West Nile Virus Subgenomic RNAs Modulate Gene Expression in a Neuronal Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of ΔsfRNA Replicon Plasmids
2.2. Cell Cultures
2.3. Translation Efficiency of WT and ΔsfRNA Replicons
2.4. RNA Extraction and qPCR for the Estimation of Replication Efficiency
2.5. Generation of the WT and ΔsfRNA Stable Cell Lines
2.6. Affinity Enrichment Protocol
2.7. RNA Sequencing of sfRNA
2.8. Estimation of the Replicon and sfRNA Abundance in Stable Cell Lines
2.9. RNA-Seq and Differential Expression Analysis
2.10. Pathway Enrichment Analysis of the Differentially Expressed Genes
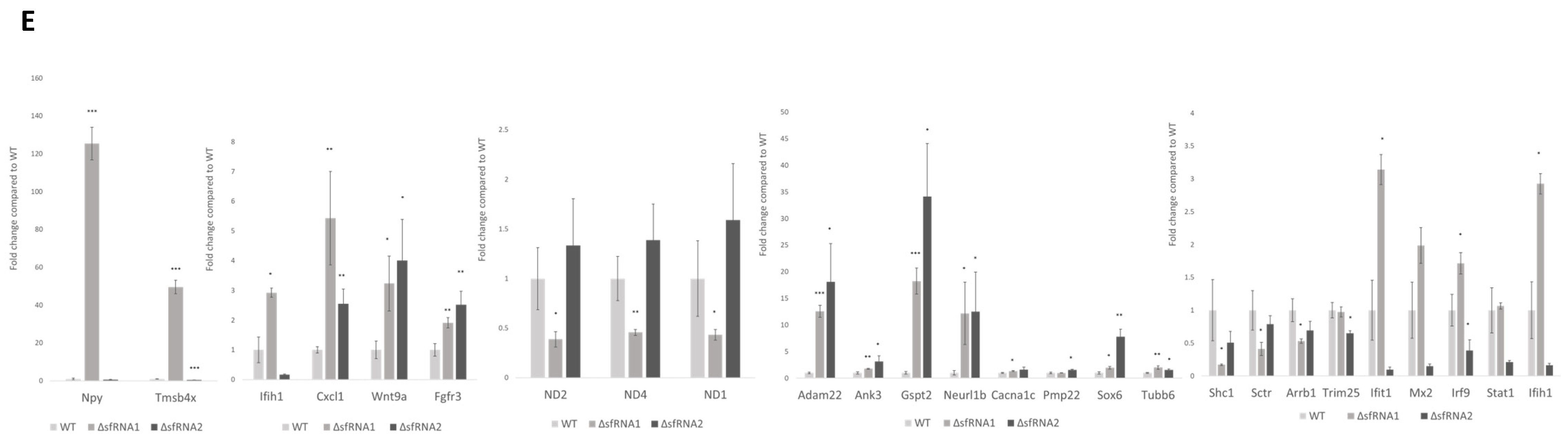
2.11. Validation of the RNA-Seq Results
2.12. Measurement of the Interferon Signaling Induction
2.13. Calculation of Viral Titer Produced in the WT and ΔsfRNA Replicon Cell Lines
3. Results
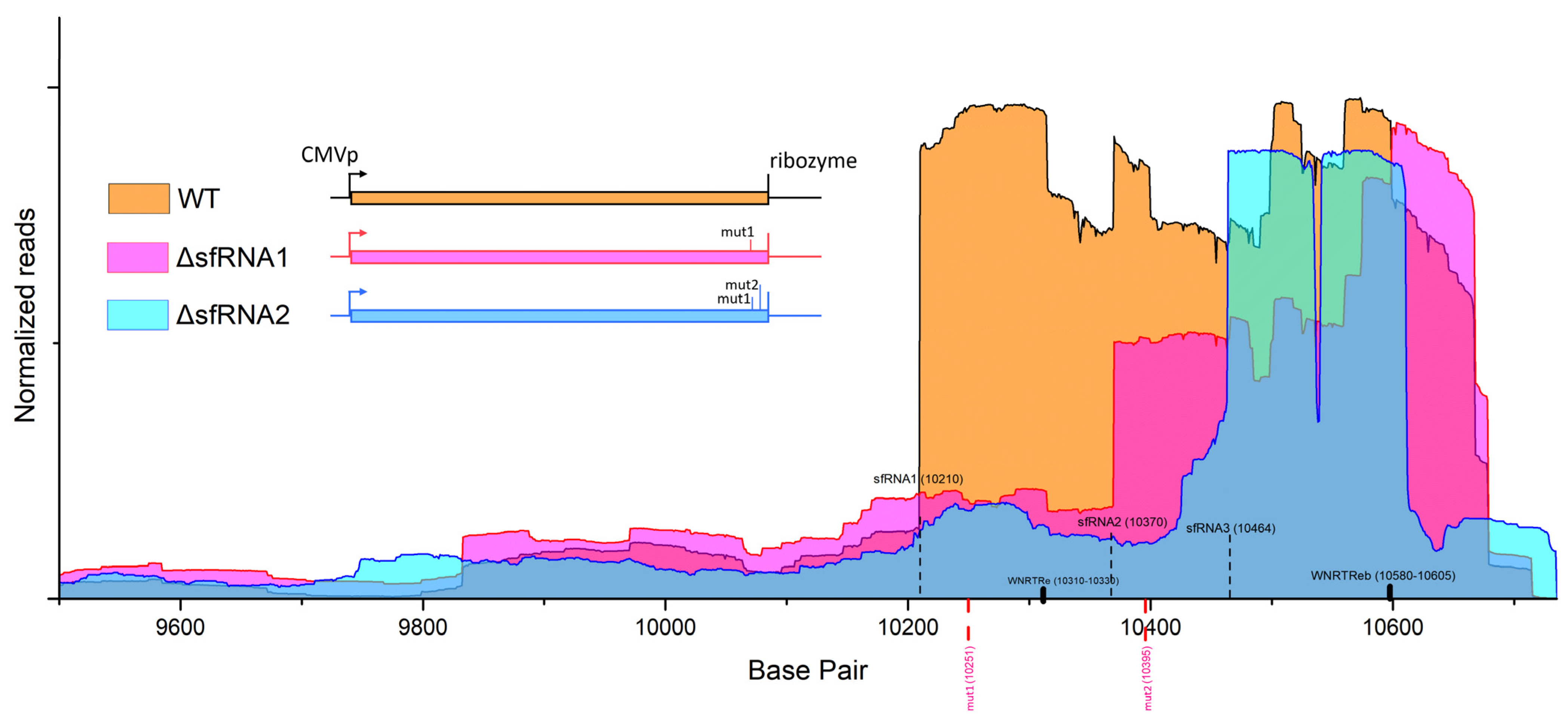
3.1. Locating sfRNA Starting Points on WNV Replicon Genome
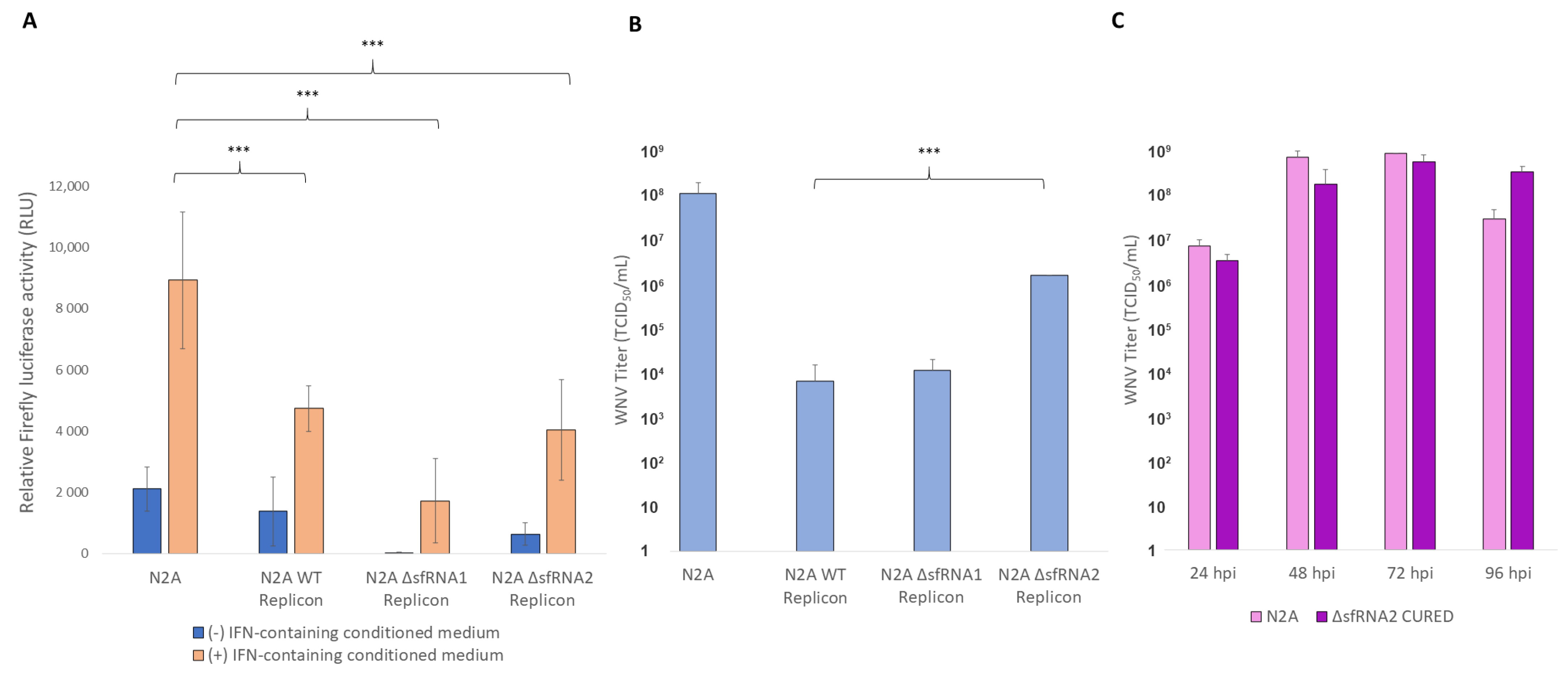
3.2. Translation and Replication Efficiency of the WT and ΔsfRNA Replicons
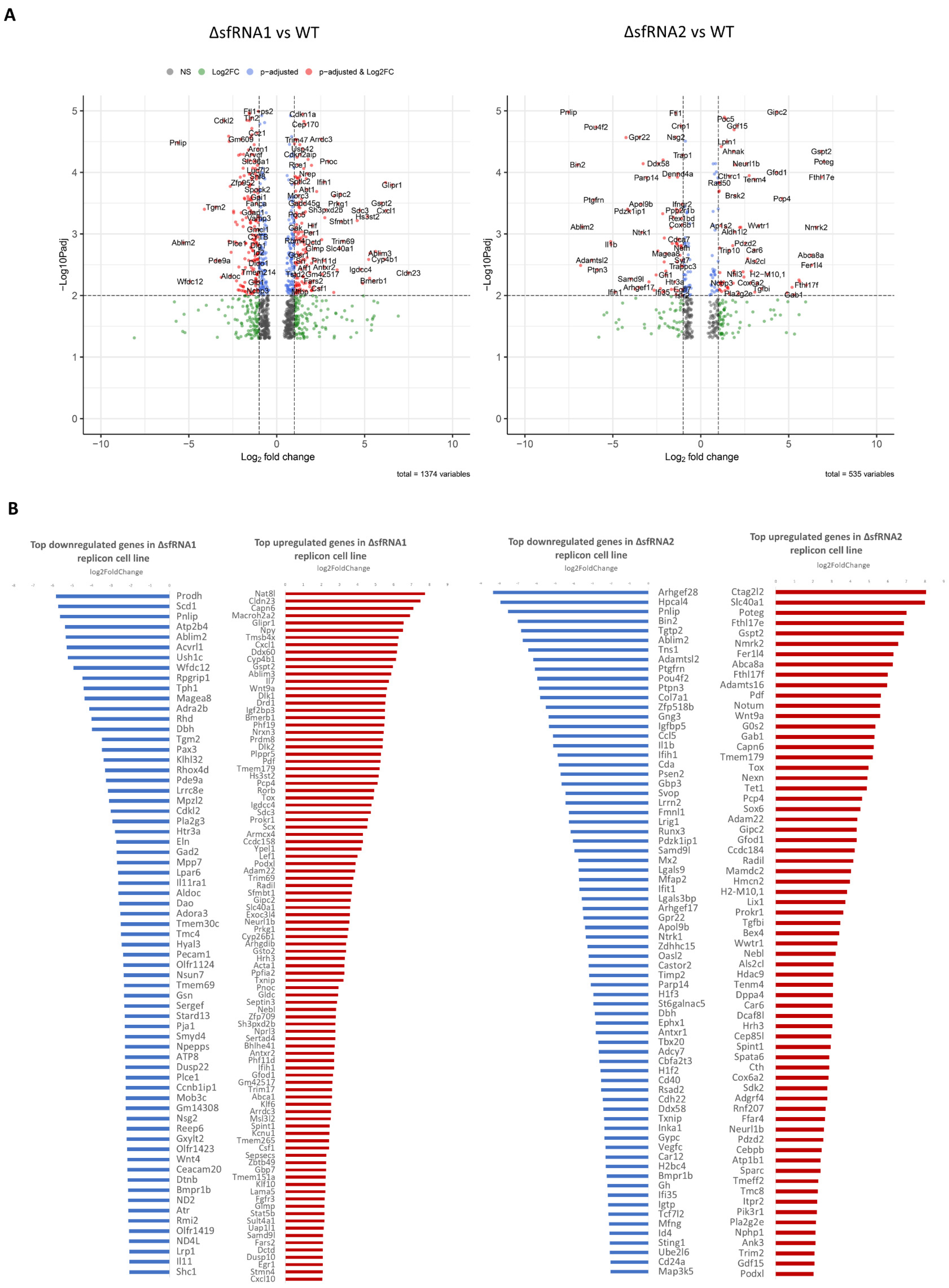
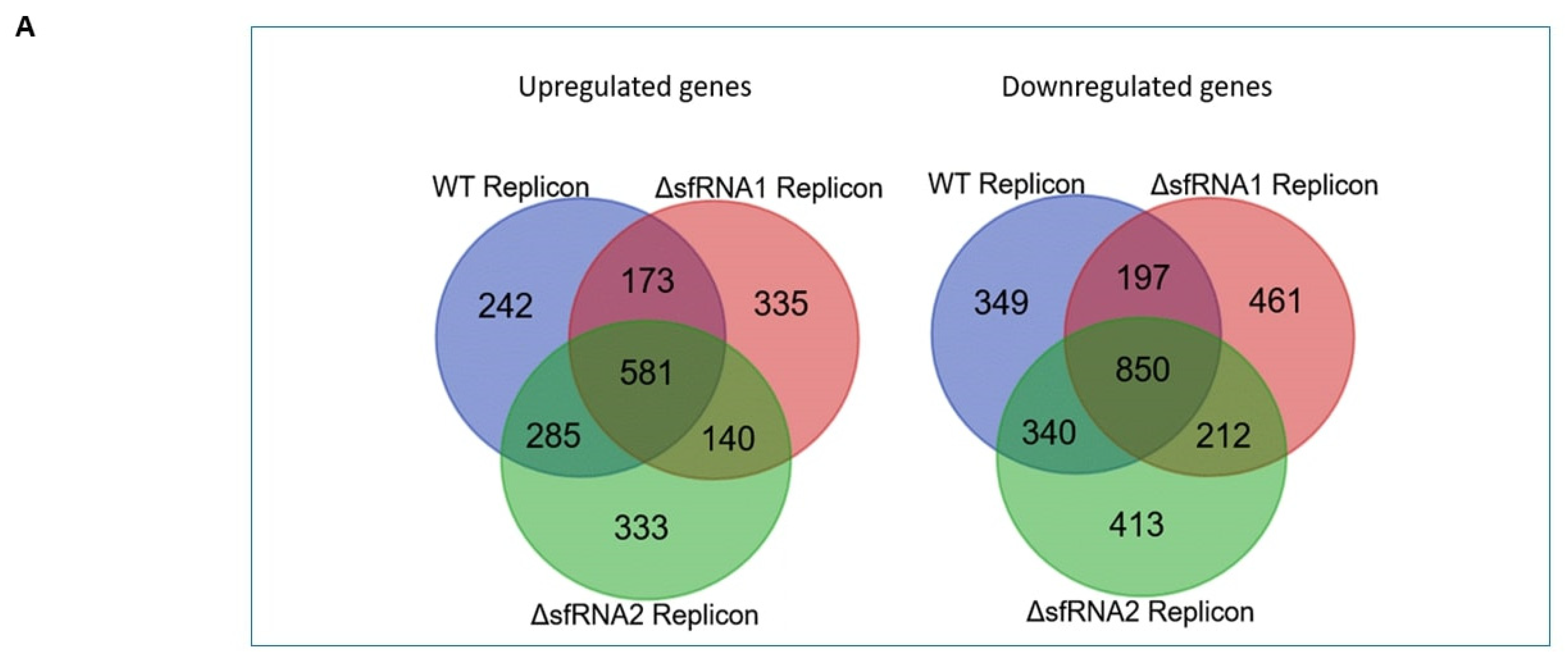
3.3. Differential Expression Analysis among the WT and ΔsfRNA Replicon Cell Lines
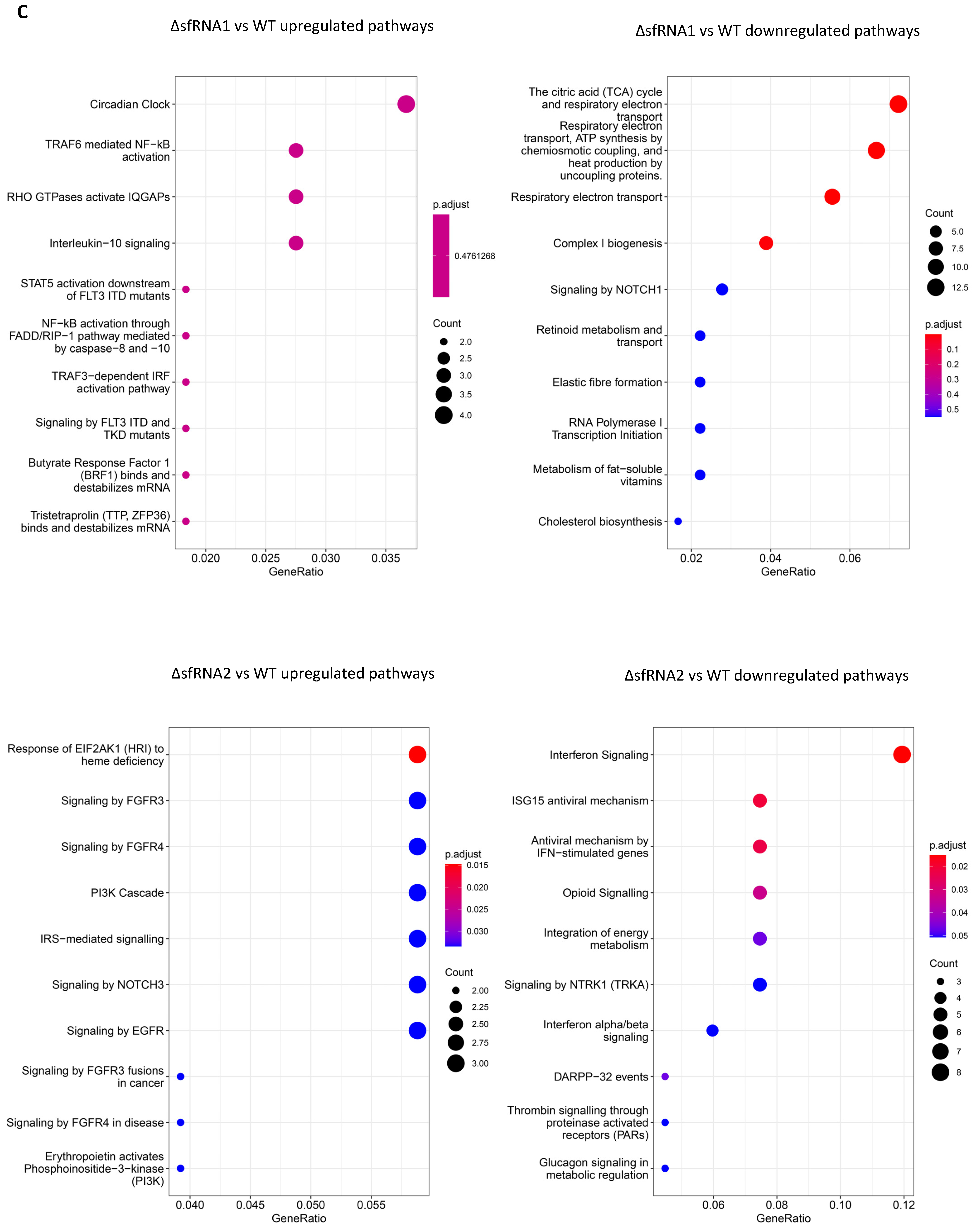
3.4. Pathway Enrichment Analysis for Differentially Regulated Genes among the WT and ΔsfRNA Replicon Cell Lines
3.5. Differential Expression Analysis among Replicon and Control Neuro2A Cell Lines
3.6. Pathway Enrichment Analysis for the Differentially Regulated Genes among the Replicon and Control Neuro2A Cell Lines
3.7. The Modulatory Role of sfRNA in the Innate immunity Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Londono-Renteria, B.; Colpitts, T.M. A Brief Review of West Nile Virus Biology. In West Nile Virus: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1435, pp. 1–13. [Google Scholar] [CrossRef]
- Bampali, M.; Konstantinidis, K.; Kellis, E.E.; Pouni, T.; Mitroulis, I.; Kottaridi, C.; Mathioudakis, A.G.; Beloukas, A.; Karakasiliotis, I. West Nile Disease Symptoms and Comorbidities: A Systematic Review and Analysis of Cases. Trop. Med. Infect. Dis. 2022, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- Ouhoumanne, N.; Lowe, A.-M.; Fortin, A.; Kairy, D.; Vibien, A.; K-Lensch, J.; Tannenbaum, T.-N.; Milord, F. Morbidity, Mortality and Long-Term Sequelae of West Nile Virus Disease in Québec. Epidemiol. Infect. 2018, 146, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Gwon, Y.-D.; Strand, M.; Lindqvist, R.; Nilsson, E.; Saleeb, M.; Elofsson, M.; Överby, A.K.; Evander, M. Antiviral Activity of Benzavir-2 against Emerging Flaviviruses. Viruses 2020, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-Year Historical Review of West Nile Virus since Its Initial Emergence in North America: Has West Nile Virus Become a Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.C.; Soto-Acosta, R.; Bradrick, S.S.; Garcia-Blanco, M.A.; Ooi, E.E. The 5’ and 3’ Untranslated Regions of the Flaviviral Genome. Viruses 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Qin, C.-F. Structure and Function of Cis-Acting RNA Elements of Flavivirus. Rev. Med. Virol. 2020, 30, e2092. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Liao, K.-C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.-C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Ramos-Lorente, S.E.; Berzal-Herranz, B.; Romero-López, C.; Berzal-Herranz, A. Recruitment of the 40S Ribosomal Subunit by the West Nile Virus 3’ UTR Promotes the Cross-Talk between the Viral Genomic Ends for Translation Regulation. Virus Res. 2024, 343, 199340. [Google Scholar] [CrossRef]
