a Novel Zoonotic Virus Responsible for Lethal Encephalitis in 2 French Patients with Hypogammaglobulinemia. Clin. Infect. Dis. 2021, 72, 1701–1708. [Google Scholar] [CrossRef]
- Balenghien, T.; Cardinale, E.; Chevalier, V.; Elissa, N.; Failloux, A.-B.; Jean Jose Nipomichene, T.N.; Nicolas, G.; Rakotoharinome, V.M.; Roger, M.; Zumbo, B. Towards a Better Understanding of Rift Valley Fever Epidemiology in the South-West of the Indian Ocean. Vet. Res. 2013, 44, 78. [Google Scholar] [CrossRef]
- Peyrefitte, C.N.; Devetakov, I.; Pastorino, B.; Villeneuve, L.; Bessaud, M.; Stolidi, P.; Depaquit, J.; Segura, L.; Gravier, P.; Tock, F.; et al. Toscana Virus and Acute Meningitis, France. Emerg. Infect. Dis. 2005, 11, 778–780. [Google Scholar] [CrossRef]
- Baldelli, F.; Grazia Ciufolini, M.; Francisci, D.; Marchi, A.; Venturi, G.; Fiorentini, C.; Laura Luchetta, M.; Bruto, L.; Pauluzzi, S. Unusual Presentation of Life-Threatening Toscana Virus Meningoencephalitis. Clin. Infect. Dis. 2004, 38, 515–520. [Google Scholar] [CrossRef]
- Gratz, N.G. The Vector-Borne Human Infections of Europe: Their Distribution and Burden on Public Health/by Norman G. Gratz; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2004. [Google Scholar]
- Menghani, S.; Chikhale, R.; Raval, A.; Wadibhasme, P.; Khedekar, P. Chandipura Virus: An Emerging Tropical Pathogen. Acta Trop. 2012, 124, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.-H.; Zhai, Y.-G.; Meng, W.-S.; Sun, X.-H.; Cao, Y.-X.; Fu, S.-H.; Wang, H.-Y.; Xu, L.-H.; Tang, Q.; et al. Banna Virus, China, 1987–2007. Emerg. Infect. Dis. 2010, 16, 514–517. [Google Scholar] [CrossRef]
- Carrera, J.-P.; Forrester, N.; Wang, E.; Vittor, A.Y.; Haddow, A.D.; López-Vergès, S.; Abadía, I.; Castaño, E.; Sosa, N.; Báez, C.; et al. Eastern Equine Encephalitis in Latin America. N. Engl. J. Med. 2013, 369, 732–744. [Google Scholar] [CrossRef]
- García, M.; Cisneros, J.; Carrera, J.-P.; Luciani, K.; Guerra, I.; Abadía, I.; Martínez-Torres, A.O.; Estripeaut, D. Madariaga Virus Infection Associated with a Case of Acute Disseminated Encephalomyelitis. Am. J. Trop. Med. Hyg. 2015, 92, 1130–1132. [Google Scholar] [CrossRef]
- Blohm, G.M.; Lednicky, J.A.; White, S.K.; Mavian, C.N.; Márquez, M.C.; González-García, K.P.; Salemi, M.; Morris, J.G.; Paniz-Mondolfi, A.E. Madariaga Virus: Identification of a Lineage III Strain in a Venezuelan Child with Acute Undifferentiated Febrile Illness, in the Setting of a Possible Equine Epizootic. Clin. Infect. Dis. 2018, 67, 619–621. [Google Scholar] [CrossRef]
- Lednicky, J.A.; White, S.K.; Mavian, C.N.; El Badry, M.A.; Telisma, T.; Salemi, M.; OKech, B.A.; Beau De Rochars, V.M.; Morris, J.G. Emergence of Madariaga Virus as a Cause of Acute Febrile Illness in Children, Haiti, 2015–2016. PLoS Negl. Trop. Dis. 2019, 13, e0006972. [Google Scholar] [CrossRef]
- Vosoughi, R.; Walkty, A.; Drebot, M.A.; Kadkhoda, K. Jamestown Canyon Virus Meningoencephalitis Mimicking Migraine with Aura in a Resident of Manitoba. CMAJ 2018, 190, E262–E264. [Google Scholar] [CrossRef]
- Fagre, A.C.; Lyons, S.; Staples, J.E.; Lindsey, N. West Nile Virus and Other Nationally Notifiable Arboviral Diseases—United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 901–906. [Google Scholar] [CrossRef]
- van Niekerk, S.; Human, S.; Williams, J.; van Wilpe, E.; Pretorius, M.; Swanepoel, R.; Venter, M. Sindbis and Middelburg Old World Alphaviruses Associated with Neurologic Disease in Horses, South Africa. Emerg. Infect. Dis. 2015, 21, 2225–2229. [Google Scholar] [CrossRef]
- Fourie, I.; Williams, J.; Ismail, A.; Jansen van Vuren, P.; Stoltz, A.; Venter, M. Detection and Genome Characterization of Middelburg Virus Strains Isolated from CSF and Whole Blood Samples of Humans with Neurological Manifestations in South Africa. PLoS Negl. Trop. Dis. 2022, 16, e0010020. [Google Scholar] [CrossRef]
- Lucas, R.E.; Qiao, M. A Case of Encephalitis in Central Australia Due to Ross River Virus? Aust. N. Z. J. Med. 1999, 29, 268–270. [Google Scholar] [CrossRef]
- Harley, D.; Sleigh, A.; Ritchie, S. Ross River Virus Transmission, Infection, and Disease: A Cross-Disciplinary Review. Clin. Microbiol. Rev. 2001, 14, 909–932. [Google Scholar] [CrossRef]
- Lednicky, J.A.; White, S.K.; Stephenson, C.J.; Cherabuddi, K.; Loeb, J.C.; Moussatche, N.; Lednicky, A.; Morris, J.G. Keystone Virus Isolated From a Florida Teenager with Rash and Subjective Fever: Another Endemic Arbovirus in the Southeastern United States? Clin. Infect. Dis. 2019, 68, 143–145. [Google Scholar] [CrossRef]
- Azar, S.R.; Campos, R.K.; Bergren, N.A.; Camargos, V.N.; Rossi, S.L. Epidemic Alphaviruses: Ecology, Emergence and Outbreaks. Microorganisms 2020, 8, 1167. [Google Scholar] [CrossRef]
- Hommel, D.; Heraud, J.M.; Hulin, A.; Talarmin, A. Association of Tonate Virus (Subtype IIIB of the Venezuelan Equine Encephalitis Complex) with Encephalitis in a Human. Clin. Infect. Dis. 2000, 30, 188–190. [Google Scholar] [CrossRef]
- Coimbra, T.L.M.; Nassar, E.S.; Nagamori, A.H.; Ferreira, I.E.; Pereira, L.E.; Rocco, I.M.; Ueda-Ito, M.; Romano, N.S. Iguape: A Newly Recognized Flavivirus from São Paulo State, Brazil. Intervirology 1993, 36, 144–152. [Google Scholar] [CrossRef]
- Trivedi, S.; Chakravarty, A. Neurological Complications of Dengue Fever. Curr. Neurol. Neurosci. Rep. 2022, 22, 515–529. [Google Scholar] [CrossRef]
- Li, G.-H.; Ning, Z.-J.; Liu, Y.-M.; Li, X.-H. Neurological Manifestations of Dengue Infection. Front. Cell. Infect. Microbiol. 2017, 7, 449. [Google Scholar] [CrossRef]
- Withana, M.; Rodrigo, C.; Chang, T.; Karunanayake, P.; Rajapakse, S. Dengue Fever Presenting with Acute Cerebellitis: A Case Report. BMC Res. Notes 2014, 7, 125. [Google Scholar] [CrossRef]
- Osnaya-Romero, N.; Perez-Guille, M.-G.; Andrade-García, S.; Gonzalez-Vargas, E.; Borgaro-Payro, R.; Villagomez-Martinez, S.; de Jesús Ortega-Maldonado, J.; Arredondo-García, J.L. Neurological Complications and Death in Children with Dengue Virus Infection: Report of Two Cases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 25. [Google Scholar] [CrossRef]
- Murugesan, A.; Manoharan, M. Dengue Virus. Emerg. Reemerging Viral Pathog. 2020, 1, 281–359. [Google Scholar] [CrossRef]
- Kok, B.H.; Lim, H.T.; Lim, C.P.; Lai, N.S.; Leow, C.Y.; Leow, C.H. Dengue Virus Infection—A Review of Pathogenesis, Vaccines, Diagnosis and Therapy. Virus Res. 2022, 324, 199018. [Google Scholar] [CrossRef]
- Singh, S.; Alallah, J.; Amrit, A.; Maheshwari, A.; Boppana, S. Neurological Manifestations of Perinatal Dengue. Newborn 2023, 2, 158–172. [Google Scholar] [CrossRef]
- Gainor, E.M.; Harris, E.; LaBeaud, A.D. Uncovering the Burden of Dengue in Africa: Considerations on Magnitude, Misdiagnosis, and Ancestry. Viruses 2022, 14, 233. [Google Scholar] [CrossRef]
- Saivish, M.V.; Gomes da Costa, V.; de Lima Menezes, G.; Alves da Silva, R.; Dutra da Silva, G.C.; Moreli, M.L.; Sacchetto, L.; Pacca, C.C.; Vasilakis, N.; Nogueira, M.L. Rocio Virus: An Updated View on an Elusive Flavivirus. Viruses 2021, 13, 2293. [Google Scholar] [CrossRef]
- Auerswald, H.; Maquart, P.-O.; Chevalier, V.; Boyer, S. Mosquito Vector Competence for Japanese Encephalitis Virus. Viruses 2021, 13, 1154. [Google Scholar] [CrossRef]