- Villordo, S.M.; Carballeda, J.M.; Filomatori, C.V.; Gamarnik, A.V. RNA Structure Duplications and Flavivirus Host Adaptation. Trends Microbiol. 2016, 24, 270–283. [Google Scholar] [CrossRef]
- Villordo, S.M.; Filomatori, C.V.; Sánchez-Vargas, I.; Blair, C.D.; Gamarnik, A.V. Dengue Virus RNA Structure Specialization Facilitates Host Adaptation. PLoS Pathog. 2015, 11, e1004604. [Google Scholar] [CrossRef] [PubMed]
- Romero-López, C.; Roda-Herreros, M.; Berzal-Herranz, B.; Ramos-Lorente, S.E.; Berzal-Herranz, A. Inter- and Intramolecular RNA-RNA Interactions Modulate the Regulation of Translation Mediated by the 3’ UTR in West Nile Virus. Int. J. Mol. Sci. 2023, 24, 5337. [Google Scholar] [CrossRef] [PubMed]
- MacFadden, A.; O’Donoghue, Z.; Silva, P.A.G.C.; Chapman, E.G.; Olsthoorn, R.C.; Sterken, M.G.; Pijlman, G.P.; Bredenbeek, P.J.; Kieft, J.S. Mechanism and Structural Diversity of Exoribonuclease-Resistant RNA Structures in Flaviviral RNAs. Nat. Commun. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Slonchak, A.; Khromykh, A.A. Subgenomic Flaviviral RNAs: What Do We Know after the First Decade of Research. Antivir. Res. 2018, 159, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, G.P.; Funk, A.; Kondratieva, N.; Leung, J.; Torres, S.; van der Aa, L.; Liu, W.J.; Palmenberg, A.C.; Shi, P.-Y.; Hall, R.A.; et al. A Highly Structured, Nuclease-Resistant, Noncoding RNA Produced by Flaviviruses Is Required for Pathogenicity. Cell Host Microbe 2008, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.; Truong, K.; Nagasaki, T.; Torres, S.; Floden, N.; Balmori Melian, E.; Edmonds, J.; Dong, H.; Shi, P.-Y.; Khromykh, A.A. RNA Structures Required for Production of Subgenomic Flavivirus RNA. J. Virol. 2010, 84, 11407–11417. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.-Y.; Hsu, T.-W.; Chen, Y.-L.; Liu, S.-F.; Tsai, Y.-J.; Lin, Y.-T.; Chen, Y.-S.; Fan, Y.-H. Japanese Encephalitis Virus Non-Coding RNA Inhibits Activation of Interferon by Blocking Nuclear Translocation of Interferon Regulatory Factor 3. Vet. Microbiol. 2013, 166, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bidet, K.; Dadlani, D.; Garcia-Blanco, M.A. G3BP1, G3BP2 and CAPRIN1 Are Required for Translation of Interferon Stimulated mRNAs and Are Targeted by a Dengue Virus Non-Coding RNA. PLoS Pathog. 2014, 10, e1004242. [Google Scholar] [CrossRef] [PubMed]
- Manokaran, G.; Finol, E.; Wang, C.; Gunaratne, J.; Bahl, J.; Ong, E.Z.; Tan, H.C.; Sessions, O.M.; Ward, A.M.; Gubler, D.J.; et al. Dengue Subgenomic RNA Binds TRIM25 to Inhibit Interferon Expression for Epidemiological Fitness. Science 2015, 350, 217–221. [Google Scholar] [CrossRef]
- Olson, K.E.; Blair, C.D. Arbovirus-Mosquito Interactions: RNAi Pathway. Curr. Opin. Virol. 2015, 15, 119–126. [Google Scholar] [CrossRef]
- Schnettler, E.; Sterken, M.G.; Leung, J.Y.; Metz, S.W.; Geertsema, C.; Goldbach, R.W.; Vlak, J.M.; Kohl, A.; Khromykh, A.A.; Pijlman, G.P. Noncoding Flavivirus RNA Displays RNA Interference Suppressor Activity in Insect and Mammalian Cells. J. Virol. 2012, 86, 13486–13500. [Google Scholar] [CrossRef]