- Mulvey, P.; Duong, V.; Boyer, S.; Burgess, G.; Williams, D.T.; Dussart, P.; Horwood, P.F. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef]
- Pommier, J.D.; Gorman, C.; Crabol, Y.; Bleakley, K.; Sothy, H.; Santy, K.; Tran, H.T.T.; Nguyen, L.V.; Bunnakea, E.; Hlaing, C.S.; et al. Childhood Encephalitis in the Greater Mekong Region (the SouthEast Asia Encephalitis Project): A Multicentre Prospective Study. Lancet Glob. Health 2022, 10, e989–e1002. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.; Yadav, P.; Dubey, S.K.; Azhar, E.I.; Maitra, S.S.; Dwivedi, V.D. Molecular Pathogenesis of Japanese Encephalitis and Possible Therapeutic Strategies. Arch. Virol. 2022, 167, 1739–1762. [Google Scholar] [CrossRef]
- Rajaiah, P.; Kumar, A. Japanese Encephalitis Virus in India: An Update on Virus Genotypes. Indian J. Med. Res. 2022, 156, 588–597. [Google Scholar] [CrossRef]
- Chowdhury, P.; Khan, S.A. Global Emergence of West Nile Virus: Threat & Preparedness in Special Perspective to India. Indian J. Med. Res. 2021, 154, 36–50. [Google Scholar] [CrossRef]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and Transmission Dynamics of West Nile Virus Disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef]
- Antoniou, E.; Orovou, E.; Sarella, A.; Iliadou, M.; Rigas, N.; Palaska, E.; Iatrakis, G.; Dagla, M. Zika Virus and the Risk of Developing Microcephaly in Infants: A Systematic Review. IJERPH 2020, 17, 3806. [Google Scholar] [CrossRef]
- Combarnous, Y.; Guillou, F.; Martinat, N. Functional States of the Luteinizing Hormone/Choriogonadotropin-Receptor Complex in Rat Leydig Cells. J. Biol. Chem. 1986, 261, 6868–6871. [Google Scholar] [CrossRef]
- Schneider, E.F.; Robich, R.M.; Elias, S.P.; Lubelczyk, C.B.; Cosenza, D.S.; Smith, R.P. Jamestown Canyon Virus in Collected Mosquitoes, Maine, United States, 2017–2019. Emerg. Infect. Dis. 2022, 28, 2330–2333. [Google Scholar] [CrossRef]
- Weiler, N.S.; Niendorf, E.; Dumic, I. Two Insects, Two Bites, One Patient: A Lyme Disease and Jamestown Canyon Co-Infection. Cureus 2023, 15, e40222. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Pillai, A.N.; Lednicky, J.A.; Morris, J.G.; Hladish, T.J. Ecology and Public Health Burden of Keystone Virus in Florida. Epidemics 2022, 39, 100555. [Google Scholar] [CrossRef]
- Artsob, H.; Spence, L. Imported Arbovirus Infections in Canada 1974-89. Can. J. Infect. Dis. 1991, 2, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gaensbauer, J.T.; Lindsey, N.P.; Messacar, K.; Staples, J.E.; Fischer, M. Neuroinvasive Arboviral Disease in the United States: 2003 to 2012. Pediatrics 2014, 134, e642–e650. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.K.; Holloway, K.; Dimitrova, K.; Rogers, L.; Chaulk, A.C.; Lang, A.S.; Whitney, H.G.; Drebot, M.A.; Chapman, T.W. The Seasonal Timing of Snowshoe Hare Virus Transmission on the Island of Newfoundland, Canada. J. Med. Entomol. 2017, 54, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Drebot, M. Emerging Mosquito-Borne Bunyaviruses in Canada. CCDR 2015, 41, 117–123. [Google Scholar] [CrossRef] [PubMed]
- May, L.P.; Watts, S.L.; Maruniak, J.E. Molecular Survey for Mosquito-Transmitted Viruses: Detection of Tensaw Virus in North Central Florida Mosquito Populations. J. Am. Mosq. Control Assoc. 2014, 30, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liu, H.; Zhao, L.; Atoni, E.; Wang, Y.; Yuan, Z. First Isolation and Characterization of a Group C Banna Virus (BAV) from Anopheles Sinensis Mosquitoes in Hubei, China. Viruses 2018, 10, 555. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.-Y.; Fu, S.-H.; Li, M.-H.; Zhai, Y.-G.; Meng, W.-S.; Sun, X.-H.; Lv, Z.; Wang, H.-Y.; Shen, X.-X.; et al. Molecular Evolution of Emerging Banna Virus. Infect. Genet. Evol. 2016, 45, 250–255. [Google Scholar] [CrossRef]
- Chinedu Eneh, S.; Uwishema, O.; Nazir, A.; El Jurdi, E.; Faith Olanrewaju, O.; Abbass, Z.; Mustapha Jolayemi, M.; Mina, N.; Kseiry, L.; Onyeaka, H. Chikungunya Outbreak in Africa: A Review of the Literature. Ann. Med. Surg. 2023, 85, 3545–3552. [Google Scholar] [CrossRef]
- Hughes, H.R.; Velez, J.O.; Davis, E.H.; Laven, J.; Gould, C.V.; Panella, A.J.; Lambert, A.J.; Staples, J.E.; Brault, A.C. Fatal Human Infection with Evidence of Intrahost Variation of Eastern Equine Encephalitis Virus, Alabama, USA, 2019. Emerg. Infect. Dis. 2021, 27, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Heberlein-Larson, L.A.; Tan, Y.; Stark, L.M.; Cannons, A.C.; Shilts, M.H.; Unnasch, T.R.; Das, S.R. Complex Epidemiological Dynamics of Eastern Equine Encephalitis Virus in Florida. Am. J. Trop. Med. Hyg. 2019, 100, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Andreadis, T.G. Eastern Equine Encephalitis Virus in Mosquitoes and Their Role as Bridge Vectors. Emerg. Infect. Dis. 2010, 16, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An Emerging Viral Threat? Emerg. Microbes Infect. 2018, 7, 163. [Google Scholar] [CrossRef]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis Virus as a Human Pathogen-Epidemiology, Clinical Picture and Pathogenesis: Sindbis Virus as a Human Pathogen. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Meno, K.; Yah, C.; Mendes, A.; Venter, M. Incidence of Sindbis Virus in Hospitalized Patients with Acute Fevers of Unknown Cause in South Africa, 2019–2020. Front. Microbiol. 2022, 12, 798810. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Terán, C.; Calderón-Rangel, A.; Rodriguez-Morales, A.; Mattar, S. Venezuelan Equine Encephalitis Virus: The Problem Is Not over for Tropical America. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.V.; Estrada-Franco, J.G.; Navarro-Lopez, R.; Ferro, C.; Haddow, A.D.; Weaver, S.C. Endemic Venezuelan Equine Encephalitis in the Americas: Hidden under the Dengue Umbrella. Future Virol. 2011, 6, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Zacks, M.A.; Paessler, S. Encephalitic Alphaviruses. Vet. Microbiol. 2010, 140, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Contigiani, M.S.; Diaz, L.A.; Spinsanti, L.I. General Aspects on Arboviruses. In Arthropod Borne Diseases; Marcondes, C.B., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–71. ISBN 978-3-319-13883-1. [Google Scholar]
- Asante, J.; Noreddin, A.; El Zowalaty, M. Systematic Review of Important Bacterial Zoonoses in Africa in the Last Decade in Light of the ‘One Health’ Concept. Pathogens 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, G.; Balamuruga, V.; Gandhale, P.N.; Singh, R.K.; Bhanupraka, V. Viral Zoonosis: A Comprehensive Review. Asian J. Anim. Vet. Adv. 2010, 5, 77–92. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, G.; Li, R.; Chanda, E.; Dean, K.R.; Dirzo, R.; Jakobsen, K.S.; Khan, I.; Leirs, H.; Shi, Z.-L.; Wolfe, N.D.; et al. The Animal Origin of Major Human Infectious Diseases: What Can Past Epidemics Teach Us About Preventing the Next Pandemic? Zoonoses 2022, 2. [Google Scholar] [CrossRef]
- Tapia-Ramírez, G.; Lorenzo, C.; Navarrete, D.; Carrillo-Reyes, A.; Retana, Ó.; Carrasco-Hernández, R. A Review of Mammarenaviruses and Rodent Reservoirs in the Americas. EcoHealth 2022, 19, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Bedi, J.S.; Vijay, D.; Dhaka, P. Textbook of Zoonoses; Wiley-Blackwell: Hoboken, NJ, USA, 2022; ISBN 978-1-119-80953-1. [Google Scholar]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus Infections in the Nervous System. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Cubitt, B.; Martinez-Sobrido, L.; de la Torre, J.C. Molecular Engineering of a Mammarenavirus with Unbreachable Attenuation. J. Virol. 2023, 97, e01385-22. [Google Scholar] [CrossRef]
- Hallam, S.J.; Koma, T.; Maruyama, J.; Paessler, S. Review of Mammarenavirus Biology and Replication. Front. Microbiol. 2018, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; de la Torre, J.C. Human Pathogenic Arenaviruses (Arenaviridae). In Encyclopedia of Virology; Elsevier: New York, NY, USA, 2019; pp. 507–517. ISBN 978-0-12-814516-6. [Google Scholar]
- Fornůsková, A.; Hiadlovská, Z.; Macholán, M.; Piálek, J.; de Bellocq, J.G. New Perspective on the Geographic Distribution and Evolution of Lymphocytic Choriomeningitis Virus, Central Europe. Emerg. Infect. Dis. 2021, 27, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Boros, Á.; Takáts, K.; Mátics, R.; Pankovics, P. A Novel Mammarenavirus (Family Arenaviridae) in Hedgehogs (Erinaceus roumanicus) in Europe. Arch. Virol. 2023, 168, 174. [Google Scholar] [CrossRef]
- Tuppeny, M. Viral Meningitis and Encephalitis. Crit. Care Nurs. Clin. N. Am. 2013, 25, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Billioux, B.J.; Smith, B.; Nath, A. Neurological Complications of Ebola Virus Infection. Neurotherapeutics 2016, 13, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Beltz, L.A. Bats and Human Health: Ebola, SARS, Rabies and Beyond; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-15004-6. [Google Scholar]
- Abir, M.H.; Rahman, T.; Das, A.; Etu, S.N.; Nafiz, I.H.; Rakib, A.; Mitra, S.; Emran, T.B.; Dhama, K.; Islam, A.; et al. Pathogenicity and Virulence of Marburg Virus. Virulence 2022, 13, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A Global Perspective on Hantavirus Ecology, Epidemiology, and Disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Бернштейн, А.Д.; Гаврилoвская, И.Н.; Апекина, Н.С.; Дзагурoва, Т.К.; Ткаченкo, Е.А. Осoбеннoсти Прирoднoй Очагoвoсти Хантавирусных Зooнoзoв. Эпидемиoлoгия и Вакцинoпрoфилактика 2010, 2, 5–13. [Google Scholar]
- Fawcett, S.J.; Chen, J.S.; Fawcett, R.W. Acute Hantavirus Infection Presenting with Fever and Altered Mentation in the Absence of Pulmonary or Renal Manifestations. Open Forum Infect. Dis. 2022, 9, ofac430. [Google Scholar] [CrossRef] [PubMed]
- Sing, A. (Ed.) Zoonoses: Infections Affecting Humans and Animals; Focus on Public Health Aspects; Springer: Dordrecht, The Netherlands, 2015; ISBN 978-94-017-9456-5. [Google Scholar]
- Monika, N.; Rohith, M.G.; Ravi, K.; Kandagal, S.A. Clinical Profile and Outcome of Patients with Meningoencephalitis in a Tertiary Care Hospital. Int. J. Res. Med. Sci. 2023, 11, 1012–1018. [Google Scholar] [CrossRef]
- Talamonti, L.; Padula, P.J.; Canteli, M.S.; Posner, F.; Marczeski, F.P.; Weller, C. Hantavirus Pulmonary Syndrome: Encephalitis Caused by Virus Andes. J. Neurovirol. 2011, 17, 189–192. [Google Scholar] [CrossRef]
- Cerar, D.; Avšič-Županc, T.; Jereb, M.; Strle, F. Case Report: Severe Neurological Manifestation of Dobrava Hantavirus Infection. J. Med. Virol. 2007, 79, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Krone, B.; Bleich, S.; Prange, H.; Paulus, W. Encephalitis Due to a Hantavirus Infection. J. Infect. 2002, 45, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Hautala, N.; Partanen, T.; Kubin, A.-M.; Kauma, H.; Hautala, T. Central Nervous System and Ocular Manifestations in Puumala Hantavirus Infection. Viruses 2021, 13, 1040. [Google Scholar] [CrossRef] [PubMed]
- Huisa, B.N.; Chapin, J.E.; Adair, J.C. Central Nervous System Complications Following Hanta Virus Cardiopulmonary Syndrome. J. Neurovirol. 2009, 15, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Lv, S.; Yi, P.; Feng, L.; Deng, X.; Xia, H.; Xu, Y. Central Nervous System Infection with Seoul Orthohantavirus in a Child after Hematopoietic Stem Cell Transplantation: A Case Report. Virol. J. 2022, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.S.; Singh, R.K.; Dhama, K. (Eds.) Animal-Origin Viral Zoonoses; Livestock Diseases and Management; Springer: Singapore, 2020; ISBN 9789811526503. [Google Scholar]
- Quarleri, J.; Galvan, V.; Delpino, M.V. Henipaviruses: An Expanding Global Public Health Concern? GeroScience 2022, 44, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Billioux, B.J.; Mbaya, O.T.; Sejvar, J.; Nath, A. Neurologic Complications of Smallpox and Monkeypox: A Review. JAMA Neurol. 2022, 79, 1180. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.; Evans, J.; Luo, T.; Fooks, A. Lyssaviruses and Bats: Emergence and Zoonotic Threat. Viruses 2014, 6, 2974–2990. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T.; Blumberg, L.H.; Liebenberg, C.; Hewlett, R.H.; Grobbelaar, A.A.; Leman, P.A.; Croft, J.E.; Nel, L.H.; Nutt, L.; Swanepoel, R. Fatal Human Infection with Rabies-Related Duvenhage Virus, South Africa. Emerg. Infect. Dis. 2006, 12, 1965–1967. [Google Scholar] [CrossRef] [PubMed]
- van Thiel, P.-P.A.M.; de Bie, R.M.A.; Eftimov, F.; Tepaske, R.; Zaaijer, H.L.; van Doornum, G.J.J.; Schutten, M.; Osterhaus, A.D.M.E.; Majoie, C.B.L.M.; Aronica, E.; et al. Fatal Human Rabies Due to Duvenhage Virus from a Bat in Kenya: Failure of Treatment with Coma-Induction, Ketamine, and Antiviral Drugs. PLoS Negl. Trop. Dis. 2009, 3, e428. [Google Scholar] [CrossRef]
- Shipley, R.; Wright, E.; Selden, D.; Wu, G.; Aegerter, J.; Fooks, A.R.; Banyard, A.C. Bats and Viruses: Emergence of Novel Lyssaviruses and Association of Bats with Viral Zoonoses in the EU. Trop. Med. Infect. Dis. 2019, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Carney, I.K.; Deverill, J.E.; Botha, J.A.; Smith, G.A.; Serafin, I.L.; Harrower, B.J.; Tannenberg, A.E.G.; Fitzpatrick, P.F.; Searle, J.W. Australian Bat Lyssavirus Infection: A Second Human Case, with a Long Incubation Period. Med. J. Aust. 2000, 172, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; McIntyre, P.G.; White, K.; Shearer, A.J.; Reynolds, N.; Walker, D.; Orange, G.V.; Fooks, A.R. Fatal Human Rabies Caused by European Bat Lyssavirus Type 2a Infection in Scotland. Clin. Infect. Dis. 2003, 37, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Irani, D.N. Aseptic Meningitis and Viral Myelitis. Neurol. Clin. 2008, 26, 635–655. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.A.E.; MacMahon, E. Viral Meningitis. BMJ 2008, 336, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Mohammadi Pour, P.; Piri, S.; Farzaei, M.H.; Echeverría, J. Modulating Neurological Complications of Emerging Infectious Diseases: Mechanistic Approaches to Candidate Phytochemicals. Front. Pharmacol. 2021, 12, 742146. [Google Scholar] [CrossRef] [PubMed]
- Guidance Ebola: Overview, History, Origins and Transmission. GOV.UK; UK Health Security Agency 2023. Available online: https://www.gov.uk/government/publications/ebola-origins-reservoirs-transmission-and-guidelines/ebola-overview-history-origins-and-transmission (accessed on 7 May 2024).
- History of Ebola Disease Outbreaks. CDC. 2023. Available online: https://www.cdc.gov/vhf/ebola/history/chronology.html (accessed on 7 May 2024).