- Pierson, T.C.; Sánchez, M.D.; Puffer, B.A.; Ahmed, A.A.; Geiss, B.J.; Valentine, L.E.; Altamura, L.A.; Diamond, M.S.; Doms, R.W. A Rapid and Quantitative Assay for Measuring Antibody-Mediated Neutralization of West Nile Virus Infection. Virology 2006, 346, 53–65. [Google Scholar] [CrossRef]
- Chapman, E.G.; Moon, S.L.; Wilusz, J.; Kieft, J.S. RNA Structures That Resist Degradation by Xrn1 Produce a Pathogenic Dengue Virus RNA. eLife 2014, 3, e01892. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A Resource of Human and Mouse PCR Primer Pairs for Gene Expression Detection and Quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-Performance Genomics Data Visualization and Exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, Vienna, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 6 June 2023).
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome Pathway Analysis: A High-Performance in-Memory Approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innov. Camb. Mass 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- El-Merhie, N.; Baumgart-Vogt, E.; Pilatz, A.; Pfreimer, S.; Pfeiffer, B.; Pak, O.; Kosanovic, D.; Seimetz, M.; Schermuly, R.T.; Weissmann, N.; et al. Differential Alterations of the Mitochondrial Morphology and Respiratory Chain Complexes during Postnatal Development of the Mouse Lung. Oxid. Med. Cell. Longev. 2017, 2017, 9169146. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.C.; Killington, R.A. Virus Isolation and Quantitation. In Virology Methods Manual; Elsevier: Amsterdam, The Netherlands, 1996; pp. 25–46. ISBN 978-0-12-465330-6. [Google Scholar]
- Wang, Y.; Luo, W.; Wang, X.; Ma, Y.; Huang, L.; Wang, Y. MAMDC2, a Gene Highly Expressed in Microglia in Experimental Models of Alzheimers Disease, Positively Regulates the Innate Antiviral Response during Neurotropic Virus Infection. J. Infect. 2022, 84, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Paez-Gonzalez, P.; Abdi, K.; Luciano, D.; Liu, Y.; Soriano-Navarro, M.; Rawlins, E.; Bennett, V.; Garcia-Verdugo, J.M.; Kuo, C.T. Ank3-Dependent SVZ Niche Assembly Is Required for the Continued Production of New Neurons. Neuron 2011, 71, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.R.; O’Donovan, M.C.; Meng, Y.A.; Jones, I.R.; Ruderfer, D.M.; Jones, L.; Fan, J.; Kirov, G.; Perlis, R.H.; Green, E.K.; et al. Collaborative Genome-Wide Association Analysis Supports a Role for ANK3 and CACNA1C in Bipolar Disorder. Nat. Genet. 2008, 40, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; de Sessions, P.F.; Leon, M.A.; Scholle, F. West Nile Virus Nonstructural Protein 1 Inhibits TLR3 Signal Transduction. J. Virol. 2008, 82, 8262–8271. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Chen, H.B.; Wang, X.J.; Huang, H.; Khromykh, A.A. Analysis of Adaptive Mutations in Kunjin Virus Replicon RNA Reveals a Novel Role for the Flavivirus Nonstructural Protein NS2A in Inhibition of Beta Interferon Promoter-Driven Transcription. J. Virol. 2004, 78, 12225–12235. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Boer, E.F.; Lubick, K.J.; Wolfinbarger, J.B.; Carmody, A.B.; Rockx, B.; Liu, W.; Ashour, J.; Shupert, W.L.; Holbrook, M.R.; et al. The NS5 Protein of the Virulent West Nile Virus NY99 Strain Is a Potent Antagonist of Type I Interferon-Mediated JAK-STAT Signaling. J. Virol. 2010, 84, 3503–3515. [Google Scholar] [CrossRef]