- Reuben, R.C.; Abunike, S.A. Marburg Virus Disease: The Paradox of Nigeria’s Preparedness and Priority Effects in Co-Epidemics. Bull. Natl. Res. Cent. 2023, 47, 10. [Google Scholar] [CrossRef] [PubMed]
- Ferrés, M.; Martínez-Valdebenito, C.; Angulo, J.; Henríquez, C.; Vera-Otárola, J.; Vergara, M.J.; Pérez, J.; Fernández, J.; Sotomayor, V.; Valdés, M.F.; et al. Mother-to-Child Transmission of Andes Virus through Breast Milk, Chile1. Emerg. Infect. Dis. 2020, 26, 1885–1888. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.B.; Gryseels, S.; Otto, H.W.; Worobey, M. Evolution and Diversity of Bat and Rodent Paramyxoviruses from North America. J. Virol. 2022, 96, e01098-21. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Ferrés, M. Person-to-Person Household and Nosocomial Transmission of Andes Hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Papa, A. Dobrava-Belgrade Virus: Phylogeny, Epidemiology, Disease. Antivir. Res. 2012, 95, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Lvov, D.K.; Shchelkanov, M.Y.; Alkhovsky, S.V.; Deryabin, P.G. Introduction. In Zoonotic Viruses in Northern Eurasia; Elsevier: New York, NY, USA, 2015; pp. 3–15. ISBN 978-0-12-801742-5. [Google Scholar]
- Steiner, T.; Ettinger, J.; Peng, Z.; Hofmann, J.; Hartmann, M.; Burkhardt, U.; Schnitzler, P. Hyperintense Lesion in the Corpus Callosum Associated with Puumala Hantavirus Infection. J. Neurol. 2012, 259, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.A.; Netter, H.J. Henipavirus Infection: Natural History and the Virus-Host Interplay. Curr. Treat. Options Infect. Dis. 2018, 10, 197–216. [Google Scholar] [CrossRef]
- Clayton, B.A.; Wang, L.F.; Marsh, G.A. Henipaviruses: An Updated Review Focusing on the Pteropid Reservoir and Features of Transmission: Henipaviruses: The Pteropid Reservoir and Features of Transmission. Zoonoses Public Health 2013, 60, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Calderon, A.; Guzman, C.; Salazar-Bravo, J.; Figueiredo, L.T.; Mattar, S. Viral Zoonoses That Fly with Bats: A Review. MANTER J. Parasite Biodivers, 2016. [Google Scholar] [CrossRef]
- Wong, K.T.; Robertson, T.; Ong, B.B.; Chong, J.W.; Yaiw, K.C.; Wang, L.F.; Ansford, A.J.; Tannenberg, A. Human Hendra Virus Infection Causes Acute and Relapsing Encephalitis. Neuropathol. Appl. Neurobiol. 2009, 35, 296–305. [Google Scholar] [CrossRef]
- Ong, K.C.; Wong, K.T. Henipavirus Encephalitis: Recent Developments and Advances: Update on Henipavirus Encephalitis. Brain Pathol. 2015, 25, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.M. Nipah Virus, an Emerging Zoonotic Disease Causing Fatal Encephalitis. Clin. Med. 2022, 22, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.S.P.; Lim, T.C.C.; Wang, L. Nipah Virus Infection. J. Clin. Microbiol. 2018, 56, e01875-17. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Ashayeri Ahmadabad, R.; Sahab-Negah, S. Monkeypox Virus from Neurological Complications to Neuroinvasive Properties: Current Status and Future Perspectives. J. Neurol. 2023, 270, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Money, K.M.; Barnett, T.A.; Rapaka, S.; Osborn, R.; Kitani, T.; Fuguet, D.; Amjad, F.; Clark, J.R.; Chakravarty, D.; Copeland, M.J.; et al. Monkeypox-Associated Central Nervous System Disease: A Case Series and Review. Ann. Neurol. 2023, 93, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Pastula, D.M.; Copeland, M.J.; Hannan, M.C.; Rapaka, S.; Kitani, T.; Kleiner, E.; Showler, A.; Yuen, C.; Ferriman, E.M.; House, J.; et al. Two Cases of Monkeypox-Associated Encephalomyelitis—Colorado and the District of Columbia, July–August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Hayman, D.; Johnson, N.; McElhinney, L.; Fooks, A.R. Bats and Lyssaviruses. In Advances in Virus Research; Elsevier: New York, NY, USA, 2011; Volume 79, pp. 239–289. ISBN 978-0-12-387040-7. [Google Scholar]
- Francis, J.R.; McCall, B.J.; Hutchinson, P.; Powell, J.; Vaska, V.L.; Nourse, C. Australian Bat Lyssavirus: Implications for Public Health. Med. J. Aust. 2014, 201, 647–649. [Google Scholar] [CrossRef]
- Kohl, C.; Kurth, A. European Bats as Carriers of Viruses with Zoonotic Potential. Viruses 2014, 6, 3110–3128. [Google Scholar] [CrossRef]
- Poleshchuk, E.M.; Tagakova, D.N.; Sidorov, G.N.; Orlova, T.S.; Gordeiko, N.S.; Kaisarov, A.Z. Lethal Cases of Lyssavirus Encephalitis in Humans after Contact with Bats in the Russian Far East in 2019–2021. Probl. Virol. 2023, 68, 45–58. [Google Scholar] [CrossRef]
- Leonova, G.N.; Somova, L.M.; Belikov, S.I.; Kondratov, I.G.; Plekhova, N.G.; Krylova, N.V.; Pavlenko, E.V.; Tiunov, M.P.; Thachev, S.E. The Fatal Case of Lyssavirus Encephalitis in the Russian Far East. In Encephalitis; Tkachev, S., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0925-9. [Google Scholar]
- Kgaladi, J.; Wright, N.; Coertse, J.; Markotter, W.; Marston, D.; Fooks, A.R.; Freuling, C.M.; Müller, T.F.; Sabeta, C.T.; Nel, L.H. Diversity and Epidemiology of Mokola Virus. PLoS Neglected Trop. Dis. 2013, 7, e2511. [Google Scholar] [CrossRef]
- Le Govic, Y.; Demey, B.; Cassereau, J.; Bahn, Y.-S.; Papon, N. Pathogens Infecting the Central Nervous System. PLoS Pathog. 2022, 18, e1010234. [Google Scholar] [CrossRef]
- Johnson, N.; Aréchiga-Ceballos, N.; Aguilar-Setien, A. Vampire Bat Rabies: Ecology, Epidemiology and Control. Viruses 2014, 6, 1911–1928. [Google Scholar] [CrossRef]
- Rhabdoviridae. In Fenner’s Veterinary Virology; Elsevier: New York, NY, USA, 2017; pp. 357–372. ISBN 978-0-12-800946-8.
- Soler-Rangel, S.; Jiménez-Restrepo, N.; Nariño, D.; Rosselli, D. Rabies Encephalitis and Extra-Neural Manifestations in a Patient Bitten by a Domestic Cat. Rev. Inst. Med. Trop. São Paulo 2020, 62, e1. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Lynch, J.; Kajon, A. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Khanal, S.; Ghimire, P.; Dhamoon, A. The Repertoire of Adenovirus in Human Disease: The Innocuous to the Deadly. Biomedicines 2018, 6, 30. [Google Scholar] [CrossRef]
- Saint-Pierre Contreras, G.; Conei Valencia, D.; Lizama, L.; Vargas Zuñiga, D.; Avendaño Carvajal, L.F.; Ampuero Llanos, S. An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat? Viruses 2023, 15, 330. [Google Scholar] [CrossRef]
- Tamiya, M.; Komatsu, H.; Hirabayashi, M.; Imura, M.; Hoshino, H. Neonatal Meningoencephalitis Caused by Human Adenovirus Species F Infection. Pediatr. Int. 2019, 61, 99–101. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Feng, Z.; Feng, Q.; Li, K.; Gao, H.; Qian, S.; Xu, L.; Xie, Z. A Fatal Case of Viral Sepsis and Encephalitis in a Child Caused by Human Adenovirus Type 7 Infection. Virol. J. 2022, 19, 154. [Google Scholar] [CrossRef]
- Robinson, C.P.; Busl, K.M. Neurologic Manifestations of Severe Respiratory Viral Contagions. Crit. Care Explor. 2020, 2, e0107. [Google Scholar] [CrossRef]
- Ivanova, O.E.; Yurashko, O.V.; Eremeeva, T.P.; Baikova, O.Y.; Morozova, N.S.; Lukashev, A.N. Adenovirus Isolation Rates in Acute Flaccid Paralysis Patients. J. Med. Virol. 2012, 84, 75–80. [Google Scholar] [CrossRef]
- Bosch, A.; Guix, S.; Krishna, N.K.; Méndez, E.; Monroe, S.S.; Pantin-Jackwood, M.; Schultz-Cherry, S.; Family: Astroviridae Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release. International Committee on Taxonomy of Viruses: ICTV. Available online: https://ictv.global/report_9th/RNApos/Astroviridae (accessed on 7 May 2024).