- Schuessler, A.; Funk, A.; Lazear, H.M.; Cooper, D.A.; Torres, S.; Daffis, S.; Jha, B.K.; Kumagai, Y.; Takeuchi, O.; Hertzog, P.; et al. West Nile Virus Noncoding Subgenomic RNA Contributes to Viral Evasion of the Type I Interferon-Mediated Antiviral Response. J. Virol. 2012, 86, 5708–5718. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Li, X.-F.; Niu, X.; Li, N.; Wang, H.-J.; Deng, C.-L.; Ye, H.-Q.; Huang, X.-Y.; Chen, Q.; Xu, Y.-P.; et al. Short Direct Repeats in the 3’ Untranslated Region Are Involved in Subgenomic Flaviviral RNA Production. J. Virol. 2020, 94, e01175-19. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Fros, J.J.; Miesen, P.; Vogels, C.B.F.; van der Bent, M.L.; Geertsema, C.; Koenraadt, C.J.M.; van Rij, R.P.; van Oers, M.M.; Pijlman, G.P. Noncoding Subgenomic Flavivirus RNA Is Processed by the Mosquito RNA Interference Machinery and Determines West Nile Virus Transmission by Culex Pipiens Mosquitoes. J. Virol. 2016, 90, 10145–10159. [Google Scholar] [CrossRef] [PubMed]
- Bidet, K.; Garcia-Blanco, M.A. Flaviviral RNAs: Weapons and Targets in the War between Virus and Host. Biochem. J. 2014, 462, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Zou, J.; Zhang, B.; Yuan, Z. Dengue Virus Subgenomic RNA Induces Apoptosis through the Bcl-2-Mediated PI3k/Akt Signaling Pathway. Virology 2014, 448, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.G.C.; Pereira, C.F.; Dalebout, T.J.; Spaan, W.J.M.; Bredenbeek, P.J. An RNA Pseudoknot Is Required for Production of Yellow Fever Virus Subgenomic RNA by the Host Nuclease XRN1. J. Virol. 2010, 84, 11395–11406. [Google Scholar] [CrossRef] [PubMed]
- Slonchak, A.; Parry, R.; Pullinger, B.; Sng, J.D.J.; Wang, X.; Buck, T.F.; Torres, F.J.; Harrison, J.J.; Colmant, A.M.G.; Hobson-Peters, J.; et al. Structural Analysis of 3’UTRs in Insect Flaviviruses Reveals Novel Determinants of sfRNA Biogenesis and Provides New Insights into Flavivirus Evolution. Nat. Commun. 2022, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- Slonchak, A.; Hugo, L.E.; Freney, M.E.; Hall-Mendelin, S.; Amarilla, A.A.; Torres, F.J.; Setoh, Y.X.; Peng, N.Y.G.; Sng, J.D.J.; Hall, R.A.; et al. Zika Virus Noncoding RNA Suppresses Apoptosis and Is Required for Virus Transmission by Mosquitoes. Nat. Commun. 2020, 11, 2205. [Google Scholar] [CrossRef]
- Slonchak, A.; Wang, X.; Aguado, J.; Sng, J.D.J.; Chaggar, H.; Freney, M.E.; Yan, K.; Torres, F.J.; Amarilla, A.A.; Balea, R.; et al. Zika Virus Noncoding RNA Cooperates with the Viral Protein NS5 to Inhibit STAT1 Phosphorylation and Facilitate Viral Pathogenesis. Sci. Adv. 2022, 8, eadd8095. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Freppel, W.; Sow, A.A.; Chatel-Chaix, L. The Interplay between Dengue Virus and the Human Innate Immune System: A Game of Hide and Seek. Vaccines 2019, 7, 145. [Google Scholar] [CrossRef]
- Donald, C.L.; Brennan, B.; Cumberworth, S.L.; Rezelj, V.V.; Clark, J.J.; Cordeiro, M.T.; Freitas De Oliveira França, R.; Pena, L.J.; Wilkie, G.S.; Da Silva Filipe, A.; et al. Full Genome Sequence and sfRNA Interferon Antagonist Activity of Zika Virus from Recife, Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0005048. [Google Scholar] [CrossRef]
- Slonchak, A.; Chaggar, H.; Aguado, J.; Wolvetang, E.; Khromykh, A.A. Noncoding RNA of Zika Virus Affects Interplay between Wnt-Signaling and Pro-Apoptotic Pathways in the Developing Brain Tissue. Viruses 2023, 15, 1062. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Pijlman, G.P. Dengue Non-Coding RNA: TRIMmed for Transmission. Cell Host Microbe 2015, 18, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Corder, K.M.; Li, Q.; Cortes, M.A.; Bartley, A.F.; Davis, T.R.; Dobrunz, L.E. Overexpression of Neuropeptide Y Decreases Responsiveness to Neuropeptide Y. Neuropeptides 2020, 79, 101979. [Google Scholar] [CrossRef] [PubMed]