- Koukou, G.; Niendorf, S.; Hornei, B.; Schlump, J.-U.; Jenke, A.C.; Jacobsen, S. Human Astrovirus Infection Associated with Encephalitis in an Immunocompetent Child: A Case Report. J. Med. Case Rep. 2019, 13, 341. [Google Scholar] [CrossRef]
- Naccache, S.N.; Peggs, K.S.; Mattes, F.M.; Phadke, R.; Garson, J.A.; Grant, P.; Samayoa, E.; Federman, S.; Miller, S.; Lunn, M.P.; et al. Diagnosis of Neuroinvasive Astrovirus Infection in an Immunocompromised Adult with Encephalitis by Unbiased Next-Generation Sequencing. Clin. Infect. Dis. 2015, 60, 919–923. [Google Scholar] [CrossRef]
- Brown, J.R.; Morfopoulou, S.; Hubb, J.; Emmett, W.A.; Ip, W.; Shah, D.; Brooks, T.; Paine, S.M.L.; Anderson, G.; Virasami, A.; et al. Astrovirus VA1/HMO-C: An Increasingly Recognized Neurotropic Pathogen in Immunocompromised Patients. Clin. Infect. Dis. 2015, 60, 881–888. [Google Scholar] [CrossRef]
- Bohmwald, K.; Andrade, C.A.; Gálvez, N.M.S.; Mora, V.P.; Muñoz, J.T.; Kalergis, A.M. The Causes and Long-Term Consequences of Viral Encephalitis. Front. Cell. Neurosci. 2021, 15, 755875. [Google Scholar] [CrossRef]
- Baig, A.M.; Sanders, E.C. Potential Neuroinvasive Pathways of SARS-CoV-2: Deciphering the Spectrum of Neurological Deficit Seen in Coronavirus Disease-2019 (COVID-19). J. Med. Virol. 2020, 92, 1845–1857. [Google Scholar] [CrossRef]
- Haddadi, K.; Asadian, L. Coronavirus Disease 2019: Latest Data on Neuroinvasive Potential. Iran. J. Med. Sci. 2020, 45, 325–332. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.-J.; Tu, D.-Z.; Li, X.-L.; Wei, B. Advances in Viral Encephalitis: Viral Transmission, Host Immunity, and Experimental Animal Models. Zool. Res. 2023, 44, 525–542. [Google Scholar] [CrossRef]
- Martínez-Mármol, R.; Giordano-Santini, R.; Kaulich, E.; Cho, A.-N.; Przybyla, M.; Riyadh, M.A.; Robinson, E.; Chew, K.Y.; Amor, R.; Meunier, F.A.; et al. SARS-CoV-2 Infection and Viral Fusogens Cause Neuronal and Glial Fusion That Compromises Neuronal Activity. Sci. Adv. 2023, 9, eadg2248. [Google Scholar] [CrossRef]
- Moretti, R.; Giuffrè, M.; Merli, N.; Caruso, P.; Di Bella, S.; Tiribelli, C.; Crocè, L.S. Hepatitis C Virus-Related Central and Peripheral Nervous System Disorders. Brain Sci. 2021, 11, 1569. [Google Scholar] [CrossRef]
- Mathew, S.; Faheem, M.; Ibrahim, S.M.; Iqbal, W.; Rauff, B.; Fatima, K.; Qadri, I. Hepatitis C Virus and Neurological Damage. WJH 2016, 8, 545. [Google Scholar] [CrossRef]
- Mankertz, A.; Chen, M.-H.; Goldberg, T.L.; Hübschen, J.M.; Pfaff, F.; Ulrich, R.G.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Matonaviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001817. [Google Scholar] [CrossRef]
- Woyessa, A.B.; Ali, M.S.; Korkpor, T.K.; Tuopileyi, R.; Kohar, H.T.; Dogba, J.; Baller, A.; Monday, J.; Abdullahi, S.; Nagbe, T.; et al. Rubella Transmission and the Risk of Congenital Rubella Syndrome in Liberia: A Need to Introduce Rubella-Containing Vaccine in the Routine Immunization Program. BMC Infect. Dis. 2019, 19, 813. [Google Scholar] [CrossRef]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef]
- Wright, W.F.; Pinto, C.N.; Palisoc, K.; Baghli, S. Viral (Aseptic) Meningitis: A Review. J. Neurol. Sci. 2019, 398, 176–183. [Google Scholar] [CrossRef]
- Carneiro, V.C.D.S.; Pereira, J.G.; De Paula, V.S. Family Herpesviridae and Neuroinfections: Current Status and Research in Progress. Mem. Inst. Oswaldo Cruz 2022, 117, e220200. [Google Scholar] [CrossRef]
- Meyding-Lamadé, U.; Strank, C. Herpesvirus Infections of the Central Nervous System in Immunocompromised Patients. Ther. Adv. Neurol. Disord. 2012, 5, 279. [Google Scholar] [CrossRef]
- McCauley, J.W.; Hongo, S.; Kaverin, N.V.; Kochs, G.; Lamb, R.A.; Matrosovich, M.N.; Perez, D.R.; Palese, P.; Presti, R.M.; Rimstad, E.; et al. Family: Orthomyxoviridae Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release. International Committee on Tax-onomy of Viruses: ICTV. Available online: https://ictv.global/report_9th/RNAneg/Orthomyxoviridae (accessed on 7 May 2024).
- Radzišauskienė, D.; Vitkauskaitė, M.; Žvinytė, K.; Mameniškienė, R. Neurological Complications of Pandemic A(H1N1)2009pdm, Postpandemic A(H1N1)v, and Seasonal Influenza A. Brain Behav. 2021, 11, e01916. [Google Scholar] [CrossRef]
- Davis, L.E.; Koster, F.; Cawthon, A. Neurologic Aspects of Influenza Viruses. In Handbook of Clinical Neurology; Elsevier: New York, NY, USA, 2014; Volume 123, pp. 619–645. ISBN 978-0-444-53488-0. [Google Scholar]
- Frankl, S.; Coffin, S.E.; Harrison, J.B.; Swami, S.K.; McGuire, J.L. Influenza-Associated Neurologic Complications in Hospitalized Children. J. Pediatr. 2021, 239, 24–31.e1. [Google Scholar] [CrossRef]
- Popescu, C.P.; Florescu, S.A.; Lupulescu, E.; Zaharia, M.; Tardei, G.; Lazar, M.; Ceausu, E.; Ruta, S.M. Neurologic Complications of Influenza B Virus Infection in Adults, Romania. Emerg. Infect. Dis. 2017, 23, 574–581. [Google Scholar] [CrossRef]
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV Virus Taxonomy Profile: Paramyxoviridae. J. Gen. Virol. 2019, 100, 1593–1594. [Google Scholar] [CrossRef]
- Wilson, M.R. Meningitis, Viral. In Encyclopedia of the Neurological Sciences; Elsevier: New York, NY, USA, 2014; pp. 1077–1081. ISBN 978-0-12-385158-1. [Google Scholar]
- Sumlivaya, O.N.; Vorobeva, N.N.; Zernina, M.G.; Kadebskaya, M.A. Clinical Case of Parainfluenza Meningitis. Sci. Rev. Med. Sci. 2022, 6, 65–69. [Google Scholar] [CrossRef]
- Farahmand, M.; Shatizadeh Malekshahi, S.; Jabbari, M.R.; Shayestehpour, M. The Landscape of Extrapulmonary Manifestations of Human Parainfluenza Viruses: A Systematic Narrative Review. Microbiol. Immunol. 2021, 65, 1–9. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- Vilmane, A.; Terentjeva, A.; Tamosiunas, P.L.; Suna, N.; Suna, I.; Petraityte-Burneikiene, R.; Murovska, M.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z. Human Parvoviruses May Affect the Development and Clinical Course of Meningitis and Meningoencephalitis. Brain Sci. 2020, 10, 339. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Ramalho, E.; Sousa, I.; Burlandy, F.; Costa, E.; Dias, A.; Serrano, R.; Oliveira, M.; Lopes, R.; Debur, M.; Burger, M.; et al. Identification and Phylogenetic Characterization of Human Enteroviruses Isolated from Cases of Aseptic Meningitis in Brazil, 2013–2017. Viruses 2019, 11, 690. [Google Scholar] [CrossRef]
- Chen, P.; Lin, X.; Liu, G.; Wang, S.; Song, L.; Tao, Z.; Xu, A. Analysis of Enterovirus Types in Patients with Symptoms of Aseptic Meningitis in 2014 in Shandong, China. Virology 2018, 516, 196–201. [Google Scholar] [CrossRef]
- Sousa, I.P.; Oliveira, M.D.L.A.; Burlandy, F.M.; Machado, R.S.; Oliveira, S.S.; Tavares, F.N.; Gomes-Neto, F.; Da Costa, E.V.; Da Silva, E.E. Molecular Characterization and Epidemiological Aspects of Non-Polio Enteroviruses Isolated from Acute Flaccid Paralysis in Brazil: A Historical Series (2005–2017). Emerg. Microbes Infect. 2020, 9, 2536–2546. [Google Scholar] [CrossRef]
- Bitnun, A.; Yeh, E.A. Acute Flaccid Paralysis and Enteroviral Infections. Curr. Infect. Dis. Rep. 2018, 20, 34. [Google Scholar] [CrossRef]
- Pandit, T.; Pandit, R.; Goyal, L.; Ajmera, K.; Dasari, N. Novel Presentation of Parechovirus Encephalitis in Children: Two Unique Cases. Cureus 2022, 14, e26456. [Google Scholar] [CrossRef]
- Suthar, P.P.; Hughes, K.; Kadam, G.; Jhaveri, M.; Gaddikeri, S. Human Parechovirus Meningoencephalitis. S. Afr. J. Radiol. 2023, 27, a2589. [Google Scholar] [CrossRef]
- Sarma, A.; Hanzlik, E.; Krishnasarma, R.; Pagano, L.; Pruthi, S. Human Parechovirus Meningoencephalitis: Neuroimaging in the Era of Polymerase Chain Reaction–Based Testing. AJNR Am. J. Neuroradiol. 2019, 40, 1418–1421. [Google Scholar] [CrossRef]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.-F. Problems of Classification in the Family Paramyxoviridae. Arch. Virol. 2018, 163, 1395–1404. [Google Scholar] [CrossRef]
- Saravanos, G.L.; King, C.L.; Deng, L.; Dinsmore, N.; Ramos, I.; Takashima, M.; Crawford, N.; Clark, J.E.; Dale, R.C.; Jones, C.A.; et al. Respiratory Syncytial Virus–Associated Neurologic Complications in Children: A Systematic Review and Aggregated Case Series. J. Pediatr. 2021, 239, 39–49. [Google Scholar] [CrossRef]
- Moens, U.; Calvignac-Spencer, S.; Lauber, C.; Ramqvist, T.; Feltkamp, M.C.W.; Daugherty, M.D.; Verschoor, E.J.; Ehlers, B.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Polyomaviridae. J. Gen. Virol. 2017, 98, 1159–1160. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-Associated Neurocognitive Disorder—Pathogenesis and Prospects for Treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Canet, G.; Dias, C.; Gabelle, A.; Simonin, Y.; Gosselet, F.; Marchi, N.; Makinson, A.; Tuaillon, E.; Van de Perre, P.; Givalois, L.; et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front. Cell. Neurosci. 2018, 12, 307. [Google Scholar] [CrossRef]
- Chemparthy, D.T.; Kannan, M.; Gordon, L.; Buch, S.; Sil, S. Alzheimer’s-Like Pathology at the Crossroads of HIV-Associated Neurological Disorders. Vaccines 2021, 9, 930. [Google Scholar] [CrossRef]
- Shieh, W.-J. Human Adenovirus Infections in Pediatric Population—An Update on Clinico–Pathologic Correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef]
- Vidal, L.R.; de Almeida, S.M.; Cavalli, B.M.; Dieckmann, T.G.; Raboni, S.M.; Salvador, G.L.O.; Pereira, L.A.; Rotta, I.; Nogueira, M.B. Human Adenovirus Meningoencephalitis: A 3-Years’ Overview. J. Neurovirol. 2019, 25, 589–596. [Google Scholar] [CrossRef]
- Reuter, G.; Pankovics, P.; Boros, Á. Nonsuppurative (Aseptic) Meningoencephalomyelitis Associated with Neurovirulent Astrovirus Infections in Humans and Animals. Clin. Microbiol. Rev. 2018, 31, e00040-18. [Google Scholar] [CrossRef]
- Tkachev, S. (Ed.) Encephalitis; InTech: Houston, TX, USA, 2013; ISBN 978-953-51-0925-9. [Google Scholar]
- Lizzi, J.; Hill, T.; Jakubowski, J. Varicella Zoster Virus Encephalitis. Clin. Pract. Cases Emerg. Med. 2019, 3, 380–382. [Google Scholar] [CrossRef]
- Alvarez, J.C.; Alvarez, J.; Ticono, J.; Medallo, P.; Miranda, H.; Ferrés, M.; Forero, J.; Álvarez, C. Varicella-Zoster Virus Meningitis and Encephalitis: An Understated Cause of Central Nervous System Infections. Cureus 2020, 12, e11583. [Google Scholar] [CrossRef]
- Herlin, L.K.; Hansen, K.S.; Bodilsen, J.; Larsen, L.; Brandt, C.; Andersen, C.Ø.; Hansen, B.R.; Lüttichau, H.R.; Helweg-Larsen, J.; Wiese, L.; et al. Varicella Zoster Virus Encephalitis in Denmark From 2015 to 2019—A Nationwide Prospective Cohort Study. Clin. Infect. Dis. 2021, 72, 1192–1199. [Google Scholar] [CrossRef]
- Dou, Y.; Li, Y. Influenza A H3N2-Associated Meningoencephalitis in an Older Adult with Viral RNA in Cerebrospinal Fluid: Case Report. Front. Neurol. 2022, 13, 874078. [Google Scholar] [CrossRef]
- Ferren, M.; Horvat, B.; Mathieu, C. Measles Encephalitis: Towards New Therapeutics. Viruses 2019, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Al-Qayoudhi, A.; Al-Kindi, H.; Meki, N.; Al-Maani, A. Acute Measles Encephalitis in an Immigrant Syrian Child: Case Report and Review of the Literature. Oman Med. J. 2016, 31, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Diwan, M.N.; Samad, S.; Mushtaq, R.; Aamir, A.; Allahuddin, Z.; Ullah, I.; Ullah Afridi, R.; Ambreen, A.; Khan, A.; Ehsan, N.; et al. Measles Induced Encephalitis: Recent Interventions to Overcome the Obstacles Encountered in the Management Amidst the COVID-19 Pandemic. Diseases 2022, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Saijo, M.; Fujita, K. Central nervous system infection caused by mumps virus. Nihon Rinsho 1997, 55, 870–875. [Google Scholar] [PubMed]
- Herndon, R.M. Ependymitis in Mumps Virus Meningitis: Electron Microscopical Studies of Cerebrospinal Fluid. Arch. Neurol. 1974, 30, 475. [Google Scholar] [CrossRef] [PubMed]
- Bitnun, A.; Ford-Jones, E.L.; Petric, M.; MacGregor, D.; Heurter, H.; Nelson, S.; Johnson, G.; Richardson, S. Acute Childhood Encephalitis and Mycoplasma pneumoniae. Clin. Infect. Dis. 2001, 32, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Sugai, K.; Tsukagoshi, H.; Nojima, I.; Fujiwara, K.; Kodera, A.; Kimura, N.; Tsuchimoto, K.; Sekimoto, K.; Kitada, K.; Takahashi, N.; et al. Severe Acute Encephalopathy Related to Human Parainfluenza Virus Type 2 Infection in an Infant: A Case Report. JMM Case Rep. 2015, 2, e000072. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Moon, J.; Sunwoo, J.-S.; Jun, J.-S.; Lee, S.-T.; Park, K.-I.; Jung, K.-H.; Jung, K.-Y.; Kim, M.; Lee, S.K.; et al. Respiratory Virus-Related Meningoencephalitis in Adults. Encephalitis 2020, 1, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, L.J.; Lief, F.S.; Verini, M.A.; Pienkowski, M.M.; ter Meulen, V.; Koprowski, H. Analysis of a Viral Agent Isolated from Multiple Sclerosis Brain Tissue: Characterization as a Parainfluenzavirus Type 1. J. Virol. 1974, 13, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Ter Meulen, V.; Iwasaki, Y.; Koprowski, H.; Käckell, Y.M.; Müller, D. Fusion of cultured multiple-sclerosis brain cells with indicator cells: Presence of nucleocapsids and virions and isolation of parainfluenza-type virus. Lancet 1972, 300, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Román, G.; Phillips, C.A.; Poser, C.M. Parainfluenza Virus Type 3: Isolation from CSF of a Patient with Guillain-Barré Syndrome. JAMA 1978, 240, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Arguedas, A.; Stutman, H.R.; Blanding, J.G. Parainfluenza Type 3 Meningitis: Report of Two Cases and Review of the Literature. Clin. Pediatr. 1990, 29, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Steinberg, E.; Warford, A. Parainfluenza virus type 3 meningitis in an 11-month-old infant. Pediatr. Infect. Dis. J. 1988, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Vreede, R.W.; Schellekens, H.; Zuijderwijk, M. Isolation of Parainfluenza Virus Type 3 from Cerebrospinal Fluid. J. Infect. Dis. 1992, 165, 1166. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Ranawaka, U.; Yamada, K.; Rajindrajith, S.; Miya, K.; Perera, H.K.K.; Matsumoto, T.; Dassanayake, M.; Mitui, M.T.; Mori, H.; et al. Human Bocavirus in Patients with Encephalitis, Sri Lanka, 2009–2010. Emerg. Infect. Dis. 2013, 19, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, Q.; Hao, Y.; Yu, T.; Zeng, S.; Wu, X.; Zhang, B.; Duan, Z. Identification of Human Bocaviruses in the Cerebrospinal Fluid of Children Hospitalized with Encephalitis in China. J. Clin. Virol. 2013, 57, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Ergul, A.B.; Altug, U.; Aydin, K.; Guven, A.S.; Altuner Torun, Y. Acute Necrotizing Encephalopathy Causing Human Bocavirus. Neuroradiol. J. 2017, 30, 164–167. [Google Scholar] [CrossRef]