- Malva, J.O.; Xapelli, S.; Baptista, S.; Valero, J.; Agasse, F.; Ferreira, R.; Silva, A.P. Multifaces of Neuropeptide Y in the Brain—Neuroprotection, Neurogenesis and Neuroinflammation. Neuropeptides 2012, 46, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Xapelli, S.; Santos, T.; Silva, A.P.; Cristóvão, A.; Cortes, L.; Malva, J.O. Neuropeptide Y Modulation of Interleukin-1{β} (IL-1{β})-Induced Nitric Oxide Production in Microglia. J. Biol. Chem. 2010, 285, 41921–41934. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.; Brot, S.; Gaillard, A. Neuroprotective Effects of Neuropeptide Y against Neurodegenerative Disease. Curr. Neuropharmacol. 2022, 20, 1717–1725. [Google Scholar] [CrossRef]
- Woods, T.A.; Du, M.; Carmody, A.; Peterson, K.E. Neuropeptide Y Negatively Influences Monocyte Recruitment to the Central Nervous System during Retrovirus Infection. J. Virol. 2015, 90, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Butchi, N.B.; Woods, T.; Morgan, T.W.; Peterson, K.E. Neuropeptide Y Has a Protective Role during Murine Retrovirus-Induced Neurological Disease. J. Virol. 2010, 84, 11076–11088. [Google Scholar] [CrossRef] [PubMed]
- Pardon, M.-C. Anti-Inflammatory Potential of Thymosin Β4 in the Central Nervous System: Implications for Progressive Neurodegenerative Diseases. Expert Opin. Biol. Ther. 2018, 18, 165–169. [Google Scholar] [CrossRef]
- Chopp, M.; Zhang, Z.G. Thymosin Β4 as a Restorative/Regenerative Therapy for Neurological Injury and Neurodegenerative Diseases. Expert Opin. Biol. Ther. 2015, 15, 9–12. [Google Scholar] [CrossRef]
- Wang, M.; Feng, L.-R.; Li, Z.-L.; Ma, K.-G.; Chang, K.-W.; Chen, X.-L.; Yang, P.-B.; Ji, S.-F.; Ma, Y.-B.; Han, H.; et al. Thymosin Β4 Reverses Phenotypic Polarization of Glial Cells and Cognitive Impairment via Negative Regulation of NF-κB Signaling Axis in APP/PS1 Mice. J. Neuroinflamm. 2021, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheng, X.; Yao, Q.; Li, J.; Ju, G. The Promotive Effects of Thymosin Β4 on Neuronal Survival and Neurite Outgrowth by Upregulating L1 Expression. Neurochem. Res. 2008, 33, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Scherbik, S.V.; Brinton, M.A. Virus-Induced Ca2+ Influx Extends Survival of West Nile Virus-Infected Cells. J. Virol. 2010, 84, 8721–8731. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Inagaki, H.; Hoshino, S. Calpain Mediates Processing of the Translation Termination Factor eRF3 into the IAP-Binding Isoform p-eRF3. FEBS Lett. 2015, 589, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Gao, Y.; Shen, Q.; Li, C.; Chang, W.; Chai, B. Polypeptide Chain Release Factor eRF3 Is a Novel Molecular Partner of Survivin. Cell Biol. Int. 2013, 37, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kumagai, N.; Hosoda, N.; Hoshino, S.-I. The Processed Isoform of the Translation Termination Factor eRF3 Localizes to the Nucleus to Interact with the ARF Tumor Suppressor. Biochem. Biophys. Res. Commun. 2014, 445, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Friebe, P.; Boudet, J.; Simorre, J.-P.; Bartenschlager, R. Kissing-Loop Interaction in the 3’ End of the Hepatitis C Virus Genome Essential for RNA Replication. J. Virol. 2005, 79, 380–392. [Google Scholar] [CrossRef]
- Koh, W.-L.; Ng, M.-L. Molecular Mechanisms of West Nile Virus Pathogenesis in Brain Cell. Emerg. Infect. Dis. 2005, 11, 629–632. [Google Scholar] [CrossRef]
- Maximova, O.A.; Sturdevant, D.E.; Kash, J.C.; Kanakabandi, K.; Xiao, Y.; Minai, M.; Moore, I.N.; Taubenberger, J.; Martens, C.; Cohen, J.I.; et al. Virus Infection of the CNS Disrupts the Immune-Neural-Synaptic Axis via Induction of Pleiotropic Gene Regulation of Host Responses. eLife 2021, 10, e62273. [Google Scholar] [CrossRef]
- Lim, S.M.; van den Ham, H.-J.; Oduber, M.; Martina, E.; Zaaraoui-Boutahar, F.; Roose, J.M.; van IJcken, W.F.J.; Osterhaus, A.D.M.E.; Andeweg, A.C.; Koraka, P.; et al. Transcriptomic Analyses Reveal Differential Gene Expression of Immune and Cell Death Pathways in the Brains of Mice Infected with West Nile Virus and Chikungunya Virus. Front. Microbiol. 2017, 8, 1556. [Google Scholar] [CrossRef]
- Clarke, P.; Leser, J.S.; Bowen, R.A.; Tyler, K.L. Virus-Induced Transcriptional Changes in the Brain Include the Differential Expression of Genes Associated with Interferon, Apoptosis, Interleukin 17 Receptor A, and Glutamate Signaling as Well as Flavivirus-Specific Upregulation of tRNA Synthetases. mBio 2014, 5, e00902–e00914. [Google Scholar] [CrossRef]
- Kumar, M.; Belcaid, M.; Nerurkar, V.R. Identification of Host Genes Leading to West Nile Virus Encephalitis in Mice Brain Using RNA-Seq Analysis. Sci. Rep. 2016, 6, 26350. [Google Scholar] [CrossRef]
- Clarke, P.; Leser, J.S.; Quick, E.D.; Dionne, K.R.; Beckham, J.D.; Tyler, K.L. Death Receptor-Mediated Apoptotic Signaling Is Activated in the Brain Following Infection with West Nile Virus in the Absence of a Peripheral Immune Response. J. Virol. 2014, 88, 1080–1089. [Google Scholar] [CrossRef]
- Venter, M.; Myers, T.G.; Wilson, M.A.; Kindt, T.J.; Paweska, J.T.; Burt, F.J.; Leman, P.A.; Swanepoel, R. Gene Expression in Mice Infected with West Nile Virus Strains of Different Neurovirulence. Virology 2005, 342, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, M.A.; Denslow, N.D.; Seino, K.S.; Barber, D.S.; Long, M.T. Gene Expression Analysis in the Thalamus and Cerebrum of Horses Experimentally Infected with West Nile Virus. PLoS ONE 2011, 6, e24371. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, S.D.; Weichhart, T. Effects of Interferons and Viruses on Metabolism. Front. Immunol. 2016, 7, 630. [Google Scholar] [CrossRef]
- Burke, J.D.; Platanias, L.C.; Fish, E.N. Beta Interferon Regulation of Glucose Metabolism Is PI3K/Akt Dependent and Important for Antiviral Activity against Coxsackievirus B3. J. Virol. 2014, 88, 3485–3495. [Google Scholar] [CrossRef]
- Wu, D.; Sanin, D.E.; Everts, B.; Chen, Q.; Qiu, J.; Buck, M.D.; Patterson, A.; Smith, A.M.; Chang, C.-H.; Liu, Z.; et al. Type 1 Interferons Induce Changes in Core Metabolism That Are Critical for Immune Function. Immunity 2016, 44, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Pattanakitsakul, S.; Sinchaikul, S.; Chen, S.-T.; Thongboonkerd, V. The Ubiquitin-Proteasome Pathway Is Important for Dengue Virus Infection in Primary Human Endothelial Cells. J. Proteome Res. 2010, 9, 4960–4971. [Google Scholar] [CrossRef]