- Mitui, M.T.; Shahnawaz Bin Tabib, S.M.; Matsumoto, T.; Khanam, W.; Ahmed, S.; Mori, D.; Akhter, N.; Yamada, K.; Kabir, L.; Nishizono, A.; et al. Detection of Human Bocavirus in the Cerebrospinal Fluid of Children with Encephalitis. Clin. Infect. Dis. 2012, 54, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Douvoyiannis, M.; Litman, N.; Goldman, D.L. Neurologic Manifestations Associated with Parvovirus B19 Infection. Clin. Infect. Dis. 2009, 48, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Barah, F.; Whiteside, S.; Batista, S.; Morris, J. Neurological Aspects of Human Parvovirus B19 Infection: A Systematic Review. Rev. Med. Virol. 2014, 24, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Acute Encephalitis and Encephalopathy Associated with Human Parvovirus B19 Infection in Children. World J. Clin. Pediatr. 2015, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L. Human Parvovirus 4 as Potential Cause of Encephalitis in Children, India. Emerg. Infect. Dis. 2011, 17, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Hanaoka, N.; Katano, H.; Konagaya, M.; Tanaka-Taya, K.; Shimizu, H.; Mukai, T.; Fujimoto, T. A Fatal Case of Acute Encephalopathy in a Child Due to Coxsackievirus A2 Infection: A Case Report. BMC Infect. Dis. 2021, 21, 1167. [Google Scholar] [CrossRef] [PubMed]
- Kon, Y.; Takahashi, S.; Takahata, N.; Onodera, I.; Sato, M. Case of chronic encephalitis with Coxsackie A 5 virus isolated from the cerebrospinal fluid. Rinsho Shinkeigaku 1974, 14, 752–759. [Google Scholar] [PubMed]
- Grist, N.R. Type A7 Coxsackie (Type 4 Poliomyelitis) Virus Infection in Scotland. J. Hyg. 1962, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Seitsonen, J.J.T.; Shakeel, S.; Susi, P.; Pandurangan, A.P.; Sinkovits, R.S.; Hyvönen, H.; Laurinmäki, P.; Ylä-Pelto, J.; Topf, M.; Hyypiä, T.; et al. Structural Analysis of Coxsackievirus A7 Reveals Conformational Changes Associated with Uncoating. J. Virol. 2012, 86, 7207–7215. [Google Scholar] [CrossRef] [PubMed]
- Eyckmans, T.; Wollants, E.; Janssens, A.; Schoemans, H.; Lagrou, K.; Wauters, J.; Maertens, J. Coxsackievirus A16 Encephalitis during Obinutuzumab Therapy, Belgium, 2013. Emerg. Infect. Dis. 2014, 20, 913–915. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Honarmand, S.; Glaser, C.; Yagi, S.; Schnurr, D.; Oberste, M.S.; Anderson, L.; Pallansch, M.A.; Khetsuriani, N. Enterovirus-Associated Encephalitis in the California Encephalitis Project, 1998–2005. J. Infect. Dis. 2008, 198, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Perez-Velez, C.M.; Anderson, M.S.; Robinson, C.C.; McFarland, E.J.; Nix, W.A.; Pallansch, M.A.; Oberste, M.S.; Glode, M.P. Outbreak of Neurologic Enterovirus Type 71 Disease: A Diagnostic Challenge. Clin. Infect. Dis. 2007, 45, 950–957. [Google Scholar] [CrossRef]
- Huang, C.-C.; Liu, C.-C.; Chang, Y.-C.; Chen, C.-Y.; Wang, S.-T.; Yeh, T.-F. Neurologic Complications in Children with Enterovirus 71 Infection. N. Engl. J. Med. 1999, 341, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Majer, A.; McGreevy, A.; Booth, T.F. Molecular Pathogenicity of Enteroviruses Causing Neurological Disease. Front. Microbiol. 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Chonmaitree, T.; Menegus, M.A.; Schervish-Swierkosz, E.M.; Schwalenstocker, E. Enterovirus 71 Infection: Report of an Outbreak with Two Cases of Paralysis and a Review of the Literature. Pediatrics 1981, 67, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Twu, S.-J.; Ho, M.-S.; Chang, L.-Y.; Lee, C.-Y. Enterovirus 71 Outbreaks, Taiwan: Occurrence and Recognition. Emerg. Infect. Dis. 2003, 9, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Moreau, B.; Bastedo, C.; Michel, R.P.; Ghali, P. Hepatitis and Encephalitis Due to Coxsackie Virus A9 in an Adult. Case Rep. Gastroenterol. 2011, 5, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Kamei, S.; Hersch, S.M.; Kurata, T.; Takei, Y. Coxsackie B Antigen in the Central Nervous System of a Patient with Fatal Acute Encephalitis: Immunohistochemical Studies of Formalin-Fixed Paraffin-Embedded Tissue. Acta Neuropathol. 1990, 80, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Litwicki, A.; Aliani, S.; Gärtner, B. Coxsackie Virus B 4 Encephalitis in a 7 Year Old Boy. Klin. Padiatr. 2004, 216, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Klapper, P.E.; Bailey, A.S.; Longson, M.; Barton, B.W.; Davies-Jones, G.A.B. Meningo-Encephalitis Caused by Coxsackievirus Group B Type 2. Diagnosis Confirmed by Measuring Intrathecal Antibody. J. Infect. 1984, 8, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, F.; Calleri, G.; Spezia, C.; Lipani, F.; Balbiano, R.; De Agostini, M.; Milia, M.G.; Caramello, P. Echovirus-4 Meningitis Outbreak Imported from India: Table 1. J. Travel Med. 2010, 17, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Handsher, R.; Shulman, L.M.; Abramovitz, B.; Silberstein, I.; Neuman, M.; Tepperberg-Oikawa, M.; Fisher, T.; Mendelson, E. A New Variant of Echovirus 4 Associated with a Large Outbreak of Aseptic Meningitis. J. Clin. Virol. 1999, 13, 29–36. [Google Scholar] [CrossRef]
- Smura, T.; Blomqvist, S.; Kolehmainen, P.; Schuffenecker, I.; Lina, B.; Böttcher, S.; Diedrich, S.; Löve, A.; Brytting, M.; Hauzenberger, E.; et al. Aseptic Meningitis Outbreak Associated with Echovirus 4 in Northern Europe in 2013–2014. J. Clin. Virol. 2020, 129, 104535. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, H. Echoviruses (Picornaviridae). In Encyclopedia of Virology; Elsevier: New York, NY, USA, 1999; pp. 411–417. ISBN 978-0-12-227030-7. [Google Scholar]
- Foncin, J.F.; Maurin, J.; Gaches, J.; Stilhart, B.; Le Beau, J. Curable ECHO 5 virus encephalitis. Clinical, electroencephalographic, virologic and ultrastructural study. Ann. Med. Interne 1977, 128, 335–343. [Google Scholar]
- Kim, H.-J.; Kang, B.; Hwang, S.; Hong, J.; Kim, K.; Cheon, D.-S. Epidemics of Viral Meningitis Caused by Echovirus 6 and 30 in Korea in 2008. Virol. J. 2012, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.J.S.; Smith, D.W.; Phillips, P.A.; Rouse, I.L. Viral Meningitis Due to Echovirus Types 6 and 9: Epidemiological Data from Western Australia. Epidemiol. Infect. 1996, 117, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Fratty, I.S.; Kriger, O.; Weiss, L.; Vasserman, R.; Erster, O.; Mendelson, E.; Sofer, D.; Weil, M. Increased Detection of Echovirus 6-Associated Meningitis in Patients Hospitalized during the COVID-19 Pandemic, Israel 2021–2022. J. Clin. Virol. 2023, 162, 105425. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, W.R. Echovirus Type 7 Meningitis in Young Children. Arch. Pediatr. Adolesc. Med. 1981, 135, 1009. [Google Scholar] [CrossRef]
- Lum, L.C.S.; Chua, K.B.; McMinn, P.C.; Goh, A.Y.T.; Muridan, R.; Sarji, S.A.; Hooi, P.S.; Chua, B.H.; Lam, S.K. Echovirus 7 Associated Encephalomyelitis. J. Clin. Virol. 2002, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, D.; Chen, L.; Zhang, Y.; Song, Y.; Zhu, S.; Ji, T.; Zhou, W.; Gan, F.; Wang, X.; et al. Multiple Genotypes of Echovirus 11 Circulated in Mainland China between 1994 and 2017. Sci. Rep. 2019, 9, 10583. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Yang, S.-L.; Yang, H.; Lin, T.-Y.; Hsieh, Y.-C.; Huang, K.-Y.A.; Kuo, C.-Y.; Chiu, C.-H.; Huang, Y.-C.; Chu, S.-M.; et al. Clinical Characteristics of Echovirus 11 and Coxsackievirus B5 Infections in Taiwanese Children Requiring Hospitalization. J. Microbiol. Immunol. Infect. 2021, 54, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Somekh, E.; Cesar, K.; Handsher, R.; Hanukoglu, A.; Dalal, I.; Ballin, A.; Shohat, T. An Outbreak of Echovirus 13 Meningitis in Central Israel. Epidemiol. Infect. 2003, 130, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.; Mas, P.; Goyenechea, A.; Palomera, R.; Morier, L.; Capó, V.; Quintana, I.; Santin, M. First Epidemic of Echovirus 16 Meningitis in Cuba. Emerg. Infect. Dis. 2001, 7, 887–889. [Google Scholar] [CrossRef]