- Gilfoy, F.; Fayzulin, R.; Mason, P.W. West Nile Virus Genome Amplification Requires the Functional Activities of the Proteasome. Virology 2009, 385, 74–84. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.-D.; Meertens, L.; Bonazzi, M.; Cossart, P.; Arenzana-Seisdedos, F.; Amara, A. Appraising the Roles of CBLL1 and the Ubiquitin/Proteasome System for Flavivirus Entry and Replication. J. Virol. 2011, 85, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Nag, D.K.; Finley, D. A Small-Molecule Inhibitor of Deubiquitinating Enzyme USP14 Inhibits Dengue Virus Replication. Virus Res. 2012, 165, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Basters, A.; Knobeloch, K.-P.; Fritz, G. USP18—A Multifunctional Component in the Interferon Response. Biosci. Rep. 2018, 38, BSR20180250. [Google Scholar] [CrossRef] [PubMed]
- François-Newton, V.; Magno de Freitas Almeida, G.; Payelle-Brogard, B.; Monneron, D.; Pichard-Garcia, L.; Piehler, J.; Pellegrini, S.; Uzé, G. USP18-Based Negative Feedback Control Is Induced by Type I and Type III Interferons and Specifically Inactivates Interferon α Response. PLoS ONE 2011, 6, e22200. [Google Scholar] [CrossRef] [PubMed]
- Honke, N.; Shaabani, N.; Zhang, D.-E.; Hardt, C.; Lang, K.S. Multiple Functions of USP18. Cell Death Dis. 2016, 7, e2444. [Google Scholar] [CrossRef]
- Ye, H.; Duan, X.; Yao, M.; Kang, L.; Li, Y.; Li, S.; Li, B.; Chen, L. USP18 Mediates Interferon Resistance of Dengue Virus Infection. Front. Microbiol. 2021, 12, 682380. [Google Scholar] [CrossRef]
- He, X.; Ashbrook, A.W.; Du, Y.; Wu, J.; Hoffmann, H.-H.; Zhang, C.; Xia, L.; Peng, Y.-C.; Tumas, K.C.; Singh, B.K.; et al. RTP4 Inhibits IFN-I Response and Enhances Experimental Cerebral Malaria and Neuropathology. Proc. Natl. Acad. Sci. USA 2020, 117, 19465–19474. [Google Scholar] [CrossRef]
- Choi, S.S.; Bradrick, S.; Qiang, G.; Mostafavi, A.; Chaturvedi, G.; Weinman, S.A.; Diehl, A.M.; Jhaveri, R. Up-Regulation of Hedgehog Pathway Is Associated with Cellular Permissiveness for Hepatitis C Virus Replication. Hepatology 2011, 54, 1580–1590. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Blázquez, A.-B.; Saiz, J.-C. Reconciling West Nile Virus with the Autophagic Pathway. Autophagy 2015, 11, 861–864. [Google Scholar] [CrossRef]
- Kobayashi, S.; Orba, Y.; Yamaguchi, H.; Takahashi, K.; Sasaki, M.; Hasebe, R.; Kimura, T.; Sawa, H. Autophagy Inhibits Viral Genome Replication and Gene Expression Stages in West Nile Virus Infection. Virus Res. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Bębnowska, D.; Niedźwiedzka-Rystwej, P. The Interplay between Autophagy and Virus Pathogenesis—The Significance of Autophagy in Viral Hepatitis and Viral Hemorrhagic Fevers. Cells 2022, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Beatman, E.; Oyer, R.; Shives, K.D.; Hedman, K.; Brault, A.C.; Tyler, K.L.; Beckham, J.D. West Nile Virus Growth Is Independent of Autophagy Activation. Virology 2012, 433, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Vandergaast, R.; Fredericksen, B.L. West Nile Virus (WNV) Replication Is Independent of Autophagy in Mammalian Cells. PLoS ONE 2012, 7, e45800. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Yoshii, K.; Phongphaew, W.; Muto, M.; Hirano, M.; Orba, Y.; Sawa, H.; Kariwa, H. West Nile Virus Capsid Protein Inhibits Autophagy by AMP-Activated Protein Kinase Degradation in Neurological Disease Development. PLoS Pathog. 2020, 16, e1008238. [Google Scholar] [CrossRef] [PubMed]