- Singh, D.V.; Kumar, A.; Kumar, P.; Baluni, M.; Ghildiyal, S.; Kumar, R.; Misra, U.K.; Dhole, T.N. An Outbreak of Encephalitis Associated with Echovirus 19 in Uttar Pradesh, India, in 2011. Arch. Virol. 2016, 161, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Kelen, A.E.; Lesiak, J.M.; Labzoffsky, N.A. An outbreak of aseptic meningitis due to ECHO 25 virus. Can. Med. Assoc. J. 1964, 90, 1349–1351. [Google Scholar] [PubMed]
- Peters, A.C.B.; Vielvoye, G.J.; Versteeg, J.; Bots, G.T.A.M.; Lindeman, J. ECHO 25 Focal Encephalitis and Subacute Hemichorea. Neurology 1979, 29, 676. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, P.; Perera, D.; Ooi, M.H.; Last, A.; Kumar, R.; Desai, A.; Begum, A.; Ravi, V.; Shankar, M.V.; Tio, P.H.; et al. Enterovirus 75 Encephalitis in Children, Southern India. Emerg. Infect. Dis. 2010, 16, 1780–1782. [Google Scholar] [CrossRef] [PubMed]
- Avellon, A.; Rubio, G.; Palacios, G.; Casas, I.; Rabella, N.; Reina, G.; Perez, C.; Lipkin, W.I.; Trallero, G. Enterovirus 75 and Aseptic Meningitis, Spain, 2005. Emerg. Infect. Dis. 2006, 12, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, K.; Abzug, M.J. Vaccine-Associated Poliovirus Meningitis in Children with Ventriculoperitoneal Shunts. J. Pediatr. 1990, 117, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Zevakov, V.F.; Semak, S.I.; Titarenko, V.I.; Andreĭchenko, N.V.; Gedzul, O.V. Role of poliomyelitis viruses in the etiology of serous meningitis in Odessa 1979–1983. Vopr. Virusol. 1987, 32, 459–464. [Google Scholar]
- Saslaw, S. Aseptic Meningitis and Nonparalytic Poliomyelitis. Arch. Intern. Med. 1961, 107, 568. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-S.; Lee, H.-C.; Lee, K.-M.; Gong, Y.-N.; Shih, S.-R. Enterovirus and Encephalitis. Front. Microbiol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Hixon, A.M.; Frost, J.; Rudy, M.J.; Messacar, K.; Clarke, P.; Tyler, K.L. Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review. Viruses 2019, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.D.; Barnes, A.; McCarthy, J.E.; Schwartzman, J.D.; Oberste, M.S.; Rhodes, C.H.; Modlin, J.F.; Wright, P.F. A Fatal Central Nervous System Enterovirus 68 Infection. Arch. Pathol. Lab. Med. 2011, 135, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Giombini, E.; Rueca, M.; Barberi, W.; Iori, A.P.; Castilletti, C.; Scognamiglio, P.; Vairo, F.; Ippolito, G.; Capobianchi, M.R.; Valli, M.B. Enterovirus D68–Associated Acute Flaccid Myelitis in Immunocompromised Woman, Italy. Emerg. Infect. Dis. 2017, 23, 1690–1693. [Google Scholar] [CrossRef]
- González-Sanz, R.; Taravillo, I.; Reina, J.; Navascués, A.; Moreno-Docón, A.; Aranzamendi, M.; Romero, M.P.; del Cuerpo, M.; Pérez-González, C.; Pérez-Castro, S.; et al. Enterovirus D68-Associated Respiratory and Neurological Illness in Spain, 2014–2018. Emerg. Microbes Infect. 2019, 8, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, E.P.; Mondkar, V.P.; Wadia, N.H.; Irani, P.F.; Katrak, S.M. Neurological complications of a new conjunctivitis. Lancet 1972, 300, 970–971. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kang, K.; Park, J.-M.; Kwon, O.; Kim, B.-K. Encephalitis Associated with Acute Hepatitis A. J. Epilepsy Res. 2011, 1, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Abedi, G.R.; Watson, J.T.; Pham, H.; Nix, W.A.; Oberste, M.S.; Gerber, S.I. Enterovirus and Human Parechovirus Surveillance—United States, 2009–2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Shirota, G.; Morozumi, M.; Ubukata, K.; Shiro, H. Infantile Meningitis Caused by Respiratory Syncytial Virus. J. Jpn. Assoc. Infect. Dis. 2011, 85, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, H.; Zeng, J.; Liu, J.; Lu, C.; Guan, X.; Qian, S.; Xie, Z. A Fatal Case Associated with Respiratory Syncytial Virus Infection in a Young Child. BMC Infect. Dis. 2018, 18, 217. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hua, Y.; Qian, J.; Sun, M.; Kang, Y.-J. The Presence of Human Respiratory Syncytial Virus in the Cerebrospinal Fluid of a Child with Anti-N-Methyl-D-Aspartate Receptor Encephalitis of Unknown Trigger. Virol. J. 2023, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Morichi, S.; Kashiwagi, Y.; Suzuki, S.; Nishimata, S.; Yamanaka, G.; Sawada, A.; Kawashima, H. A Case of Respiratory Syncytial Virus-Associated Encephalopathy in Which the Virus Was Detected in Cerebrospinal Fluid and Intratracheal Aspiration despite Negative Rapid Test Results. J. Infect. Chemother. 2020, 26, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Schildgen, O.; Glatzel, T.; Geikowski, T.; Scheibner, B.; Simon, A.; Bindl, L.; Born, M.; Viazov, S.; Wilkesmann, A.; Knöpfle, G.; et al. Human Metapneumovirus RNA in Encephalitis Patient. Emerg. Infect. Dis. 2005, 11, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Fok, A.; Mateevici, C.; Lin, B.; Chandra, R.V.; Chong, V.H.T. Encephalitis-Associated Human Metapneumovirus Pneumonia in Adult, Australia. Emerg. Infect. Dis. 2015, 21, 2074–2076. [Google Scholar] [CrossRef]
- Sánchez Fernández, I. Human Metapneumovirus in the Cerebrospinal Fluid of a Patient with Acute Encephalitis. Arch. Neurol. 2012, 69, 649. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Coeckelbergh, E.; De Cauwer, H.; Viaene, M.; Van der Mieren, G. An Adult Case of Metapneumovirus-Induced Acute Encephalitis. Acta Neurol. Belg. 2019, 119, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.C.; Singh, K.K.; Milder, E.; Spector, S.A.; Sawyer, M.H.; Gavali, S.; Glaser, C. Human Metapneumovirus Associated with Central Nervous System Infection in Children. Pediatr. Infect. Dis. J. 2009, 28, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.P.; Wuthrich, C.; Dang, X.; Nauen, D.; Karimi, R.; Viscidi, R.; Bord, E.; Batson, S.; Troncoso, J.; Koralnik, I.J. A Fatal Case of JC Virus Meningitis Presenting with Hydrocephalus in a Human Immunodeficiency Virus-Seronegative Patient: JCV Meningitis. Ann. Neurol. 2014, 76, 140–147. [Google Scholar] [CrossRef]
- Miskin, D.P.; Koralnik, I.J. Novel Syndromes Associated with JC Virus Infection of Neurons and Meningeal Cells: No Longer a Gray Area. Curr. Opin. Neurol. 2015, 28, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R.; Klein, J.P.; Lyons, J.L.; Milner, D.A.; Phillips, R.E.; Schutten, M.; Folkerth, R.D.; Ciarlini, P.; Henrich, T.J.; Johnson, J.A. HIV-2 Encephalitis: Case Report and Literature Review. AIDS Patient Care STDs 2012, 26, 383–387. [Google Scholar] [CrossRef] [PubMed]
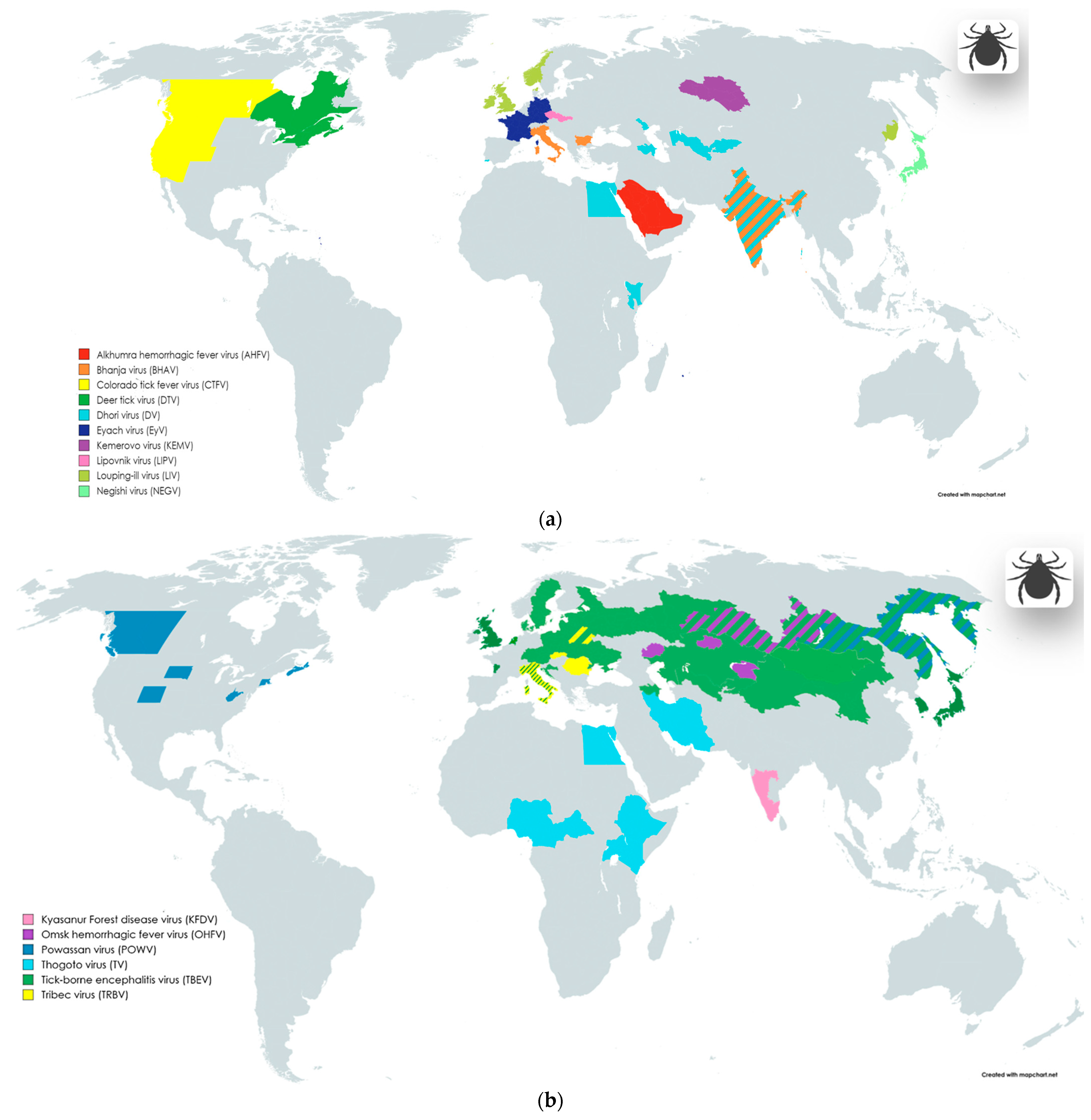






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurmukanova, V.; Matsvay, A.; Gordukova, M.; Shipulin, G. Square the Circle: Diversity of Viral Pathogens Causing Neuro-Infectious Diseases. Viruses 2024, 16, 787. https://doi.org/10.3390/v16050787
Nurmukanova V, Matsvay A, Gordukova M, Shipulin G. Square the Circle: Diversity of Viral Pathogens Causing Neuro-Infectious Diseases. Viruses. 2024; 16(5):787. https://doi.org/10.3390/v16050787
Chicago/Turabian StyleNurmukanova, Varvara, Alina Matsvay, Maria Gordukova, and German Shipulin. 2024. "Square the Circle: Diversity of Viral Pathogens Causing Neuro-Infectious Diseases" Viruses 16, no. 5: 787. https://doi.org/10.3390/v16050787
APA StyleNurmukanova, V., Matsvay, A., Gordukova, M., & Shipulin, G. (2024). Square the Circle: Diversity of Viral Pathogens Causing Neuro-Infectious Diseases. Viruses, 16(5), 787. https://doi.org/10.3390/v16050787