- Shives, K.D.; Beatman, E.L.; Chamanian, M.; O’Brien, C.; Hobson-Peters, J.; Beckham, J.D. West Nile Virus-Induced Activation of Mammalian Target of Rapamycin Complex 1 Supports Viral Growth and Viral Protein Expression. J. Virol. 2014, 88, 9458–9471. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Liao, C.-L.; Lin, Y.-L. Flavivirus Activates Phosphatidylinositol 3-Kinase Signaling to Block Caspase-Dependent Apoptotic Cell Death at the Early Stage of Virus Infection. J. Virol. 2005, 79, 8388–8399. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, V.; Cinti, A.; Amorim, R.; Mouland, A.J. Adapting the Stress Response: Viral Subversion of the mTOR Signaling Pathway. Viruses 2016, 8, 152. [Google Scholar] [CrossRef]
- Hasty, P.; Sharp, Z.D.; Curiel, T.J.; Campisi, J. mTORC1 and P53: Clash of the Gods? Cell Cycle 2013, 12, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Levine, A.J. The Regulation of Energy Metabolism and the IGF-1/mTOR Pathways by the P53 Protein. Trends Cell Biol. 2010, 20, 427–434. [Google Scholar] [CrossRef]
- Rothan, H.A.; Fang, S.; Mahesh, M.; Byrareddy, S.N. Zika Virus and the Metabolism of Neuronal Cells. Mol. Neurobiol. 2019, 56, 2551–2557. [Google Scholar] [CrossRef]
- Cui, D.; Qu, R.; Liu, D.; Xiong, X.; Liang, T.; Zhao, Y. The Cross Talk Between P53 and mTOR Pathways in Response to Physiological and Genotoxic Stresses. Front. Cell Dev. Biol. 2021, 9, 775507. [Google Scholar] [CrossRef] [PubMed]
- Fraisier, C.; Camoin, L.; Lim, S.M.; Bakli, M.; Belghazi, M.; Fourquet, P.; Granjeaud, S.; Osterhaus, A.D.M.E.; Koraka, P.; Martina, B.; et al. Altered Protein Networks and Cellular Pathways in Severe West Nile Disease in Mice. PLoS ONE 2013, 8, e68318. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; West, N.; Vider, J.; Zhang, P.; Griffiths, R.E.; Wolvetang, E.; Burtonclay, P.; Warrilow, D. Inflammatory Responses to a Pathogenic West Nile Virus Strain. BMC Infect. Dis. 2019, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Guan, C.; Jiang, Y.; Chen, G.; Zhao, C.; Cui, K.; Song, Y.; Wu, C.; Poo, M.; Yuan, X. Ca2+-Dependent Regulation of Rho GTPases Triggers Turning of Nerve Growth Cones. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 2338–2347. [Google Scholar] [CrossRef]
- Semenova, M.M.; Mäki-Hokkonen, A.M.J.; Cao, J.; Komarovski, V.; Forsberg, K.M.; Koistinaho, M.; Coffey, E.T.; Courtney, M.J. Rho Mediates Calcium-Dependent Activation of P38alpha and Subsequent Excitotoxic Cell Death. Nat. Neurosci. 2007, 10, 436–443. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bampali, M.; Kouvela, A.; Kesesidis, N.; Kassela, K.; Dovrolis, N.; Karakasiliotis, I. West Nile Virus Subgenomic RNAs Modulate Gene Expression in a Neuronal Cell Line. Viruses 2024, 16, 812. https://doi.org/10.3390/v16050812
Bampali M, Kouvela A, Kesesidis N, Kassela K, Dovrolis N, Karakasiliotis I. West Nile Virus Subgenomic RNAs Modulate Gene Expression in a Neuronal Cell Line. Viruses. 2024; 16(5):812. https://doi.org/10.3390/v16050812
Chicago/Turabian StyleBampali, Maria, Adamantia Kouvela, Nikolaos Kesesidis, Katerina Kassela, Nikolas Dovrolis, and Ioannis Karakasiliotis. 2024. "West Nile Virus Subgenomic RNAs Modulate Gene Expression in a Neuronal Cell Line" Viruses 16, no. 5: 812. https://doi.org/10.3390/v16050812
APA StyleBampali, M., Kouvela, A., Kesesidis, N., Kassela, K., Dovrolis, N., & Karakasiliotis, I. (2024). West Nile Virus Subgenomic RNAs Modulate Gene Expression in a Neuronal Cell Line. Viruses, 16(5), 812. https://doi.org/10.3390/v16050812







